In this issue of Blood, Köhler and colleagues developed a time-lapse intravital imaging method to observe BM cells within long bones of live mice, and found that aged eHPCs localized more distantly to the endosteum than young eHPCs.
The hematopoietic stem cell (HSC) niche is composed of specialized bone marrow (BM) stromal cells that play important roles in regulating adult stem cell self-renewal and differentiation.1 Thus far, at least 3 types of stromal cells including osteoblastic, sinusoidal endothelium, and CXCL12 abundant reticular (CAR) cells have been shown to be involved in the regulation of HSCs.2-5 However, these studies relied on use of genetic knockout models impacting on specific niche cell types, or immunostaining to localize putative HSCs within the marrow environment. Both of these methods have limitations. The former does not precisely define localization of HSCs, and the latter does not reveal their function.6,7 Furthermore, the identification of HSCs requires multiple markers that are feasible using flow cytometry on cells in vitro, but difficult for performing immunoassays on HSCs within intact marrow environments. To monitor the dynamic interaction between the HSC and its niche, new technology was necessary.
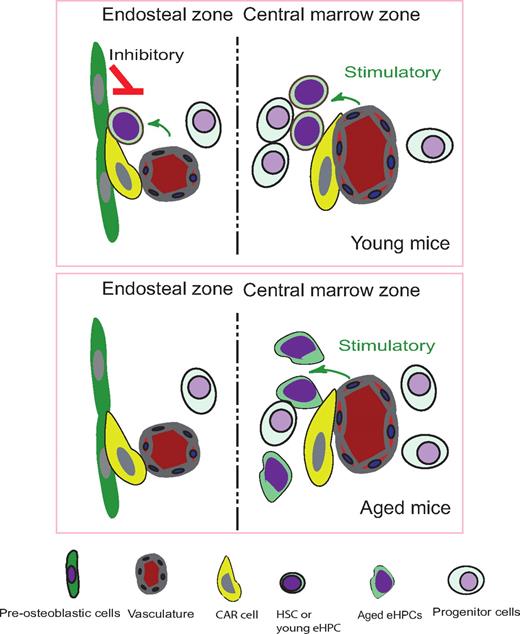
Illustration of endosteal zone and central marrow zone in transplanted mice which donor eHPCs come from young and aged mice. Fig showed young eHPCs located closer to endosteum compare to aged eHPCs, and protrusion movements and cell surface increased in aged eHPCs.
Illustration of endosteal zone and central marrow zone in transplanted mice which donor eHPCs come from young and aged mice. Fig showed young eHPCs located closer to endosteum compare to aged eHPCs, and protrusion movements and cell surface increased in aged eHPCs.
Recently, 2 labs have developed imaging technologies using 2-photon microscopy that allow for observation of HSCs in their niches in real time, both in vivo and ex vivo. Lo Celso and colleagues have monitored the behavior of individual hematopoietic stem and progenitor cells (HSPCs) in the calvarium BM of live mice.6 Xie and colleagues have established a new method for ex vivo imaging stem cells (EVISCs) to trace the homing of purified green fluorescent protein (GFP)–expressing HSCs.7 Both groups determined the distribution patterns of homed HSCs and examined the relationship of transplanted HSCs with blood vessels and osteoblastic cells. The primary conclusion obtained from these 2 studies is that HSCs localize predominantly closer to the endosteum, while more differentiated progenitors reside progressively farther away,6 indicative of the important role the endosteum plays in supporting HSCs. Surprisingly, both studies found that osteoblastic and vascular components are not far apart from each other as expected, but coexist in the endosteal zone. However, in the central marrow (CM) zone where vasculature is dominant, the endosteal and CM zones can form different microenvironments for HSCs, the former being more inhibitory and the latter more stimulatory.
In this issue of Blood, Köhler et al establish a multiphoton intravital microscopy technique to visualize the interaction of immature hematopoietic cells and stromal cells in the endosteal microenvironment of live mouse tibias.8 They find that aged early hematopoietic progenitor cells (eHPCs) increased changes in cell volume and surface area compared with young eHPCs. Furthermore, aged eHPCs localize farther away from the endosteum than do young eHPCs, with the former displaying significantly reduced adhesion to stromal cells. Using certain cytoskeletal molecules as polarization markers, they observe that aged eHPCs have defects in their adhesive ability. This reduction in adhesion is also reciprocally correlated with elevated protrusion movements, resulting in distant localization from the endosteum. These data for the first time reveal profound location differences within BM between young and aged eHPCs. Together, these findings suggest that alterations in stem cell property during aging, such as reduced self-renewal and altered differentiation, are, at least in part, due to changes in localization of hematopoietic cells within the marrow microenvironment, away from the endosteal zone. This indicates there are different zones in BM.
The novel technique discussed above and in this issue of Blood allows researchers to monitor the behavior of individual HSCs and their dynamic interactions with niches in vivo and ex vivo in various skeletal sites in mice. Although the techniques can be further improved, such as observing the behavior of endogenous HSCs and successfully conducting lineage tracing studies in vivo, this new method is ready to address many important biological questions.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal