Abstract
Antiangiogenic effects of the proteasome inhibitor bortezomib were analyzed on tumor xenografts in vivo. Bortezomib strongly inhibited angiogenesis and vascularization in the chicken chorioallantoic membrane. Bortezomib's inhibitory effects on chorioallantoic membrane vascularization were abrogated in the presence of distinct tumor xenografts, thanks to a soluble factor secreted by tumor cells. Through size-exclusion and ion-exchange chromatography as well as mass spectroscopy, we identified GRP-78, a chaperone protein of the unfolded protein response, as being responsible for bortezomib resistance. Indeed, a variety of bortezomib-resistant solid tumor cell lines (PC-3, HRT-18), but not myeloma cell lines (U266, OPM-2), were able to secrete high amounts of GRP-78. Recombinant GRP-78 conferred bortezomib resistance to endothelial cells and OPM-2 myeloma cells. Knockdown of GRP78 gene expression in tumor cells and immunodepletion of GRP-78 protein from tumor cell supernatants restored bortezomib sensitivity. GRP-78 did not bind or complex bortezomib but induced prosurvival signals by phosphorylation of extracellular signal–related kinase and inhibited p53-mediated expression of proapoptotic Bok and Noxa proteins in endothelial cells. From our data, we conclude that distinct solid tumor cells are able to secrete GRP-78 into the tumor microenvironment, thus demonstrating a hitherto unknown mechanism of resistance to bortezomib.
Introduction
Bortezomib (Velcade, formerly PS-341), a boronic acid dipeptide, is a selective but reversible proteasome inhibitor.1,2 It has been approved for clinical use in humans, in particular for treatment of multiple myeloma and mantle cell lymphoma.3,4 Besides its direct antitumor effects,5,6 antiangiogenic actions of bortezomib have recently been described in vitro and in vivo.2,7-11 Angiogenesis is critical for tumor growth, invasion, and metastasis. Thus, inhibiting angiogenesis in tumor tissue is a strategy by which solid tumor progression can be slowed down or even stopped.
In contrast to other antiangiogenic compounds, such as thalidomide or bevacizumab, the clinical use of bortezomib is not associated with vascular complications, such as bleeding and thrombosis.12 In this study, we aimed to analyze its effects on growth and angiogenesis of tumor xenografts in vivo and to characterize molecular targets in vitro on human endothelial cells.
Bortezomib has been shown to interfere in the ubiquitin-proteasome pathway by inhibiting the 26S proteasome at its “chymotrypsin-like” site.13 The 26S proteasome is responsible for degrading a variety of regulatory proteins involved in proliferation and cell cycle regulation. Moreover, misfolded proteins, such as immunoglobulins in myeloma, are degraded by the proteasome.14 Accumulation of misfolded proteins after proteasome inhibition results in activation of the unfolded protein response (UPR). The UPR is an evolutionarily conserved mechanism that activates survival pathways to allow eukaryotic cells to adapt to endoplasmic reticulum (ER) stress.15 One of the main UPR responses is induction of the chaperone protein GRP-78. This protein belonging to the heat shock protein family has a C-terminal ER retention signal and is up-regulated under stress conditions, such as hypoxia, glucose deprivation, or the presence of cytotoxic substances.15 In this study, we were able to demonstrate that GRP-78 is released from the ER by solid tumor but not by myeloma cells, and protects endothelial cells from the antiangiogenic action of bortezomib. This is a novel, hitherto unknown, mechanism of resistance to bortezomib.
Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated according to the Jaffe et al protocol.16 HUVECs were cultivated in endothelial growth medium-2 medium (Cambrex) on type I collagen (from kangaroo; Sigma-Aldrich)–coated plastic. Expanded HUVECs were detached using collagenase-I (Sigma-Aldrich) and trypsin-ethylenediaminetetraacetic acid (Invitrogen) and split in a 1:3 ratio.
To achieve cell cycle–arrested cells, HUVECs were grown to confluence. Cell-cycle arrest was assessed by flow cytometry using propidium iodide staining. Murine B16F10 melanoma cells, the glioblastoma cell line U373, the prostate cancer cell line PC-3, and the breast cancer cell line MCF-7 were purchased from ATCC. The multiple myeloma cell lines U266 and OPM-2 were a generous gift of Dr Karin Jöhrer, and HRT-18 colon carcinoma cells were obtained from Dr Albert Amberger (both Tyrolean Cancer Research Institute). All cell lines were grown in the recommended cell media (RPMI 1640; PAA Laboratories GmbH) supplemented with 10% fetal calf serum, 105 IU/L penicillin, 100 mg/L streptomycin, and 2 mmol/L glutamine in the presence of 5% CO2.
Generation of GFP-transgenic U373 and HRT-18 cells
The cDNA for green fluorescent protein (GFP) was subcloned in a pT2 transposon vector with a strong cytomegalovirus promoter as described elsewhere.17 Thereafter, nucleotide sequence was confirmed by double-strand sequencing. U373 and HRT18 cells were transfected with the pT2 GFP vector and a vector encoding the sleeping beauty transposase SB11. Clones resistant to neomycin were selected and propagated in the ClonaCell medium (Stem Cell Technologies) and then analyzed for GFP expression.
Tumor cell supernatants and bortezomib
Bortezomib (Velcade, PS341) was purchased from Janssen-Cilag GmbH and diluted with fresh medium immediately before use.
For Western blot analysis and to test their potential to inhibit bortezomib-conditioned supernatants of various tumor cell lines were obtained by culturing the cells with RPMI containing 1% fetal calf serum for 48 hours. For chromatographic analysis of tumor cell supernatants, PC3 were cultured after extensive washing for 72 hours in serum-free RPMI medium containing 105 IU/L penicillin, 100 mg/L streptomycin, and 2 mmol/L glutamine. Supernatants were harvested, centrifuged at 12=000g for 30 minutes to remove cell debris, and sterile-filtered (Millex GP, 0.2 μm; Millipore) to remove residual precipitates. Supernatants were concentrated using Vivaspin 20 tubes (Sartorius) according to the manufacturer's instructions.
GRP-78 denaturation, knockdown, and immunoabsorption
Recombinant GRP-78 protein purified from bacterial overexpression was purchased from Acris Antibodies Ltd. Mannitol was solved in EBM-2 culture media at a concentration of 250nM. GRP-78 protein was denaturated by heating at 100°C for 10 minutes and then cooling on ice for 10 minutes. Recombinant bacterial control protein (bacterial alkaline phosphatase; Sigma-Aldrich) was used for all experiments to exclude effects of bacterial endotoxins. A rabbit anti–GRP-78 polyclonal antibody and a rabbit polyclonal control antibody directed against Bak (both Sigma-Aldrich) were used for neutralization experiments.
A pool of GRP78 specific siRNAs (ON-TARGET plus Smart pool siRNA, HSPA5; Thermo Scientific) were used to knock down gene expression in PC-3 cells. Cells were transfected either with 100 nM GRP-78 siRNA or control nontargeting siRNA (Thermo Scientific) using Lipofectamine 2000 reagent (Invitrogen) in serum-reduced culture medium. Knockdown was controlled after 48 hours by Western blots of the respective supernatants with a rabbit anti–GRP-78 polyclonal antibody (Sigma-Aldrich).
Immunoabsorption of GRP-78 from PC-3 supernatants was performed 4 hours after incubation with 10 ng/mL bortezomib. Precipitation was done overnight by incubation with a 10 molar excess of rabbit anti–GRP-78 polyclonal antibody or a rabbit polyclonal control antibody directed against Bok (both Sigma-Aldrich) and removal of the complexes with the Immunoprecipitation Starter Pack (GE Healthcare) according to the manufacturer's instructions. Depletion of GRP-78 from supernatants was tested by Western blot analysis using a mouse polyclonal antibody directed against GRP-78 (Abnova).
Proteasome activity assay
To determine the proteasome activity, a 20S Proteasome Activity Assay Kit (Chemicon International) was used according to the manufacturer's instructions. In brief, endothelial cells were incubated with or without bortezomib (10 ng/mL) in the presence of recombinant GRP-78 or control protein (10nM each) for 12 hours. Then, cells were lysed and the total protein amount was determined (Nano-Drop; Peqlab). Equal amounts of total protein of every sample were used for incubation with the proteasome substrate. Fluorescence intensity was measured using a multilabel counter (Victor 1420; Wallac).
Cell viability
The effects of bortezomib on endothelial cell viability were analyzed by cleaving the synthetic substrate 4-[3-(iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1; Roche) using mitochondrial succinate reductase. Briefly, 5000 cells/well were plated in a 96-well plate. After adhesion, cells were incubated with the respective concentrations of bortezomib, supernatant, or fractionated supernatant for 24 hours. Then, 10 μL of the ready-to-use WST-1 solution was added to 90 μL EBM-2 medium. Extinction at 450 nm was measured with a microplate reader (ELx808; BioTek) 120 minutes after adding the WST-1 reagent.
BrdU incorporation assay
A total of 5000 cells/well were seeded in triplicates into a 96-well plate (Nunc) in culture medium and were allowed to adhere overnight before the culture medium was changed. Thereafter, cells were incubated with the respective purified proteins. After 1 day, cells were pulsed for 24 hours with a 10μM dilution of bromodeoxyuridine (BrdU; GE Healthcare) in culture medium. Then cells were washed, fixed, and incorporated BrdU detected by a specific enzyme-linked immunosorbent assay (GE Healthcare) in an enzyme-linked immunosorbent assay reader (EL808; BioTek).
Measurement of apoptosis by flow cytometry
Apoptotic cells were determined by annexin V–fluorescein isothiocyanate (Alexis) and 7-amino-actinomycin D (7-AAD; Beckman Coulter) staining in a binding buffer consisting of 2.5mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 40mM NaCl, and 0.8mM CaCl2. Stained cells were then analyzed in the Cytomics-FC-500 cytometer using Cytomics RXP software (Beckman Coulter).
Chick CAM assay
The assay was performed as described elsewhere.18 In brief, fertilized white leghorn chicken eggs (SPF eggs) were purchased from Charles River (Kiesslegg) and incubated at 37°C with 60% humidity (Compact S84; Grumbach) for 4 days. Then a Thermanox ring (Nunc) was placed on the chorioallantoic membrane (CAM), and after sealing (Durapor tape) eggs were incubated for 24 hours. Thereafter, the ring was opened and various concentrations of bortezomib, dissolved in a 10mM Tris-glycine buffer solution (pH 7.4), or buffer solution alone were applied to the ring area. After 3 days of incubation, blood vessels were analyzed using the Olympus SZX10 stereomicroscope (Olympus) equipped with an Olympus DFPL 2X-4 objective lense (numerical aperture 0.2) connected with a digital camera (Olympus E410) and flexible cold light (KL200; Olympus).
Tumor xenografts in the CAM
B16F10 xenografts were generated by grafting 106 melanoma cells within the ring area on the CAM of a 4-day-old embryo. One day after grafting, various concentrations of bortezomib were applied daily to the area within the ring. Dark-pigmented melanoma cells and the subjacent CAM were analyzed on developmental day 8 (ie, 72 hours after incubation).
Moreover, CAM xenografts of HRT-18 and U373 cells expressing the transgene GFP were generated. This allows visualization of the tumor by fluorescence stereomicroscope with a connected digital camera (same Olympus system as described in “Chick CAM assay”). For this purpose, 106 tumor cells were seeded in the ring area of 7-day-old embryos. After 2 days, the rings were treated daily with 0.5 ng of bortezomib. On day 12, tumors and subjacent CAM were analyzed.
Western blot analysis
A total of 30 μg of total protein was denaturated, separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Lonza Walkersville), and transferred to polyvinylidene difluoride membranes (Bio-Rad). After blocking, membranes were probed with primary antibodies directed against alpha tubulin (Sigma-Aldrich), phospho-p53 (S46; R&D Systems), Noxa (GeneTex), 20S proteasome core subunits (Calbiochem), Bok, phospho-AKT (both Cell Signaling), and phospho-extracellular signal–related kinase (ERK) and GAPDH (both Santa Cruz Biotechnology).
Then membranes were incubated with a 1:2500 dilution of a horseradish peroxidase–conjugated rabbit anti–mouse IgG or a 1:2500 dilution of a horseradish peroxidase–conjugated swine anti–rabbit IgG (Dako North America). After washing, chemoluminescent substrate (GE Healthcare) was added and membranes were exposed immediately to ECL Hyperfilms (GE Healthcare) and signals detected by the use of the Chemidoc XRS Workstation (Bio-Rad) or chemoluminescence films (GE Healthcare).
Concentrated tumor cell supernatants and total cell extracts were analyzed using a polyclonal antibody directed against GRP-78 (Sigma-Aldrich).
Densitometric analysis was done after scanning the chemoluminescence films. In brief, all samples were normalized with the internal housekeeper α-tubulin using the National Institutes of Health ImageJ software. Then, relative intensity units were calculated in comparison with confluent endothelial cells at time point zero before bortezomib stimulation.
High-performance liquid chromatography analysis
Size-exclusion chromatography (SEC) was performed as described elsewhere.19 In brief, concentrated supernatants were loaded on a Sephadex column (Superdex 200; GE Healthcare). Protein fractions were eluted with Hanks balanced salt solution (Invitrogen) at 0.4 mL/min. A molecular weight gel filtration calibration kit (GE Healthcare) containing blue dextran 2000 (200=000 Da), bovine serum albumin (67=000 Da), ovalbumin (43=000 Da), chymotrypsinogen A (25=000 Da), and ribonuclease A (13=700 Da) was used to estimate protein sizes of the obtained fractions.
Ion-exchange chromatography (IEC) was performed as described elsewhere.17 In brief, supernatants were buffered with a 0.020M Tris-HCl solution to pH 9.0 before loading on a BioSuite Q10 AXC column (Waters). The proteins were eluted using 1M NaCl/0.020mM Tris-HCl, pH 9.0. The elution gradient proceeded from 0% to 70% of 1M NaCl within 70 minutes. Isoelectric points (pI) were estimated with the broad pI kit (3.6-9.3; GE Healthcare).
Two-dimensional gel electrophoresis and mass spectrometry
Proteins in the active fractions from SEC and IEC were precipitated in a 10% trichloroacetic acid solution (Roth) as described previously.20 The precipitated proteins were resuspended in an isoelectric focusing sample solution (8M urea, 1% 3-(3-cholamidopropyl)dimethylammonio-1-propanesulfonate, 0.010M dithiothreitol, 0.1% [wt/vol] ampholytes [pH 3-10]; Bio-Rad). First-dimension isoelectric focusing was performed with the Ettan IPGphor system (GE Healthcare) with Immobiline DryStrip gels (GE Healthcare) having a pH gradient of 3 to 10. SDS-PAGE in the second dimension was performed with 4% to 16% gradient gels (Lonza Walkersville) in the Mine PROTEAN 3 system (Bio-Rad) as described previously.21 Protein spots were visualized using Page Blue (Fermentas) or SyproRuby (Lonza Walkersville) according to the manufacturer's protocol. Spots of interest were excised from gels and subjected to mass spectrometry (MALDI TOF/TOF 4800 plus Analyzer; Applied Biosystems), and fragments were analyzed with ProteinPilot Version 2.0 software (Applied Biosystems).
Statistical analysis
Statistical analysis of all in vitro assays was performed with Prism 5 software for Windows (GraphPad Software Inc) using the 2-sided Mann-Whitney test.
Results
Bortezomib inhibits angiogenesis in vivo in the CAM assay
The CAM assay is an established model for studying proangiogenic and antiangiogenic substances in vivo. Effects can be directly monitored by studying the formation of the vascular plexus in the CAM after treatment with bortezomib. Even lowest concentrations of bortezomib (0.1 ng/ring) were found to inhibit the formation of vascular structures in the treated area (Figure 1A). Doses of up to 10 ng were also tested but were toxic to the embryo, that is, caused growth retardation and/or death (data not shown).
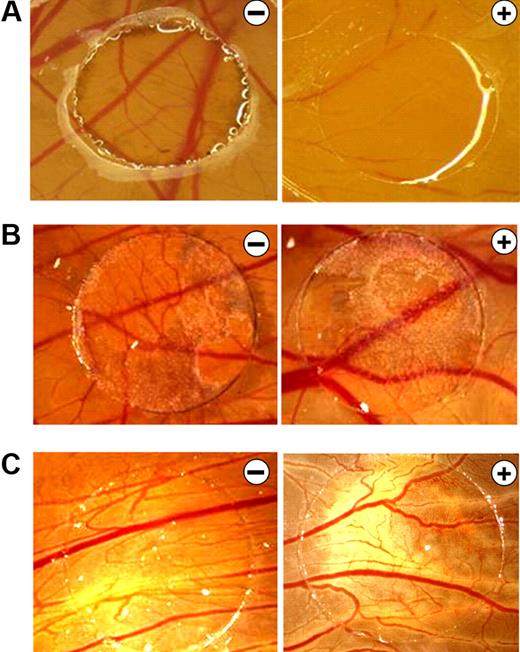
B16F10 melanoma supernatants neutralize the antiangiogenic effects of bortezomib in the CAM assay. (A) CAMs of 4-day-old chickens were incubated for 72 hours with control solution (−) or 0.1 ng of bortezomib/ring (+). Treatment with bortezomib inhibited CAM vascularization. (B) B16F10 melanoma xenografts were cultivated on the CAM for 5 days and treated daily with control solution (−) or 10 ng of bortezomib (+). Even at this high concentration, bortezomib had no antiangiogenic effects. B16F10 xenografts have a dark color. (C) CAMs were stimulated for 72 hours with supernatants of B16F10 melanoma cells in the presence of control solution (−) or 10 ng of bortezomib (+). Again, even at this high concentration, bortezomib had no antiangiogenic effects. Original magnification ×10. Images were acquired using an Olympus SZX10 stereomicroscope (2×/0.2 NA objective) with an Olympus E410 digital camera.
B16F10 melanoma supernatants neutralize the antiangiogenic effects of bortezomib in the CAM assay. (A) CAMs of 4-day-old chickens were incubated for 72 hours with control solution (−) or 0.1 ng of bortezomib/ring (+). Treatment with bortezomib inhibited CAM vascularization. (B) B16F10 melanoma xenografts were cultivated on the CAM for 5 days and treated daily with control solution (−) or 10 ng of bortezomib (+). Even at this high concentration, bortezomib had no antiangiogenic effects. B16F10 xenografts have a dark color. (C) CAMs were stimulated for 72 hours with supernatants of B16F10 melanoma cells in the presence of control solution (−) or 10 ng of bortezomib (+). Again, even at this high concentration, bortezomib had no antiangiogenic effects. Original magnification ×10. Images were acquired using an Olympus SZX10 stereomicroscope (2×/0.2 NA objective) with an Olympus E410 digital camera.
Bortezomib does not inhibit growth or angiogenesis of melanoma xenografts
To study the effects of bortezomib on tumor growth and vascularization in vivo, we used a xenograft tumor model in the chicken embryo. B16F10 melanoma cells were used because dark melanin pigments allow easy detection of tumor cells on the CAM. Moreover, the generated melanomas have a strong proangiogenic phenotype. Interestingly, even highest dosages of bortezomib (10 ng/ring) did not affect tumor growth or vascularization of the subjacent CAM (Figure 1B). We thus hypothesized that tumors secrete substances able to block the inhibitory effects of bortezomib on the subjacent vascular plexus. Indeed, application of purified B16F10 melanoma cell supernatants blocked the antiangiogenic activity of bortezomib on the CAM (Figure 1C).
Solid tumor cell lines secrete factors inhibiting the antiangiogenic activity of bortezomib
To test whether this observation is unique for B16F10 mouse melanoma cells or also holds true for human tumor cells, we tested a panel of myeloma and solid tumor cell lines in vitro. Supernatants were collected and applied together with bortezomib to human endothelial cells (Figure 2A). Neither myeloma cell lines U266 or OPM-2 nor the breast cancer cell line MCF-7 or the glioblastoma cell line U373 were able to block the antiangiogenic activity of bortezomib. However, the colorectal carcinoma cell line HRT-18 and the prostate carcinoma cell line PC-3 strongly inhibited the antiangiogenic action of bortezomib.
Screening for human tumor cell supernatants that block the antiangiogenic activity of bortezomib. (A) HUVECs were incubated with either control solution (−) or 10 ng/mL bortezomib (+) in the presence of the respective tumor cell culture supernatant. Cell viability was determined after 24 hours in a WST-1 assay monitoring the mitochondrial succinate reductase activity at 450 nm. Cells in standard culture medium served as control. Among the analyzed cell lines were: myeloma (U266 and OPM-2), breast carcinoma (MCF-7), glioblastoma (U373), colorectal cancer (HRT-18), and prostate carcinoma (PC-3). Bars represent mean ± SEM. *P < .05. (B) Growth and vascularization of human tumor xenografts in the CAM assay. Bortezomib-sensitive glioblastoma (U373) and bortezomib-resistant colorectal cancer (HRT-18) cells with stable overexpression of GFP were grown on the CAM for 2 days. Then xenografts were treated daily with control solution (−) or with 5 ng/mL bortezomib (+). After 3 days, tumors were analyzed under the fluorescence stereomicroscope to visualize GFP-positive tumor cells on the CAM. Bortezomib displayed marked antitumor effects against U373 but not against HRT-18 xenografts on the CAM. Original magnification ×10. Images were acquired using an Olympus SZX10 stereomicroscope (2×/0.2 NA objective) with an Olympus E410 digital camera.
Screening for human tumor cell supernatants that block the antiangiogenic activity of bortezomib. (A) HUVECs were incubated with either control solution (−) or 10 ng/mL bortezomib (+) in the presence of the respective tumor cell culture supernatant. Cell viability was determined after 24 hours in a WST-1 assay monitoring the mitochondrial succinate reductase activity at 450 nm. Cells in standard culture medium served as control. Among the analyzed cell lines were: myeloma (U266 and OPM-2), breast carcinoma (MCF-7), glioblastoma (U373), colorectal cancer (HRT-18), and prostate carcinoma (PC-3). Bars represent mean ± SEM. *P < .05. (B) Growth and vascularization of human tumor xenografts in the CAM assay. Bortezomib-sensitive glioblastoma (U373) and bortezomib-resistant colorectal cancer (HRT-18) cells with stable overexpression of GFP were grown on the CAM for 2 days. Then xenografts were treated daily with control solution (−) or with 5 ng/mL bortezomib (+). After 3 days, tumors were analyzed under the fluorescence stereomicroscope to visualize GFP-positive tumor cells on the CAM. Bortezomib displayed marked antitumor effects against U373 but not against HRT-18 xenografts on the CAM. Original magnification ×10. Images were acquired using an Olympus SZX10 stereomicroscope (2×/0.2 NA objective) with an Olympus E410 digital camera.
To monitor tumor growth in vivo, bortezomib-sensitive U373 and bortezomib-resistant HRT-18 cells were genetically modified to stably overexpress GFP. This transgene allows tumors to be monitored after xenotransplantation in the CAM. Growth of HRT-18 tumors was not affected by bortezomib treatment. By contrast, the growth of U373 tumors was markedly inhibited by bortezomib (Figure 2B).
GRP-78: an acidic 72-kDa protein of the unfolded protein response is secreted by bortezomib-resistant tumor cells
The supernatant of the bortezomib-resistant prostate cancer cell line PC-3 was further analyzed to identify factors responsible for blocking the antiangiogenic activity of bortezomib. This was done by separating the serum-free secretome according to the molecular weight using SEC. All fractions were subsequently tested on HUVECs to identify those able to inhibit the antiangiogenic action of bortezomib. A fraction with the molecular size of approximately 70 kDa showed the strongest inhibitory effect on endothelial cell growth (Figure 3A). In parallel, proteins of the same PC-3 supernatant were separated according to pI using anion-exchange chromatography (IEC). Obtained fractions were again tested on HUVECs to identify those that block the antiangiogenic effect of bortezomib on endothelial cells (Figure 3B). Only a very acidic fraction with a pI of less than 4.5 was able to block the antiangiogenic effect on HUVECs, whereas the other fractions were ineffective. Identified fractions of size-exclusion or anion-exchange chromatography were further analyzed using 2-dimensional gel electrophoresis, and stained spots were analyzed by mass spectrometry. The main component of the identified SEC (Fr.8) and IEC fractions (Fr.15) was GRP-78, a protein of the ER that has been shown to function as a chaperone in unfolded protein transport and recognition. Immunoreactive GRP-78 protein was present in both biologically active fractions of SEC and IEC (Figure 3C right panel) at concentrations lower than 100nM. GRP-78 was also the major protein compound in both fractions analyzed as shown by SDS-PAGE and Coomassie staining (Figure 3C left panel).
Identification of GRP-78 in the prostate carcinoma cell supernatant. (A) SEC of PC-3 supernatants was performed, and single fractions were tested on HUVECs in the WST-1 cell viability assay. All fractions were tested in the presence of 10 ng/mL bortezomib in direct comparison with unfractionated supernatant (SN). Untreated HUVECs served as positive control (co.+) and HUVECs stimulated with bortezomib in the absence of tumor supernatant as negative control (co.−). The scale indicates the molecular weight (kDa) of the individual fractions. A fraction with the molecular size of approximately 70 kDa showed the strongest inhibitory effect on endothelial cell growth. (B) IEC of PC-3 supernatants was performed, and the fractions were analyzed in HUVECs in the WST-1 assay. The scale indicates the approximate pI of the various fractions. Only a very acidic fraction with a pI of less than 4.5 was able to block the antiangiogenic effect on HUVECs. Bars represent mean ± SEM. *P < .05. (C) GRP-78 was the main compound in both biologically active fractions (Fr.8 from SEC and Fr.15 from IEC). Fractions were analyzed with recombinant GRP-78 (100-nM solution) either by SDS-PAGE stained with Coomassie (left panels) or by Western blot (right panel) to estimate GRP-78 concentrations and purity.
Identification of GRP-78 in the prostate carcinoma cell supernatant. (A) SEC of PC-3 supernatants was performed, and single fractions were tested on HUVECs in the WST-1 cell viability assay. All fractions were tested in the presence of 10 ng/mL bortezomib in direct comparison with unfractionated supernatant (SN). Untreated HUVECs served as positive control (co.+) and HUVECs stimulated with bortezomib in the absence of tumor supernatant as negative control (co.−). The scale indicates the molecular weight (kDa) of the individual fractions. A fraction with the molecular size of approximately 70 kDa showed the strongest inhibitory effect on endothelial cell growth. (B) IEC of PC-3 supernatants was performed, and the fractions were analyzed in HUVECs in the WST-1 assay. The scale indicates the approximate pI of the various fractions. Only a very acidic fraction with a pI of less than 4.5 was able to block the antiangiogenic effect on HUVECs. Bars represent mean ± SEM. *P < .05. (C) GRP-78 was the main compound in both biologically active fractions (Fr.8 from SEC and Fr.15 from IEC). Fractions were analyzed with recombinant GRP-78 (100-nM solution) either by SDS-PAGE stained with Coomassie (left panels) or by Western blot (right panel) to estimate GRP-78 concentrations and purity.
GRP-78 is up-regulated after bortezomib treatment in solid tumor cells
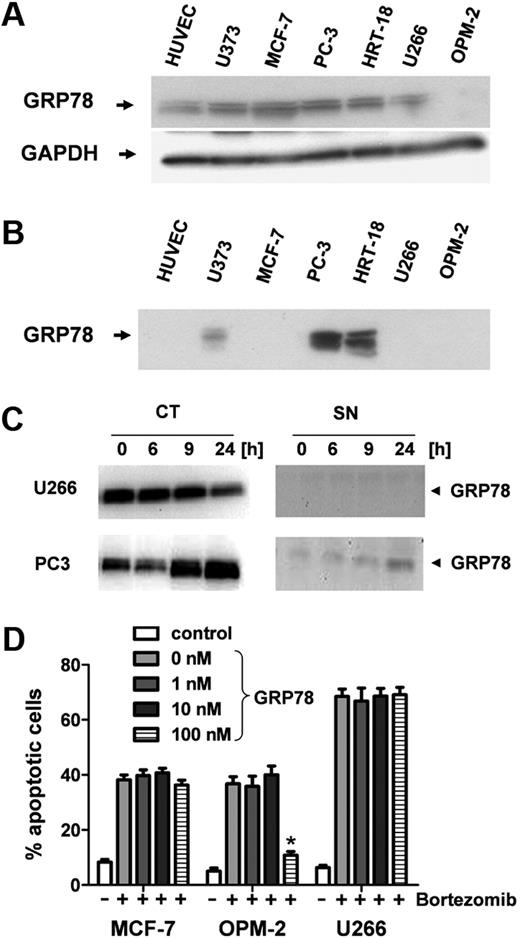
Although almost all tumor cell lines analyzed and also HUVECs contain GRP-78 intracellularly (Figure 4A), only the bortezomib-resistant lines were found to secrete it into the supernatant. Notably, GRP-78 protein was detectable in high amounts in the supernatants of both bortezomib-resistant cell lines PC-3 and HRT-18 but not or only faintly in the supernatants of MCF7, U373, U266, and OPM-2, respectively (Figure 4B). Whereas U266 myeloma cells down-regulated intracellular GRP-78 expression 24 hours after bortezomib stimulation, PC-3 prostate cancer cells showed a significant up-regulation of cytosolic GRP-78 (CT; Figure 4C). Moreover, PC3 cells also increased GRP-78 secretion into the supernatant on bortezomib treatment (SN, Figure 4C).
GRP-78 is up-regulated and secreted from bortezomib-resistant tumor cells. (A) Western blot analysis of intracellular protein levels of GRP-78 in solid tumor cell lines (U373, MCF-7, PC-3, and HRT-18), myeloma cells (U266, OPM-2), and human endothelial cells (HUVECs). GADPH served as internal loading control. (B) GRP-78 analysis in conditioned supernatants of human solid tumor cell lines and HUVECs. (C) Myeloma (U266) and prostate carcinoma (PC-3) cells were stimulated with 5 ng/mL bortezomib for 24 hours. GRP-78 protein levels were analyzed by Western blot in cytosolic extracts (CT) and supernatant (SN). (D) Different concentrations of recombinant GRP-78 ranging from 0 to 100nM were used to inhibit the antiangiogenic action of bortezomib. Bortezomib-sensitive MCF7 and myeloma cells (U266 and OPM-2) were stimulated with 10 ng/mL bortezomib. Apoptotic cells (percentage) were determined by flow cytometry after annexin V/7-AAD staining. Bars represent mean ± SEM. *P < .05.
GRP-78 is up-regulated and secreted from bortezomib-resistant tumor cells. (A) Western blot analysis of intracellular protein levels of GRP-78 in solid tumor cell lines (U373, MCF-7, PC-3, and HRT-18), myeloma cells (U266, OPM-2), and human endothelial cells (HUVECs). GADPH served as internal loading control. (B) GRP-78 analysis in conditioned supernatants of human solid tumor cell lines and HUVECs. (C) Myeloma (U266) and prostate carcinoma (PC-3) cells were stimulated with 5 ng/mL bortezomib for 24 hours. GRP-78 protein levels were analyzed by Western blot in cytosolic extracts (CT) and supernatant (SN). (D) Different concentrations of recombinant GRP-78 ranging from 0 to 100nM were used to inhibit the antiangiogenic action of bortezomib. Bortezomib-sensitive MCF7 and myeloma cells (U266 and OPM-2) were stimulated with 10 ng/mL bortezomib. Apoptotic cells (percentage) were determined by flow cytometry after annexin V/7-AAD staining. Bars represent mean ± SEM. *P < .05.
GRP-78 confers resistance to OPM-2 myeloma cells
Finally, recombinant full-length GRP-78 was tested for inhibition of the proapoptotic action of bortezomib (10 ng/mL) on solid tumor (MCF7) and myeloma cells (OPM-2, U266) that are not able to secrete this chaperone. When stimulated simultaneously, GRP-78 concentrations of 100 nM were sufficient to completely inhibit bortezomib-induced death in OPM-2 cells but were not effective in U266 or MCF7 cells (Figure 4D). However, when added 12 hours after bortezomib, GRP-78 was not able to rescue cells from bortezomib-induced apoptosis (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The protective action of GRP-78 on endothelial cells can be significantly blocked by a neutralizing antibody
Application of recombinant GRP-78 was tested on HUVECs. Whereas the control protein (alkaline phosphatase) and mannitol (added as a stabilizer to commercially available bortezomib) had no effect on cell viability, recombinant GRP-78 efficiently prevented loss of cell viability after bortezomib treatment (Figure 5A). Concentrations of 10nM were sufficient to antagonize 26nM bortezomib (10 ng/mL, P < .05). GRP-78 still retained its protective activity even after denaturation and refolding (10 minutes, 100°C). Addition of a GRP-78–neutralizing polyclonal antibody together with GRP-78 rendered cells significantly more sensitive to bortezomib treatment than an isotype control antibody (Figure 5A, P < .05). Exogenous application of the GRP-78–neutralizing polyclonal antibody to PC3 and HRT18 cells did not render cells more sensitive to bortezomib-induced apoptosis (supplemental Figure 1B).
GRP-78 does not interact with bortezomib or affect proteasome activity. (A) Endothelial cells were stimulated with or without 10 ng/mL bortezomib in the presence of mannitol (260nM), a control protein (10nM), or GRP-78 (10nM). In contrast to controls, GRP-78 blocked the action of bortezomib in the WST-1 cell viability assay. This effect was significantly inhibited by the addition of a polyclonal antibody (PAB) directed against GRP-78 (10nM). The control antibody (10nM) did not show any effect. (B) PC-3 cells were transfected either with control siRNA (si control) or siRNA mix down-regulating GRP78 gene expression (si GRP-78). After 2 days, respective supernatants were collected. Moreover, PC-3 cell supernatant was spiked with 10 ng/mL bortezomib (PC-3 SN) and then GRP-78 removed by immunoprecipitation using a polyclonal antibody directed against GRP-78 (IP GRP-78). A rabbit polyclonal antibody isotype not binding GRP-78 served as control (IP-control). Absorption and knockdown were controlled by Western blot analysis. (C) Immunoprecipitation experiments testing the hypothesis of a direct interaction of GRP-78 as being responsible for bortezomib inhibition. Bortezomib was incubated together with GRP-78 in PC-3 tumor cell supernatants. After immunoprecipitation using a GRP-78 binding antibody and Sepharose beads in excess amounts, viability of HUVECs was determined to test for biologically active bortezomib present in the supernatant. (D) All collected supernatants were tested on HUVECs in a WST-1 cell viability assay. In PC-3 supernatants containing no GRP-78 resulting from knockdown or immunoabsorption, viability of HUVECs was significantly decreased in the presence of 10 ng/mL bortezomib. (E) GRP-78 did not show any influence on proteasome activity, either in the presence or absence of bortezomib. The 26S proteasome activity was quantified after cleavage of a synthetic substrate. Bars represent mean ± SEM. *P < .05.
GRP-78 does not interact with bortezomib or affect proteasome activity. (A) Endothelial cells were stimulated with or without 10 ng/mL bortezomib in the presence of mannitol (260nM), a control protein (10nM), or GRP-78 (10nM). In contrast to controls, GRP-78 blocked the action of bortezomib in the WST-1 cell viability assay. This effect was significantly inhibited by the addition of a polyclonal antibody (PAB) directed against GRP-78 (10nM). The control antibody (10nM) did not show any effect. (B) PC-3 cells were transfected either with control siRNA (si control) or siRNA mix down-regulating GRP78 gene expression (si GRP-78). After 2 days, respective supernatants were collected. Moreover, PC-3 cell supernatant was spiked with 10 ng/mL bortezomib (PC-3 SN) and then GRP-78 removed by immunoprecipitation using a polyclonal antibody directed against GRP-78 (IP GRP-78). A rabbit polyclonal antibody isotype not binding GRP-78 served as control (IP-control). Absorption and knockdown were controlled by Western blot analysis. (C) Immunoprecipitation experiments testing the hypothesis of a direct interaction of GRP-78 as being responsible for bortezomib inhibition. Bortezomib was incubated together with GRP-78 in PC-3 tumor cell supernatants. After immunoprecipitation using a GRP-78 binding antibody and Sepharose beads in excess amounts, viability of HUVECs was determined to test for biologically active bortezomib present in the supernatant. (D) All collected supernatants were tested on HUVECs in a WST-1 cell viability assay. In PC-3 supernatants containing no GRP-78 resulting from knockdown or immunoabsorption, viability of HUVECs was significantly decreased in the presence of 10 ng/mL bortezomib. (E) GRP-78 did not show any influence on proteasome activity, either in the presence or absence of bortezomib. The 26S proteasome activity was quantified after cleavage of a synthetic substrate. Bars represent mean ± SEM. *P < .05.
Depletion of GRP-78 protein from tumor supernatants restores sensitivity of endothelial cells to bortezomib
Depletion of GRP-78 from tumor cell supernatant was performed by 2 approaches. First, endogenous gene expression was down-regulated by 100 nM siRNA, resulting in loss of GRP-78 secretion within 72 hours of incubation. Second, GRP-78 was depleted from the tumor cell supernatant by immunoprecipitation (IP) with a GRP-78–specific polyclonal antibody. Compared with the control siRNA and control IP using an isotype antibody, both techniques were successful to eliminate GRP-78 protein from the tumor supernatant (Figure 5B). All supernatants were tested together with 10 ng/mL bortezomib on HUVECs in a cell viability assay. As expected, depletion of GRP-78 resulted in a significant loss of protection from bortezomib-induced cell death (Figure 5D, P < .05).
GRP-78 does not bind bortezomib and does not affect S20 proteasome activity
To study the possibility of a direct interaction of GRP-78 with bortezomib, immunoabsorption assays using a GRP-78–binding antibody were carried out after incubation of the respective supernatant (HRT-18 and PC-3 cells, respectively) with 10 ng/mL bortezomib (Figure 5C). After effective GRP-78 depletion using Sepharose beads (Figure 5B), the supernatant was tested on endothelial cells. GRP-78 was not found to inhibit bortezomib by direct interaction with the compound itself (eg, binding or complex formation) because GRP-78–depleted supernatants still contained the drug and induced cell death on exposed HUVECs (Figure 5D). Moreover, GRP-78 had no significant impact on the overall proteasome activity of HUVECs with or without the presence of bortezomib (Figure 5E).
GRP-78 increased phosphorylation of ERK and AKT and inhibited p53-mediated induction of BOK and NOXA
Bortezomib induces apoptosis in proliferating but not in confluent endothelial cells as determined by annexin V–fluorescein isothiocyanate staining, not even at concentrations as high as 1000 ng/mL. In contrast to the control protein, GRP-78 (10nM) significantly protected endothelial cells from bortezomib-induced apoptosis (Figure 6A).
GRP-78 inhibits p53-mediated induction of BOK and NOXA and induces phosphorylation of ERK. (A) HUVECs were incubated with increasing concentrations of bortezomib (0.01-1000 ng/mL) for 24 hours. Then the percentage of apoptotic cells was determined by flow cytometry after annexin V and 7-AAD staining. Confluent cells were directly compared with proliferating cells (log phase) and proliferating cells treated with 10nM control protein or GRP-78. *P < .05. (B) Molecular proapoptotic targets of bortezomib and GRP-78 on endothelial cells: p53 phosphorylation and NOXA and BOK expression were analyzed in confluent and log phase growing cells 3 to 12 hours after bortezomib stimulation (10 ng/mL). In contrast to control protein, GRP-78 (10nM) prevented the phosphorylation of p53 (serine 46) and induction of BOK and NOXA. Tubulin-α served as internal loading control. (C) DNA synthesis was studied by the BrdU incorporation assay. In contrast to control cells and cells treated with control protein, 10nM GRP-78 significantly blocked the antiproliferative effect of bortezomib on endothelial cells. Bars represent mean ± SEM. *P < .05. (D) Phosphorylation of the ERK and protein kinase B (AKT) was analyzed on endothelial cells 5 to 90 minutes after treatment with 10 ng/mL bortezomib and GRP-78 or control protein (each 10nM). Tubulin-α served as internal loading control. The presence of GRP-78 resulted in a strong phosphorylation of ERK and to a lesser extent of AKT despite treatment with bortezomib.
GRP-78 inhibits p53-mediated induction of BOK and NOXA and induces phosphorylation of ERK. (A) HUVECs were incubated with increasing concentrations of bortezomib (0.01-1000 ng/mL) for 24 hours. Then the percentage of apoptotic cells was determined by flow cytometry after annexin V and 7-AAD staining. Confluent cells were directly compared with proliferating cells (log phase) and proliferating cells treated with 10nM control protein or GRP-78. *P < .05. (B) Molecular proapoptotic targets of bortezomib and GRP-78 on endothelial cells: p53 phosphorylation and NOXA and BOK expression were analyzed in confluent and log phase growing cells 3 to 12 hours after bortezomib stimulation (10 ng/mL). In contrast to control protein, GRP-78 (10nM) prevented the phosphorylation of p53 (serine 46) and induction of BOK and NOXA. Tubulin-α served as internal loading control. (C) DNA synthesis was studied by the BrdU incorporation assay. In contrast to control cells and cells treated with control protein, 10nM GRP-78 significantly blocked the antiproliferative effect of bortezomib on endothelial cells. Bars represent mean ± SEM. *P < .05. (D) Phosphorylation of the ERK and protein kinase B (AKT) was analyzed on endothelial cells 5 to 90 minutes after treatment with 10 ng/mL bortezomib and GRP-78 or control protein (each 10nM). Tubulin-α served as internal loading control. The presence of GRP-78 resulted in a strong phosphorylation of ERK and to a lesser extent of AKT despite treatment with bortezomib.
Next, the molecular targets of bortezomib were investigated up to 12 hours after bortezomib treatment on confluent and proliferating HUVECs. Moreover, effects of GRP-78 were studied in direct comparison with a control protein on proliferating HUVECs in the presence of bortezomib. Compared with arrested cells, bortezomib induced phosphorylation of p53 (serine 46; 6- to 7-fold) and induced the proapoptotic p53 target proteins BOK (4- to 6-fold) and NOXA (7- to 8-fold) in proliferating HUVECs (Figure 6B). The addition of GRP-78 significantly inhibited p53 phosphorylation (7.4- vs 2.3-fold) and induction of BOK (5.4- vs 0.9-fold) and NOXA (8.4- vs 3.2-fold). However, GRP-78 had no impact on the amount of 26S proteasome subunits in endothelial cells. Notably, the overall amount of 26S proteasome subunits was 6- to 7-fold higher in proliferating than in confluent cells.
Treatment with bortezomib significantly reduced DNA synthesis of proliferating endothelial cells (Figure 6C). In contrast to the control protein, the addition of GRP-78 to bortezomib resulted in an increased DNA synthesis, whereas GRP-78 alone had no impact on DNA metabolism.
To test the hypothesis that, in the presence of bortezomib, exogenous GRP-78 may activate prosurvival pathways, we analyzed the expression of ERK and AKT at various time points after exposure to bortezomib in combination with GRP-78 (Figure 6D). Indeed, the addition of GRP-78 to bortezomib resulted in an increase of phosphorylated isoforms of ERK in endothelial cells compared with control protein-treated cells, although, to a lesser extent, phosphorylation of AKT in HUVECs was also increased after coincubation of bortezomib with GRP-78.
Discussion
Analysis of various tumor and myeloma cell lines revealed that supernatants of distinct solid tumor cell lines, such as HRT-18 and PC-3, conferred bortezomib resistance to endothelial cells. However, other cell lines, such as MCF-7 and the multiple myeloma cell lines U266 and OPM-2, did not protect endothelial cells from bortezomib-induced apoptosis. The finding that tumor cells secrete factors that render adjacent cells of the tumor microenvironment resistant to bortezomib treatment has not been described to date. However, several other mechanisms that render tumor cells resistant to bortezomib have been found recently. As an example, mutations in the β5 subunit of the proteasome prevented bortezomib from binding to the proteasome.22 Furthermore, tumor cells have been shown to acquire resistance by up-regulating subunits of the proteasome after prolonged sublethal proteasome inhibition.23,24 Moreover, overexpression of heat shock protein (HSP) 27 has been reported in some tumor cell types that inhibited the apoptotic activity of bortezomib. However, the mechanisms mediating the cytoprotective function of HSP27 are still unclear.25 Analysis of supernatant proteins using various chromatographic separation methods followed by mass spectrometry identified GRP-78, a member of the heat shock family HSP70, as the main compound of the biologically active fractions. This protein, also named BiP or HSP70A5, is normally localized in the ER and belongs to a group of chaperones recognizing and transporting unfolded proteins.26 It has been shown that GRP-78 is especially up-regulated on accumulation of misfolded proteins or ER stress.27 Under genotoxic stress, tumor cells are able to localize this protein on the cell surface, where it binds to other proteins (eg, Cripto-1), thereby preventing proapoptotic pathways.28 Overexpression of GRP-78 in the ER of tumor cells has been observed in a variety of cancer entities, thus rendering these cells more resistant to chemotherapy.29 To date, no secretion of GRP-78 by tumor cells has been reported in the literature. Interestingly, we found large amounts of GRP-78 to be secreted by various tumor cell types. A recent publication by Virrey et al identified the protein as also being overexpressed in the tumor endothelium in situ and in primary endothelial cultures of the tumor tissue in vitro.27
There are at least 2 possible means by which secreted GRP-78 blocks bortezomib-associated apoptosis in endothelial cells. On one hand, GRP-78 may bind directly to bortezomib and sequester it before it can enter the endothelial cell. Our data exclude this possibility because absorption of GRP-78 in the presence of bortezomib does not complex or bind the drug. Biologically active unbound bortezomib was still present in the supernatant, entered into endothelial cells, and inhibited proteasome activity. On the other hand, GRP-78 may bind and activate an antiapoptotic receptor on the cell surface (eg, Cripto-1), thereby suppressing proapoptotic signals induced by the UPR.28 This possibility seems to be more probable and remains to be studied in future investigations. Our data give evidence that GRP-78 interferes in p53-mediated induction of proapoptotic BOK and NOXA. Notably, GRP-78 can rescue cells from bortezomib-induced apoptosis only when added simultaneously. Later application does not reverse the initiated apoptotic process. With regard to myeloma cells, only OPM-2 cells, but not U266 cells, can be protected by the presence of GRP-78 from bortezomib-induced apoptosis. The reason for this heterogeneous response pattern to GRP-78 is currently unclear. GRP-78 confers resistance to apoptosis not only as a chaperone but also by preserving ER calcium homeostasis, direct inhibition of proapaptotic effectors, such as caspase-7, BIK, or BAK, and prevention of cytochrome c release from the mitochondria.29 Thus, one could speculate that distinct cells may lack important interaction or binding partners of GRP-78 either specifically or in particular in tumor cells because of general loss of genomic material, gene inactivation, or mutations resulting in failure to mediate prosurvival signals necessary to antagonize the UPR-induced apoptosis.
Moreover, despite the presence of bortezomib, GRP-78 induced a strong phosphorylation of ERK in endothelial cells and, although weakly, also of AKT. Again, GRP-78 might bind to cell surface receptors, such as cripto-1, which is highly expressed on the cell surface of endothelial cells and induces a strong prosurvival signal by activating the ERK and possibly also the AKT signaling pathway.30
In conclusion, distinct tumor but not myeloma cells are able to inhibit bortezomib by secretion of GRP-78 on proteasome inhibition, thus demonstrating a hitherto unknown mechanism of resistance to bortezomib. Future studies will have to focus on the potential clinical relevance of these findings with regard to the administration of bortezomib, possibly in combination with HSP inhibitors in solid tumors and lymphoproliferative disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cornelia Heis, Monika Bauer, and Martina Zimmermann for their excellent technical help.
This work was supported by Verein zur Förderung der Krebsforschung an der Universitätsklinik Innsbruck, Krebshilfe Tirol, Fellinger Krebsforschungsverein, ONCOTYROL Research Consortium, and Janssen-Cilag Pharma GmbH.
Authorship
Contribution: J.K., G.U., and M.S. designed the study, carried out experiments, data analysis, and statistical evaluation, and wrote the manuscript; C.Z. and B.S. carried out size-exclusion and ion-exchange chromatography and mass spectroscopy; G.G., E.G., G.U., and M.S. interpreted study data; and E.G. and M.S. provided funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Steurer, Department of Internal Medicine V, Innsbruck Medical University, Anichstr 35, A-6020 Innsbruck, Austria; e-mail: michael.steurer@i-med.ac.at.
References
Author notes
J.K. and G.U. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal