We recently showed that granzyme A (GzmA) cleaves poly(adenosine 5′-diphosphate-ribose) polymerase-1 (PARP-1) to promote cell death and inhibit DNA repair.1 PARP-1 cleavage is required for apoptotic cell death, which reduces inflammation, because scavenger macrophages phagocytose apoptotic cells.
Our paper discussed a recent study from Froelich and colleagues2 that asserts that native GzmA from human killer cells is not cytotoxic at nanomolar concentrations, contradicting our results using recombinant GzmA (rGzmA). There is considerable evidence that native GzmA and GzmB both independently activate cell death.3 Mice genetically deficient in either GzmA or GzmB are essentially immunocompetent, whereas mice lacking both Gzms are profoundly immunodeficient. Moreover, as shown in Figure 7 of our paper,1 cytotoxic T lymphocytes (CTLs) lacking GzmA or GzmB induce comparable cytotoxicity.
The Froelich study also could not verify previous work that native GzmA cleaves and activates pro–IL-1β.4 However, they showed that native human GzmA was proinflammatory, because it induced monocytes to secrete IL-1β. Because the GzmA concentration that activates cytokine secretion was approximately 2 to 3 logs less than the cytotoxic concentration, they speculated that GzmA's primary function is proinflammatory, rather than cytotoxic. However, because Gzms are secreted into a small enclosed space (the immunologic synapse) and only small amounts leak out into extracellular fluids, the local synapse concentration is relatively much higher. During chronic inflammation (rheumatoid arthritis joints, AIDS patient blood), GzmA can reach low-nanomolar extracellular levels (vs < 1pM in normal blood).5 Based on the GzmA yield from killer cells (∼ 20 μg/109 cells)6 and conservative estimates that approximately one-tenth of CTL granule contents are released into a single synapse with a volume of less than 5 μm3, we estimate that GzmA synapse concentrations are vastly greater (∼ 2 × 10−9 μg/5 μm3 or ∼ 8μM). Therefore, the local synapse concentration is more than adequate for inducing cytotoxicity, activated at 250nM concentrations (Figure 1).1,7-9
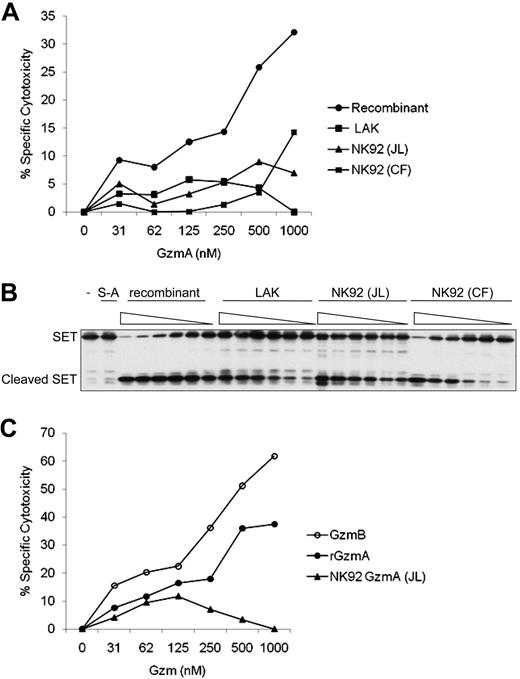
Recombinant human granzyme A induces cell death more potently than granzyme A isolated from lymphokine-activated killer or NK cells. (A) Four sources of GzmA were added with a sublytic concentration of perforin to K562 cells at the indicated concentrations. Percent specific cytotoxicity was calculated by 51Cr release after 4 hours. Background cytolysis of cells treated with perforin without GzmA was subtracted. GzmA was either rGzmA or native GzmA purified from human lymphokine-activated killer (LAK) cells or NK92 cells either by us (JL) or from NK92 cells by the Froelich laboratory (CF). (B) The 4 sources of GzmA were also compared for in vitro cleavage of recombinant SET protein using 2-fold serial dilutions at concentrations ranging from 31nM to 1000nM. Reactions were incubated for 1 hour at 37°C. Enzymatically inactive rGzmA in which the active site Ser is mutated to Ala (S-A) was an additional negative control. (C) Cytotoxicity by rGzmA was compared with cytotoxicity induced by native GzmB purified from an NK-cell line (YT-Indy) and native GzmA from NK92 cells. 51Cr release assay performed as in panel A.
Recombinant human granzyme A induces cell death more potently than granzyme A isolated from lymphokine-activated killer or NK cells. (A) Four sources of GzmA were added with a sublytic concentration of perforin to K562 cells at the indicated concentrations. Percent specific cytotoxicity was calculated by 51Cr release after 4 hours. Background cytolysis of cells treated with perforin without GzmA was subtracted. GzmA was either rGzmA or native GzmA purified from human lymphokine-activated killer (LAK) cells or NK92 cells either by us (JL) or from NK92 cells by the Froelich laboratory (CF). (B) The 4 sources of GzmA were also compared for in vitro cleavage of recombinant SET protein using 2-fold serial dilutions at concentrations ranging from 31nM to 1000nM. Reactions were incubated for 1 hour at 37°C. Enzymatically inactive rGzmA in which the active site Ser is mutated to Ala (S-A) was an additional negative control. (C) Cytotoxicity by rGzmA was compared with cytotoxicity induced by native GzmB purified from an NK-cell line (YT-Indy) and native GzmA from NK92 cells. 51Cr release assay performed as in panel A.
In the absence of a defined mechanism, we are uncertain whether GzmA has additional proinflammatory effects, besides activating pro–IL-1β4 (which we recently confirmed using rGzmA). Monocytes/macrophages are exquisitely endotoxin-sensitive. It is impossible to verify endotoxin elimination from GzmA preparations. This is an issue for both recombinant and purified enzymes, considering that buffers, water, and glassware can be contaminated. Commercial kits do not completely remove endotoxin. Moreover, the Limulus clot assay for endotoxin, which measures tryptase activity, cannot be performed on GzmA preparations. Therefore, because there may be residual endotoxin in all GzmA preparations, it will be important to define the molecular basis for additional proinflammatory mechanisms of GzmA.
The Froelich group recently kindly provided us with the native natural killer (NK) cell GzmA that they used. We compared the cytotoxicity of human GzmA from 4 sources6 : rGzmA,1 native NK92 cell GzmA purified by us and Froelich,2 and native lymphokine-activated killer cell GzmA purified by us. rGzmA induced significant cytotoxicity at approximately 250nM concentrations (consistent with our previous publications1,7-9 ), whereas native GzmA had limited cytotoxic activity (consistent with Froelich's published study2 ) (Figure 1A). rGzmA was also substantially more active at cleaving SET, the inhibitor of GzmA-activated DNA damage7 (Figure 1B). Moreover, rGzmA and native GzmB from YT-Indy NK cells6 were active at similar concentrations (∼ 250nM; Figure 1C). Therefore native GzmA from human killer cell granules is less cytolytic than rGzmA.
Further work is needed to understand why native GzmA has reduced cytotoxicity. GzmA is synthesized as an inactive pro-enzyme. One potential explanation could be that GzmA might be stored mostly as the pro-enzyme, which is activated only during CTL degranulation.
Authorship
D.M. and M.W. contributed equally to this study.
Contribution: D.M., M.W., D.K.J., and J.T. designed and performed the experiments and analyzed the data; and J.L. designed the experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judy Lieberman, MD, PhD, Immune Disease Institute, 200 Longwood Ave, Boston MA 02115; e-mail: lieberman@idi.harvard.edu.
References
National Institutes of Health