Abstract
Lymphopenia enhances the effectiveness of adoptive immunotherapy by facilitating expansion of transferred T cells but also limits the T-cell repertoire available to mediate immune responses and, in humans, is associated with chronic immune dysfunction. Previous studies concluded that lymphopenia augments adoptive immunotherapy by diminishing Tregs and increasing homeostatic cytokines. We sought to determine whether targeted therapies that replicate the physiology of lymphopenia in lymphoreplete hosts could provide a similarly supportive milieu. Pmel-1 T cells were transferred to B16-bearing lymphopenic versus lymphoreplete mice receiving αCD25 and/or recombinant human interleukin-7. Although CD25-based Treg depletion was inefficient because of peripheral expansion of CD4+CD25−FOXP3+ cells, outcomes were better in αCD25-treated lymphoreplete hosts than in lymphopenic hosts, and adoptive immunotherapy was most effective in lymphoreplete hosts receiving αCD25 plus recombinant human interleukin-7. Lymphopenic hosts supported increased proliferation of adoptively transferred antigen-specific T cells, but cells transferred to lymphoreplete recipients receiving targeted therapies showed superior function. Further, determinant spreading was substantial in lymphoreplete hosts but absent in lymphopenic hosts. These results demonstrate that targeted therapies delivered to mimic the “physiology of lymphopenia” enhance the efficacy of adoptive immunotherapy in lymphoreplete hosts and provide a potentially superior alternative to the induction of lymphopenia.
Introduction
T-cell depletion induces profound changes in lymphocyte physiology and has long been known to augment T-cell proliferation in vivo via homeostatic peripheral expansion (HPE).1-3 Recent preclinical and clinical studies have concluded that HPE renders adoptive cell therapies more effective in lymphopenic, compared with lymphoreplete, hosts.4-7 This has led some to recommend the deliberate induction of T-cell depletion before adoptive immunotherapy as a means to enhance effectiveness.6,8 Although such approaches may improve outcomes in the short term, T-cell depletion in humans is associated with prolonged immune dysfunction. Because of impaired thymic regenerative capacity, most adult patients rendered lymphopenic with cytotoxic therapies, bone marrow transplantation, or monoclonal antibody (mAb) therapy for inflammatory diseases show persistent CD4+ lymphopenia and limited immune competence when examined more than 2 years after completion of therapy.9,10 CD4+ depletion is associated with enhanced susceptibility to common and opportunistic infections,11,12 and potentially increases the risk for tumor recurrence or second malignant neoplasms. Furthermore, therapies used to induce lymphodepletion induce toxic side effects.8,13 Thus, although the altered immune physiology associated with lymphopenia provides an attractive adjunct for improving the effectiveness of adoptive immunotherapy, the negative consequences of T-cell depletion in humans could offset the benefits.
During the last 10 years, specific factors that drive HPE during lymphopenia have been more completely characterized. Briefly, lymphopenic hosts show augmented T-cell responses to cognate antigens,14,15 enhanced T-cell proliferation to low-affinity self-antigens,16,17 and enhanced antigen-independent cycling of memory CD8+ T cells.18 Current models hold that increased levels of homeostatic cytokines present in lymphopenic hosts, including but not limited to interleukin-7 (IL-7), contribute to these effects.3,4,13,19 Diminished regulatory T-cell numbers are also thought to play a role in augmenting the effectiveness of adoptive immunotherapy in lymphopenic hosts.4,20 Based on this paradigm, we sought to determine whether the specific administration of targeted therapies, which replicate the “physiology of lymphopenia” in lymphoreplete hosts, could enhance the effectiveness of adoptive immunotherapy while avoiding the detrimental effects of lymphopenia.
Methods
Mice and tumor lines
Female C57BL/6 mice were obtained from the Animal Production Unit, National Cancer Institute (NCI). Where noted, at 4 to 5 weeks of age, thymectomized (TXY) mice underwent vacuum suction removal of the thymus according standard protocol. In experiments using TXY groups, a postmortem evaluation of each mouse was performed at the completion of each experiment to verify the completeness of thymectomy. Rag1−/− mice were purchansed from The Jackson Laboratory. C57BL/6-pmel-1-Thy1.1 Tg mice were a generous gift from Nick Restifo (Surgery Branch in NCI, National Institutes of Health). Mice were housed in a strict pathogen-free environment at the NCI animal facility at the National Institutes of Health. All studies were approved by the NCI Animal Care and Use Committee. The murine melanoma cell line B16/F1-luc was transduced with cDNA encoding firefly (Photinus puralis) luciferase as previously described.21
Flow cytometric analysis
Cells were stained and analyzed on a FACSCaliber or FACSAria (BD Biosciences). The following mAbs were used: anti–CD25-fluorescein isothiocyanate (FITC) (7D4), anti–CD4-peridinin chlorophyll protein (PerCP)/Cy5.5 (RM4-5), anti–CD62L-allophycocyanin (APC; MEL-14), anti–CD8-APC (53-6.7), anti–Thy1.1-PerCP (OX-7), anti–CD4-phycoerythrin (PE) (RM4-5), anti–Vβ13-FITC (MR12-3), and anti–CD4-Pacific Blue (RM4-5) from BD Biosciences; anti–Foxp3-PE (FJK-16s) and anti–CD127-AlexaFluor 750 (A7R34) were from eBioscience. Intracellular Foxp3 (FJK-16s; eBioscience) and bromodeoxyuridine (BrdU) incorporation (APC BrdU flow kit; BD Biosciences) were detected according to the manufacturer's instructions.
Treg depletion, recombinant human IL-7 therapy, and irradiation
Treg depletion was accomplished using affinity-purified rat anti–mouse anti-CD25 (clone PC61) antibody purchased from the Biologic Resources Branch, NCI. Anti-CD25 was administered as 3 injections of 0.3 mg given intraperitoneally 72 hours apart. Doses and schedules of antibody administration were determined based on its capacity to deplete more than 75% of CD25+ T cells. To allow for clearance of monoclonal antibodies, at least 10 days elapsed after antibody therapy before further experimental manipulation. For analysis of Treg depletion and for numbers of antigen-specific T cells, single-cell suspensions were prepared from bilateral inguinal lymph nodes pooled for individual mice; then cells were stained with anti–CD25-FITC (7D4), anti–Foxp3-PE, anti–CD4-PerCP/Cy5.5, and anti–CD62L-APC. Lyophilized recombinant human IL-7 (rhIL-7; kindly provided by Cytheris Inc) was reconstituted with sterile water and resuspended in phosphate-buffered saline (PBS) buffer before injection. A total of 10 μg/mouse rhIL-7 was administered intraperitoneally once a day, Monday through Friday, for 6 weeks. Where indicated, mice were irradiated with 500 cGy 24 hours before tumor inoculation and 4 days before adoptive cell transfer.
Pmel-1 cells isolation and labeling with carboxyfluorescein succinimidyl ester
Axillary and inguinal lymph nodes were harvested from C57BL/6-pmel-1-Thy1.1 Tg mice. The nodes were teased apart to obtain a single-cell suspension, and the cells were passed through 70-μm nylon mesh. The cells were analyzed on FACSCaliber by staining with anti-Vβ13-FITC, anti–CD4-PE, anti–CD8-APC, and anti–Thy1.1-PerCP antibodies. The pmel-1 cells accounted for 95% of CD8+ cells and 30% of total lymph node cells. Where indicated, pmel-1 cells were activated before adoptive transfer as described previously.22 Before injection, the lymph node cells were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) for 15 minutes at room temperature, washed twice in PBS, and then resuspended in serum-free RPMI 1640.
Immunotherapy of B16 melanoma
B16-luc cells were grown to confluence and harvested by trypsin digestion, and a single-cell suspension was prepared. Animals were treated with anti-CD25 mouse antibody (PC61) as described in “Methods” (Figure 2). A total of 4 × 105 B16-luc cells was injected subcutaneously on day 10 after the last dose of PC61. At 3 days after tumor challenge, 1 to 2 × 106 naive pmel-1-1 cells, which were freshly isolated from lymph nodes of C57BL/6-pmel-1-Thy1.1 Tg mice, were injected intravenously in the tail vein of recipient C57BL/6 mice where designated. Tumors were measured twice weekly and animals were humanely killed according to NCI Animal Care and Use guidelines (maximal tumor dimension of 2 cm, tumor ulceration, or severe morbidity resulting from tumor growth).
In vivo bioluminescence imaging
Starting at day 10 after B16-luc cells inoculation, bioluminescence imaging was performed every week to monitor tumor progression. Ten minutes before imaging, mice were injected intraperitoneally with 100 μL of 30 mg/mL of the luciferase substrate, D-Luciferin (Xenogen Corp) in PBS, and shaved over the inoculation area (right flank area) to minimize the amount of light absorbed by black fur. Mice were anesthetized with isoflurane in the knockdown chamber and then transferred to the imaging chamber (Xenogen IVIS imaging system) for imaging. A cooled charge-coupled device camera apparatus (Xenogen IVIS imaging system) was used to detect photon emission from tumor-bearing mice with an acquisition time of 60 seconds. The emitted photons were displayed as a pseudocolor graphic, superimposed over a conventional photographic image. Display and analyses of the images were performed using Living Image software (Xenogen) and Igor Image analysis software (Wave Metrics).
ELISpot assay
Mouse interferon-γ (IFN-γ) ELISpot plates were purchased from R&D Systems. Freshly isolated lymphocytes from mouse spleen, inguinal lymph node, and tumor draining inguinal lymph node were resuspended in HL-1 serum-free medium (BioWhittaker) supplemented with 1% penicillin/streptomycin/l-glutamine, and were plated at 106/well in duplicate. Synthetic melanoma-specific peptides were used as follows: (1) Gp100: the immunodominant Db restricted peptide corresponding to amino acids 25 to 33 (EGSRNQDWL) of the mouse gp100 gene; (2) Trp-2: the subdominant Kb restricted peptide corresponding to amino acids 180 to 188 (SVYDFFVWL) of the mouse Trp-2 gene; (3) Trp-1: the immunodominant I-Ab restricted peptide corresponding to amino acids 113 to 128 (CRPGWRGAACNQKILT) of the mouse Trp-1 gene; and as specific controls for (4) Db binding: the E7 peptide (RAHYNIVTF) derived from HPV and (5) I-Ab peptide: the Trypanosoma cruzi peptide (SHNFTLVASVIIEEA). Peptides were used as stimulators to each well at a final concentration of 10 μM. The experiments were performed according to manufacturer's instruction.
Image analysis of ELISpot plates was performed as already described with Series 1 Immunospot Satellite Analyzer (Cellular Technology) using software specifically designed for the ELISpot assay. Briefly, digitized images were analyzed for the presence of areas in which the color density exceeded that of the background by a preset factor based on the comparison of control wells and experimental wells. Specific spots were calculated by subtracting the average number of spots formed in specific control wells from the average number of spots formed in each experimental well.
Immunostaining
Frozen tissues were sectioned at 5-μm intervals and fixed using acetone. After blocking for 30 minutes in PBS supplemented with 5% goat serum, sections were incubated with primary antibodies. Rat monoclonal Foxp3 (FJK-16s, eBioscience) at 100 μg/mL was added for 30 minutes at room temperature. Goat anti–rat IgG Alexa Fluor 594 secondary antibodies at 1:400 were applied to sections for 30 minutes in the dark at room temperature and mounted with VectaShield containing 4,6-diamidino-2-phenylindole nuclear stain (Vector Laboratories). Sections were viewed using a Zeiss AxioObserver Z1 microscope (Zeiss Inc) using a 20× differential interference contrast objective and appropriate filter sets. Images were captured with a Zeiss AxioCam MRm monochrome digital camera and analyzed using AxioVision version 4.6 software.
Statistical analysis
Statistical tests were performed using GraphPad Prism, Version 4.0a for Macintosh (GraphPad Software Inc). Significant differences for comparisons of 2 groups were determined by the Mann-Whitney test and survival curves were analyzed using a Wilcoxon log-rank test. P values less than .05 were considered significant.
Results
Anti-CD25 and/or rhIL-7 induce modest decreases in Treg frequency
The goal of this work was to explore whether αCD25-mediated Treg depletion and/or the provision of supranormal levels of rhIL-7, a prototypic homeostatic cytokine, enhances the effectiveness of adoptive immunotherapy and to compare outcomes in T-cell–replete recipients treated in this manner with lymphopenic recipients of adoptive immunotherapy. We first enumerated sequential changes in Treg frequency on days 3, 10, 14, and 28 after administration of the αCD25 mAb PC61 (0.3 mg on days −6, −3, and 0) and/or rhIL-7 therapy (10 μg/day on days 0-28). Because Tregs can be regenerated via thymic-dependent and/or thymic-independent pathways23 and the efficiency of each pathway differs, we treated both thymus-bearing (Thy-B) and adult TXY hosts in these studies. Treg numbers nadired approximately 10 days after completion of αCD25, but the degree of depletion was modest, with Thy-B and TXY hosts showing mean nadir Treg frequencies of 35% and 45% those found in controls, and mean nadir of absolute Treg numbers that were 29% and 47% of controls (Figure 1A-B). Regeneration of CD25+FOXP3+ Tregs was rapid with absolute Treg numbers returning to baseline by day 28 after completion of αCD25, and thymic-independent because the pace and degree of recovery were essentially the same in Thy-B and TXY mice (Figure 1B). In contrast, regeneration of the CD62L+ subset of CD25+FOXP3+ cells was thymic-dependent as this subset was rapidly reconstituted in Thy-B hosts but was subnormal in TXY mice before depletion and remained depleted after αCD25 (Figure 1C).
Changes in Treg number and frequency after αCD25 and/or rhIL-7 in tumor-free animals. Where indicated, Thy-B and TXY C57BL/6 mice received 0.3 mg αCD25 on days −6, −3, and 0 and/or 10 μg rhIL-7 on days 0 to 28. (A) Percentage CD4+FOXP3+ Tregs in inguinal lymph nodes. (B) Absolute numbers of CD4+FOXP3+CD62L+ Tregs in inguinal lymph nodes. Results in panels A and B show the mean plus or minus SEM of the groups (n = 3-10/group), and results were pooled from 2 separate experiments. *P < .05, **P < .005, compared with control untreated Thy-B or TXY animals. (C) Representative fluorescence-activated cell sorter plots illustrating CD62L expression on FOXP3+ cells in gated CD4+ lymph node cells in Thy-B (left panel) and TXY hosts (right panel) at baseline and on the days indicated after αCD25. (D-E) BrdU incorporation in CD25+ and CD25− subsets of FOXP3+ LN cells in (D) untreated mice and (E) 6 days after αCD25 therapy. Blue line represents BrdU-injected animals; red line, control animals. Results are representative of 3 experiments with 10 to 13 mice/group.
Changes in Treg number and frequency after αCD25 and/or rhIL-7 in tumor-free animals. Where indicated, Thy-B and TXY C57BL/6 mice received 0.3 mg αCD25 on days −6, −3, and 0 and/or 10 μg rhIL-7 on days 0 to 28. (A) Percentage CD4+FOXP3+ Tregs in inguinal lymph nodes. (B) Absolute numbers of CD4+FOXP3+CD62L+ Tregs in inguinal lymph nodes. Results in panels A and B show the mean plus or minus SEM of the groups (n = 3-10/group), and results were pooled from 2 separate experiments. *P < .05, **P < .005, compared with control untreated Thy-B or TXY animals. (C) Representative fluorescence-activated cell sorter plots illustrating CD62L expression on FOXP3+ cells in gated CD4+ lymph node cells in Thy-B (left panel) and TXY hosts (right panel) at baseline and on the days indicated after αCD25. (D-E) BrdU incorporation in CD25+ and CD25− subsets of FOXP3+ LN cells in (D) untreated mice and (E) 6 days after αCD25 therapy. Blue line represents BrdU-injected animals; red line, control animals. Results are representative of 3 experiments with 10 to 13 mice/group.
Increasing the dose of αCD25 did not improve the efficiency of Treg depletion, and this was not the result of ineffectiveness of the antibody, as very few CD25+ cells remained and most FOXP3+ cells were CD25− after completion of αCD25 therapy (Figure 1D-E). BrdU labeling demonstrated that the CD25−FOXP3+ subset has a substantial proliferative rate at baseline compared with CD25+FOXP3+ cells, and after αCD25 therapy, cycling of both CD25+ and CD25−FOXP3+ cells increases substantially (Figure 1D-E). Thus, CD25-based depletion of the Treg pool is limited by rapid thymic-independent peripheral expansion of CD25−FOXP3+ cells, which presumably regenerates the CD25+FOXP3+ pool on clearance of the αCD25 mAb. In summary, Thy-B and TXY hosts experience modest transient depletion of peripheral Tregs after αCD25 therapy, and TXY hosts experience persistent depletion of naive CD4+FOXP3+CD62L+ regulatory cells.
We also analyzed the effects of rhIL-7 treatment, alone and in combination with αCD25, on Treg frequency and number. Consistent with observations made after rhIL-7 therapy in humans,24,25 rhIL-7 treatment led to transient declines in Treg frequencies in both Thy-B and TXY hosts resulting from expansion of non-Treg T cells (Figure 1A), but absolute numbers were not reduced (Figure 1B). Interestingly, at late time points, IL-7 therapy increased Tregs in Thy-B hosts but not in TXY hosts, suggesting an IL-7–mediated effect on thymic throughput, egress, or expansion of recent thymic emigrant Tregs as previously reported for human CD4+ and CD8+ naive T cells.25 When αCD25 and IL-7 were combined, the nadir of Treg depletion, pace, and degree of recovery were similar to that observed in animals treated with αCD25 alone, except that late IL-7–mediated increases in Treg numbers occurred in Thy-B hosts.
Lymphoreplete hosts treated with targeted therapy to replicate the physiology of lymphopenia show superior outcomes compared with lymphopenic hosts after adoptive immunotherapy
To determine whether targeted therapies delivered to mimic the physiology of lymphopenia, namely, Treg depletion and/or increased availability of IL-7, enhanced the effectiveness of adoptive immunotherapy, we administered these therapies to B16-bearing mice receiving T-cell receptor (TCR) Tg+pmel-1 cells with specificity for gp100, the immunodominant tumor antigen in this model system26 (Figure 2A). Pmel-1 cells have been shown previously to be capable of inducing B16 tumor regression in lymphopenic mice when expanded ex vivo and combined with vaccines and IL-2.5 Because the effects of the αCD25 mAb could diminish antigen-specific activation and/or expansion of activated T cells, pmel-1 cells were not transferred until 13 days after the last dose of αCD25, which roughly corresponded to the point of Treg nadir. Treg depletion in tumor-draining lymph nodes was similar in pace and extent to that observed in spleens and lymph nodes of non–tumor-bearing hosts (Figure 2B-D), with the caveat that IL-7 did not induce the significant declines in Treg frequency in tumor-draining lymph nodes that were observed in lymph nodes of non–tumor-bearing mice (Figure 1A). Tregs also infiltrated the B16 tumors, and this infiltration was diminished in tissue sections of tumors from animals receiving αCD25 therapy plus rhIL-7 (Figure 2C bottom panel). Thus, αCD25 plus rhIL-7 administered to Thy-B and TXY mice induces modest, transient Treg depletion in tumors and tumor-draining lymph nodes.
Treg depletion after αCD25 and/or rhIL-7 in B16 tumor-bearing hosts. (A) Timing of tumor inoculation in relation to αCD25, rhIL-7, and adoptive transfer of pmel-1 LN cells. (B) Representative fluorescence-activated cell sorter plots on day 20 showing Treg frequencies in tumor-draining lymph nodes (TDLN) of treated mice. Results were consistent in 3 experiments. (C) Mean plus or minus SEM Treg frequencies in TDLNs of treated animals on day 16 (n = 6 or 7/group, results confirmed in 2 experiments). **P < .005. (D) Representative tissue sections on day 20 from TDLN and B16 tumors of animals from each treatment group showing FOXP3+ cells (red) versus nucleated cells (blue). Original magnification ×200.
Treg depletion after αCD25 and/or rhIL-7 in B16 tumor-bearing hosts. (A) Timing of tumor inoculation in relation to αCD25, rhIL-7, and adoptive transfer of pmel-1 LN cells. (B) Representative fluorescence-activated cell sorter plots on day 20 showing Treg frequencies in tumor-draining lymph nodes (TDLN) of treated mice. Results were consistent in 3 experiments. (C) Mean plus or minus SEM Treg frequencies in TDLNs of treated animals on day 16 (n = 6 or 7/group, results confirmed in 2 experiments). **P < .005. (D) Representative tissue sections on day 20 from TDLN and B16 tumors of animals from each treatment group showing FOXP3+ cells (red) versus nucleated cells (blue). Original magnification ×200.
We next compared tumor growth and survival after B16 inoculation plus or minus adoptive transfer of pmel-1 cells in Rag1−/− mice, a prototypic lymphopenic host versus lymphoreplete Thy-B or TXY hosts. Lymphopenic Rag1−/− animals died more quickly to growth of B16 melanoma compared with T-cell–replete Thy-B (median survival, 24 days vs 31 days in Rag1−/− vs Thy-B, P < .001) and TXY (median survival, 24 days vs 31 days in Rag1−/− vs TXY hosts, P = .004) hosts, consistent with modest immune surveillance in lymphoreplete mice (Figure 3). We next investigated the impact of adoptive transfer of pmel-1 LN cells on tumor growth and survival in each host. As shown in Figure 3, transfer of pmel-1 cells to B16-bearing hosts modestly diminished tumor growth rates and improved survival in lymphopenic Rag1−/− mice from 24 days to 30 days median survival (P = .015), whereas tumor growth rates and survival were not significantly changed in Thy-B (median survival, 31 days vs 31 days, P = not significant) or TXY mice (median survival, 31 days vs 31 days, P = not significant) receiving pmel-1 cells alone. Thus, the lymphopenic milieu showed superior support for adoptively transferred cells because adoptive transfer of pmel-1 cells alone improved outcomes in Rag1−/− lymphopenic recipients, but not in unmanipulated lymphoreplete hosts. However, the effect of the adoptively transferred cells in Rag1−/− mice was not sufficient to overcome the increased susceptibility to tumor growth these animals display at baseline. As a result, lymphopenic Rag1−/− hosts did not experience superior survival compared with unmanipulated Thy-B or TXY hosts receiving adoptive T-cell therapy for B16 melanoma (30 days vs 31 days vs 31 days, P = not significant).
Adoptive immunotherapy for B16 melanoma slows tumor growth and improves survival in lymphoreplete hosts versus lymphopenic Rag1−/− hosts. (A) Tumor growth curves (left) and survival curves (right) of treated groups as shown. B16 tumor cells were inoculated subcutaneously at day 0 in the graph. Statistically significant differences (*P < .05, **P < .005), compared with pmel-1 cells transferred mice. (B) Bioluminescence imaging of luciferase-expressing B16 tumors in Thy-B, TXY, and Rag1−/− mice imaged on day 20 after tumor cells inoculation. Tumor cells injection area (right flank area) were shaved to minimize the amount of light absorbed by black fur. Crosses pointing to necrotic tumors indicate dead mice resulting from tumor progression.
Adoptive immunotherapy for B16 melanoma slows tumor growth and improves survival in lymphoreplete hosts versus lymphopenic Rag1−/− hosts. (A) Tumor growth curves (left) and survival curves (right) of treated groups as shown. B16 tumor cells were inoculated subcutaneously at day 0 in the graph. Statistically significant differences (*P < .05, **P < .005), compared with pmel-1 cells transferred mice. (B) Bioluminescence imaging of luciferase-expressing B16 tumors in Thy-B, TXY, and Rag1−/− mice imaged on day 20 after tumor cells inoculation. Tumor cells injection area (right flank area) were shaved to minimize the amount of light absorbed by black fur. Crosses pointing to necrotic tumors indicate dead mice resulting from tumor progression.
We next sought to determine whether targeted therapies that mimic the lymphopenic milieu, namely, Treg depletion and/or the administration of rhIL-7, would improve the effectiveness of adoptive immunotherapy in lymphoreplete hosts. In this regard, the effect of αCD25-mediated Treg depletion in this model system was substantial (Figure 3). Anti-CD25 therapy significantly diminished tumor growth (P < .001 in Thy-B and TXY hosts) and improved median survival after pmel-1 transfer into lymphoreplete Thy-B (31 days vs 47 days, P < .001) and TXY hosts (31 days vs 53 days, P < .001), resulting in survival that was far superior to that seen in Rag1−/− hosts (Figure 3, P < .005 for αCD25 in Thy-B and TXY recipients vs Rag1−/−). This is remarkable considering that Rag1−/− hosts are essentially devoid of Treg populations, whereas αCD25 therapy in this regimen induced only limited, transient Treg depletion as shown in Figure 1. Notably, this effect was not unique to the B16 model system, as similar results were seen when αCD25 was administered to mice receiving TCR Tg+ T cells that recognize the immunodominant Uty peptide in the HY antigen complex were administered to female mice inoculated with MB49, an HY-expressing tumor27 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, even inefficient Treg depletion using αCD25 is sufficient to substantially enhance the effectiveness of adoptive immunotherapy for solid tumors.
rhIL-7 administration also diminished the rate of tumor growth in Rag1−/− and thymus-bearing mice (P < .01 in both) and improved survival after adoptive immunotherapy in Rag1−/− hosts (30 days vs 37 days, P = .004), with a trend toward improved survival in Thy-B hosts (31 vs 36 days, P = .07). Furthermore, in both Thy-B and TXY lymphoreplete hosts, Treg depletion combined with rhIL-7 led to diminished tumor growth rates (P < .05) and significantly greater survival (P < .001) compared with Rag1−/− hosts plus or minus rhIL-7 (Figure 3). In the clinical setting, lymphopenia is typically induced using cytotoxic therapy and/or irradiation, which could potentially enhance the effectiveness of adoptive immunotherapy by activation of innate immunity after lipopolysaccharide leakage across damaged gut mucosa or by inducing expression of damage-associated molecular patterns.28 We therefore compared outcomes after adoptive immunotherapy administered to hosts treated with sublethal irradation (500 cGy). Surprisingly, irradiated Rag1−/− hosts or Thy-B hosts receiving αCD25 showed enhanced tumor growth and diminished survival (Figure 4). Because the pmel-1 cells used here were obtained from TCR Tg+ mice and therefore represent largely naive populations whereas clinical protocols typically use activated effectors in adoptive immunotherapy, we also evaluated the efficacy of activated pmel-1 T cells in these models. Compared with resting pmel-1 cells, administration of activated pmel-1 cells administered to Rag1−/− mice did slow tumor growth modestly, but these results were not significant and overall survival remained inferior to that observed in lymphoreplete hosts treated with targeted therapies (Figure 4). Therefore, adoptive immunotherapy is more effective at eradicating B16 melanoma in lymphoreplete hosts treated with targeted therapies to mimic the “physiology of lymphopenia” than in lymphopenic Rag1−/− hosts that are completely devoid of regulatory T cells and that have physiologic elevations in IL-729 or in animals receiving sublethal irradiation before adoptive transfer.
Lymphoreplete hosts tyreated with Treg depletion show superior survivals compared with hosts receiving preimmunotherapy irradiation or activated T cells. Tumor growth curves (left) and survival curves (right) of treated groups as shown. Radiation therapy (500 cGy) administered to groups shown on day −1, B16 tumors inoculated subcutaneously on day 0, and pmel-1 cells administered on day 3. Where indicated, pmel-1 cells were activated before transfer as described in “Methods.” Results representative of 2 separate experiments (n = 4 or 5/group in each experiment). *P < .05.
Lymphoreplete hosts tyreated with Treg depletion show superior survivals compared with hosts receiving preimmunotherapy irradiation or activated T cells. Tumor growth curves (left) and survival curves (right) of treated groups as shown. Radiation therapy (500 cGy) administered to groups shown on day −1, B16 tumors inoculated subcutaneously on day 0, and pmel-1 cells administered on day 3. Where indicated, pmel-1 cells were activated before transfer as described in “Methods.” Results representative of 2 separate experiments (n = 4 or 5/group in each experiment). *P < .05.
Effective immunotherapy in lymphoreplete hosts is associated with enhanced functionality of transferred cells and enhanced epitope spreading
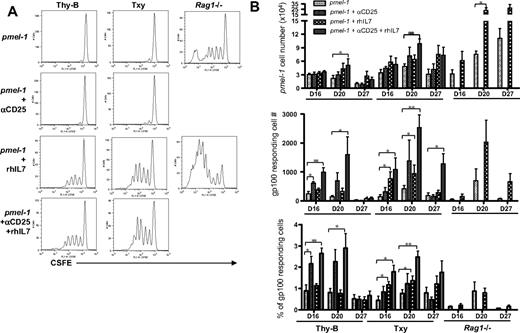
To investigate the biology responsible for these results, we studied expansion and function of the pmel-1 antigen-specific T cells after adoptive transfer into the various hosts. As shown in Figure 5A, the lymphopenic milieu present in Rag1−/− hosts provided a superior environment for proliferation of adoptively transferred CD8+ T cells. Whereas only approximately 2% of adoptively transferred pmel-1 T cells proliferated by day 20 in lymphoreplete Thy-B or TXY animals plus or minus αCD25, approximately 25% of pmel-1 T cells proliferated after transfer into Rag1−/− mice at the same time point. Moreover, the extent of cell proliferation was greatest in Rag1−/− mice with some pmel-1 cells demonstrating complete dilution of the CFSE dye by day 20 after transfer into the lymphopenic milieu, whereas pmel-1 cells found in the tumor-draining lymph nodes of untreated Thy-B and TXY hosts showed evidence for a maximum of 2 divisions at the same time point. Interestingly, although Treg depletion substantially diminished tumor growth and improved survival (Figure 3), this maneuver had little effect on the proliferation of adoptive transferred pmel-1 T cells. rhIL-7 substantially augmented proliferation in all hosts, but even rhIL-7–induced proliferation was greater in Rag1−/− hosts compared with lymphoreplete hosts.
High proliferation rates but low functionality of antigen-specific T cells in lymphopenic Rag1−/− hosts versus low proliferation but high functionality after Treg depletion plus or minus rhIL-7. (A) Representative CFSE profiles of pmel-1 T cells transferred into hosts with B16 melanoma treated as shown and gated using CD8/Thy1.1/Vβ13.1 on day 20 in schema shown in Figure 2A. Pmel-1 T cell transferred into Rag1−/− hosts show substantially increased numbers of cells with CFSE dilution compared with those transferred into lymphoreplete hosts. rhIL-7 increased pmel-1 proliferation in all groups. (B top panel) Absolute numbers of adoptively transferred pmel-1 T cells recovered from the TDLNs on day 20 of B16-bearing hosts treated as shown. Pmel-1 T cells were identified as per panel A. (Middle panel) Net number of cells producing IFN-γ in response to gp100 peptide, control Db126 peptide in groups, and at time points shown. (Bottom panel) Percentage of pmel-1 T cells producing IFN-γ in response to gp100 on day 20 calculated by dividing the number shown in the middle panel/total pmel-1 cells determined as per top panel. Thy-B (days 16, 20, and 27) and TXY (days 16 and 27) groups receiving αCD25 plus rhIL-7 show significantly higher IFN-γ production than Rag1−/− groups at the same time points. Results shown represent the mean ± SEM of the groups (n = 3-8/group), and consistent results were seen in 2 separate experiments. *P < .05. **P < .005.
High proliferation rates but low functionality of antigen-specific T cells in lymphopenic Rag1−/− hosts versus low proliferation but high functionality after Treg depletion plus or minus rhIL-7. (A) Representative CFSE profiles of pmel-1 T cells transferred into hosts with B16 melanoma treated as shown and gated using CD8/Thy1.1/Vβ13.1 on day 20 in schema shown in Figure 2A. Pmel-1 T cell transferred into Rag1−/− hosts show substantially increased numbers of cells with CFSE dilution compared with those transferred into lymphoreplete hosts. rhIL-7 increased pmel-1 proliferation in all groups. (B top panel) Absolute numbers of adoptively transferred pmel-1 T cells recovered from the TDLNs on day 20 of B16-bearing hosts treated as shown. Pmel-1 T cells were identified as per panel A. (Middle panel) Net number of cells producing IFN-γ in response to gp100 peptide, control Db126 peptide in groups, and at time points shown. (Bottom panel) Percentage of pmel-1 T cells producing IFN-γ in response to gp100 on day 20 calculated by dividing the number shown in the middle panel/total pmel-1 cells determined as per top panel. Thy-B (days 16, 20, and 27) and TXY (days 16 and 27) groups receiving αCD25 plus rhIL-7 show significantly higher IFN-γ production than Rag1−/− groups at the same time points. Results shown represent the mean ± SEM of the groups (n = 3-8/group), and consistent results were seen in 2 separate experiments. *P < .05. **P < .005.
Consistent with the CFSE profiles, larger numbers of pmel-1 cells were recovered after transfer into Rag1−/− hosts and rhIL-7–treated hosts (Figure 5B top panel), whereas Treg depletion did not significantly increase the number of pmel-1 cells present in the tumor-draining lymph nodes, spleen, or tumors themselves (data not shown). Interestingly, however, although the extensive division of pmel-1 cells transferred into Rag1−/− hosts illustrated by the CFSE profiles would predict substantially increased recovery on day 20 in Rag1−/− host, the increases in pmel-1 cells present in Rag1−/− hosts were only approximately 2- to 4-fold that found in TXY and Thy-B hosts, respectively. Previous studies have demonstrated that, in lymphopenic settings, dramatic T-cell proliferation is offset by a high rate of programmed cell death,30,31 and it is possible that the accumulation of pmel-1 cells in Rag1−/− hosts in this model system was also limited by a high rate of apoptosis. Regardless of the mechanism, these results suggest that the degree of proliferation experienced by antigen-specific T cells after adoptive transfer is not the primary factor determining antitumor effectiveness after adoptive immunotherapy; proliferation was clearly superior in lymphopenic hosts, but this did not correlate with the greatest antitumor effects.
We next sought to investigate whether the functional activity of adoptively transferred cells correlated with tumor growth and survival outcomes. Using the ELISpot assay, IFN-γ production was measured in response to gp100 after adoptive transfer. Figure 5B (middle panel) shows the results of tumor-draining lymph nodes harvested from each group directly. Anti-CD25 as a single maneuver increased early (day 16) responses to gp100 in Thy-B animals; and when αCD25 was combined with rhIL-7, responses were significantly increased at several time points in both Thy-B and TXY hosts. In contrast, despite higher numbers of pmel-1 cells in Rag1−/− mice, low rates of IFN-γ production in response to gp100 were observed on day 16 and day 27. Thus, the highly proliferative cells present in Rag1−/− mice showed low antigen-specific reactivity; Treg depletion did not significantly change the proliferative rate but substantially augmented antigen-specific reactivity.
To more directly compare the functionality of pmel-1 T cells across hosts in this model system, independent of differences in the number of antigen-specific T cells, we calculated the percentage of pmel-1 T cells producing IFN-γ in response to cognate peptide antigen (Figure 5B bottom panel). This analysis demonstrates that Treg depletion significantly enhanced pmel-1 function in Thy-B hosts, and rhIL-7 plus or minus Treg depletion enhanced pmel-1 function in both Thy-B and TXY hosts. In contrast, the percentage of pmel-1 T cells capable of responding to gp100 peptide was the lowest in Rag1−/− mice at all time points, regardless of rhIL-7 therapy. These results demonstrate that cells undergoing massive proliferation in the lymphopenic environment show substantially reduced functionality compared with those transferred into hosts treated with targeted therapy to induce Treg depletion plus or minus rhIL-7, and that the enhanced functionality correlates with improved antitumor effects in vivo.
Interestingly, in irradiated hosts, Tregs showed relative radioresistance, resulting in substantially increased Treg frequencies compared with normal or αCD25-treated hosts (supplemental Figure 2A). Although proliferation was substantial in irradiated hosts, transferred pmel-1 cells showed diminished antigen-specific reactivity. Thus, radioresistant Tregs may adversely affect that ability for irradiation to effectively prepare hosts to receive adoptive immunotherapy (supplemental Figure 2B-C).
We also evaluated the effects of adoptive immunotherapy on the generation of de novo responses to other antigens expressed by B16, but not targeted by the adoptively transferred cells. Whereas the Thy-B and TXY hosts showed substantial reactivity to the subdominant class I-restricted antigen Trp-2 and the class II-restricted antigen Trp-1, no determinant spreading was observed in Rag1−/− mice (Figure 6) presumably because of the lack of cells available to respond to these antigens. Thus, the absence of an endogenous repertoire in lymphopenic hosts prevents determinant spreading within the inflammatory tumor microenvironment, thus potentially limiting the overall efficacy of the adoptive immunotherapy. Further evidence suggesting an important role for determinant spreading in this model system comes from analysis of irradiated hosts, where high Treg frequencies and poor antitumor effects were accompanied by diminished epitope spreading (supplemental Figure 2D-E).
Determinant spreading is evident in T-cell–replete hosts receiving adoptive immunotherapy with Treg depletion and rhIL-7 but is not evident in lymphopenic Rag1−/− adoptive immunotherapy recipients. Mean net number of splenocytes from treated groups producing IFN-γ in response to Trp1 (top panels) and Trp2 (bottom panels) are shown at time points according to schema in Figure 2A. *P < .05, **P < .005, results in tumor plus pmel-1 plus αCD25 plus IL-7 compared with the same time point in Rag1−/− recipients of adoptive immunotherapy.
Determinant spreading is evident in T-cell–replete hosts receiving adoptive immunotherapy with Treg depletion and rhIL-7 but is not evident in lymphopenic Rag1−/− adoptive immunotherapy recipients. Mean net number of splenocytes from treated groups producing IFN-γ in response to Trp1 (top panels) and Trp2 (bottom panels) are shown at time points according to schema in Figure 2A. *P < .05, **P < .005, results in tumor plus pmel-1 plus αCD25 plus IL-7 compared with the same time point in Rag1−/− recipients of adoptive immunotherapy.
Discussion
Progress in understanding the dynamic interactions between cancer and the immune system continues to fuel hopes that effective immune-based cancer therapies will ultimately emerge.32 Many groups are currently focused on optimizing adoptive immunotherapy to improve outcomes.33-36 Successful adoptive immunotherapy requires transfer of potent antitumor effectors into a host milieu that supports expansion, function, and persistence in vivo. It is well known that lymphopenia augments T-cell expansion and increases T-cell responsiveness to self-antigens,16,17 and some nonrandomized clinical studies have concluded that lymphoablation enhances the effectiveness of adoptive immunotherapy for cancer,6,8,13 leading some to suggest that lymphopenia is a prerequisite for effective adoptive immunotherapy. At the same time, impressive results have been observed after adoptive immunotherapy without specific measures to induce lympodepletion.36 Furthermore, humans inefficiently regenerate T cells and experience prolonged periods of immune dysfunction after lymphopenia-inducing therapies,9,37 raising concerns regarding whether it is logical or prudent to induce chronic immune depletion to effect short-term immune amplification.
The studies presented here sought to evaluate the relative merits of lymphopenia for supporting adoptively transferred cells and to determine whether therapies that replicate the “physiology of lymphopenia” could provide equal or superior support for adoptive immunotherapy in T-cell–replete hosts. Diminished Treg populations combined with an increased availability of homeostatic cytokines have been postulated as primary factors enhancing the effectiveness of adoptive immunotherapy in lymphopenic hosts.4,5,20 We observed robust proliferation of adoptively transferred cells in lymphopenic hosts and confirmed work showing that adoptive immunotherapy in lymphopenic hosts improved survival over baseline, whereas similar effects were not seen when pmel-1 cells alone were transferred into T-cell–replete hosts. However, the results also clearly demonstrated that lymphopenic hosts show poor tumor control at baseline and have inferior overall outcomes compared with lymphoreplete hosts receiving the same adoptive immunotherapy. Thus, these results do not support the notion that lymphopenia, per se, improves outcomes in hosts treated with adoptive immunotherapy for cancer (Figure 3).
In addition to the likelihood that diminished immune surveillance adversely impacted outcomes in lymphopenic hosts, Rag1−/− mice also did not experience determinant spreading after adoptive immunotherapy, whereas substantial priming of cells recognizing the Trp-1 and Trp-2 subdominant tumor antigens occurred in lymphoreplete mice receiving pmel-1 cells after Treg depletion plus or minus rhIL-7 (Figure 6). Determinant spreading is recognized to be a functionally important component of the development of autoimmune pathophysiology,38-41 and current concepts hold that effective tumor rejection is tantamount to autoimmunity.22 Recent studies have also shown a critical role for CD4+ cells in breaking immune tolerance during lymphopenia, and it is possible that the lack of CD4+ cells in the Rag1−/− hosts limited the capacity to generate effective antitumor immunity.42 Thus, profoundly lymphopenic hosts have diminished immune surveillance and an inability to generate determinant spreading during the evolution of antitumor immune responses. Furthermore, whereas the lymphopenic Rag1−/− environment supported increased proliferation of adoptively transferred pmel-1 cells compared with cells transferred into lymphoreplete hosts, the highly proliferated cells showed diminished antigen-specific IFN-γ production on a per-cell basis, compared with those transferred into lymphoreplete hosts (Figure 5). Similar evidence for dysfunction of highly proliferated cells has been observed in other states associated with excessive or prolonged TCR triggering43,44 and in T cells obtained from lymphopenic humans,31,45 raising the prospect that the permissive environment supporting unbridled proliferation in lymphopenic hosts may be a double-edged sword that ultimately predisposes to immune exhaustion/senescence. We postulate that the combination of diminished immune surveillance, lack of determinant spreading, and immune exhaustion serve to limit the effectiveness of adoptive immunotherapy in profoundly lymphopenic hosts.
Targeted therapies administered to replicate the “physiology of lymphopenia” in lymphoreplete hosts improved outcomes after adoptive immunotherapy. Treg depletion, albeit incomplete and transient, was a singularly potent maneuver, substantially improving survival in B16- and MB49-bearing (supplemental Figure 1) hosts receiving adoptive immunotherapy. Interestingly, we observed no benefit to incorporating sublethal irradiation into the preparative regimen for immunotherapy, and total body irradiation-induced significant increases in Treg frequencies, presumably because of relative radioresistance of this subset. Together, these results demonstrate that Tregs potently modulate the effectiveness of adoptive immunotherapy and suggest that clinical studies should incorporate therapies aimed at depleting this subset before adoptive immunotherapy. We and others saw impressive augmentation of T-cell–based immunotherapies despite incomplete Treg depletion,46 and recent clinical trials have documented similar changes in Treg populations after CD25-based targeting using CD25-directed immunotoxins LMB-247 or RFT5-SMPT-dgA.48,49 We posit that neither complete nor prolonged Treg depletion is necessary to improve the efficacy of adoptive immunotherapy and that Treg depletion mirroring the degree we show here to improve outcomes in the context of adoptive immunotherapy is readily translatable to the clinic.
RhIL-7 therapy, administered without Treg depletion, enhanced both the number and function of adoptively transferred T cells and significantly enhanced outcomes in both Rag1−/− and Thy-B recipients. Further, in adult TXY mice, full delivery of the “lymphopenic physiology” via the combination of αCD25 plus rhIL-7 led to outcomes far superior to that observed in lymphopenic mice and significantly improved compared with results after Treg depletion alone.
In conclusion, this work demonstrates that lymphopenia is not a prerequisite for effective adoptive immunotherapy and that manipulation of the lymphoid milieu in T-cell–replete hosts can significantly enhance the effectiveness of adoptive immunotherapy. These studies also illustrate several inherent limitations of lymphopenia in supporting adoptive immunotherapy. Conceptually, this opens the door for development of more directed, less toxic approaches for enhancing the effectiveness of adoptive therapies in the clinic. Both rhIL-7- and CD25-targeted agents that induce Treg depletion, similar to that induced here, have already been shown to be safe and biologically active in clinical trials. At the same time, we want to emphasize that these results provide proof of principle, and it remains possible that systematic manipulation of specific variables in preclinical models or in the clinical setting could further improve outcomes beyond those presented here. For instance, it remains possible that rhIL-15 and/or rhIL-21 may have similar or superior effects, or that the cytokine combinations may be even more potent.50 Moreover, subcutaneous tumor models as used here do not accurately mimic the microenvironmental milieu present in spontaneously occurring cancer. Nonetheless, these results suggest that future preclinical and clinical studies should seek to optimize targeted regimens that can specifically modulate regulatory cells and homeostatic cytokines in patients receiving adoptive immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Ron Gress and Dan Fowler for their careful reviews of the manuscript. C57BL/6-Pmel-1-1-Thy1.1 Tg mice were a generous gift from Nick Restifo, Surgery Branch, National Cancer Institute, National Institutes of Health. The murine melanoma cell line B16/F1-luc was kindly provided by Sam Hwang, Dermatology Branch, National Cancer Institute, National Institutes of Health.
This work was supported by the Intramural Research Program of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: Y.C., H.Z., M.G., and C.L.M. designed the experiments; Y.C., J.M., and R.P. performed the experiments; Y.C., H.Z., M.G., and C.L.M. performed data analysis; Y.C., H.Z., M.G., and C.L.M. interpreted the results; and Y.C. and C.L.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Crystal L. Mackall, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, 10CRC/1W-3750, 10 Center Dr, MSC 1104, Bethesda, MD 20892; e-mail: cm35c@nih.gov.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal