Abstract
In platelets stimulated by thrombin to secrete and aggregate, cofilin is rapidly dephosphorylated leading to its activation. Cofilin by severing existing actin filaments and stimulating F-actin polymerization on newly created barbed ends dynamizes the actin cytoskeleton. We previously found that cofilin dephosphorylation is Ca2+-dependent and occurs upstream of degranulation in stimulated platelets. We report now in thrombin-stimulated platelets that Rac1 and class II PAKs (PAK4/5/6) were rapidly (within 5 seconds) activated, whereas PAK1/2 (class I PAKs) phosphorylation was slower. The Rac1-specific inhibitor NSC23766 blocked phosphorylation of class II PAKs, but not PAK1/2. Moreover, inhibition of the Ca2+/calmodulin-dependent phosphatase calcineurin inhibited Rac1 activation and class II PAKs phosphorylation. Prevention of Rac1 activation by calcineurin inhibition or NSC23766 also blocked cofilin dephosphorylation and platelet granule secretion indicating that a calcineurin/Rac1/class II PAKs pathway regulates cofilin dephosphorylation leading to secretion. We further found that PI3-kinases were activated downstream of Rac1, but were not involved in regulating cofilin dephosphorylation and secretion in thrombin-stimulated platelets. Our study unravels a Ca2+-dependent pathway of secretion in stimulated platelets as a signaling pathway linking Rac1 activation to actin dynamics: calcineurin→Rac1→class II PAKs→cofilin activation. We further demonstrate that this pathway is separate and independent of the protein kinase C (PKC) pathway mediating secretion.
Introduction
Activation of platelets at sites of vascular injury plays a key role in hemostasis. A rapid remodeling of the actin cytoskeleton is required for the functional and morphologic platelet responses such as shape change, spreading, secretion, and aggregation induced by various stimuli. Rho-GTPases such as Rho, Rac, and Cdc42 are key regulators of rapid actin dynamics in cells. These Rho-GTPases differentially regulate actin assembly leading to different cellular structures. Rho is known for spheration and contractility during platelet shape change,1 Rac1 regulates the formation of lamellipodia,2 whereas Cdc42 controls the formation of filopodia during platelet spreading.3 RhoA mediates Rho-kinase activation, which in turn phosphorylates and activates the LIMK family kinases.4 LIMKs phosphorylate cofilin, a small actin dynamizing protein, at its Ser-3 position. Rac and Cdc42 activation in cells have also been shown to stimulate LIMKs-mediated cofilin phosphorylation through activation of p21-activated kinases (PAKs).5
Phosphorylation of cofilin at its Ser3 position inhibits its F-actin binding, severing and depolymerizing activities. Although cofilin is known for its actin disassembling properties, it shows a complex actin dynamizing behavior by promoting lamellipodial assembly through generation of free barbed ends. In platelets, the regulation of cofilin during various platelet responses such as secretion, aggregation, and store-operated calcium entry received recent attention.6-9 We unraveled in thrombin- and lysophosphatidic acid (LPA)-stimulated platelets a 2-step regulation of the cofilin phospho-cycle; a rapid first phase of Ca2+-dependent cofilin dephosphorylation (activation) through an unknown cofilin phosphatase was followed by a second phase of Rho-kinase/LIMK-1–mediated slow cofilin rephosphorylation (inactivation). The rapid cofilin dephosphorylation was upstream of dense granule secretion and aggregation.7,8 The ability of active cofilin to dynamize actin (ie, to sever and depolymerize actin filaments as well as to stimulate actin polymerization) might be critical for platelet granule secretion.
The signaling pathway(s) regulating cofilin dephosphorylation in platelets is not fully understood. Pioneering experiments using electropermeabilized platelets showed that cofilin dephosphorylation could be stimulated by GTPγS as well as by cytosolic Ca2+ after treatment with ionophore A23187.10 We wondered, whether among the GTP-binding proteins orchestrating actin dynamics, Rac1 could be involved in regulating cofilin dephosphorylation for the following reasons: Rac1 activation in platelets is Ca2+-dependent,11,12 and we showed that cofilin dephosphorylation in LPA-stimulated platelets was Ca2+-dependent and upstream of secretion, and therefore, probably leading to secretion.8 Moreover, Rac1 has recently been shown to be involved in regulating secretion in human and mice platelets stimulated with thrombin,13 although the findings in mice platelets could not be confirmed in another study.2
In this study, we unraveled that Rac1 activation in thrombin-stimulated platelets regulates the rapid cofilin dephosphorylation leading to secretion. Interestingly this pathway is mediated through activation of class II PAKs (PAK4/5/6), but not the PAK1/2. Rac1-mediated cofilin dephosphorylation was found to be independent of PKC activation. Although Rac1 activation also led to stimulation of PI3-kinases, PI3-kinase activation was not involved in cofilin dephosphorylation and secretion.
Methods
Reagents
Human thrombin (T-7009) and EHT1864 were from Sigma-Aldrich. NSC23766 was from Tocris Bioscience. Gö6983, Gö6976, LY294002, PDBu, wortmannin, calcineurin autoinhibitory domain (CAID) peptide, CAID peptide fused to a cell-permeant polyarginine peptide (CAID-11R) and nuclear factor of activated T-cell inhibitor fused to 11R (NFAT-11R) were from Calbiochem, Merck Biosciences GmbH. RGDS peptide was from Bachem Biochemica. Chrono-Lumi luciferase luciferin reagent was from Chrono-Log Corporation. Anti–phospho-myosin phosphatase targeting subunit (MYPT; Thr853) was from Upstate Biotechnology. Anti-cofilin and anti-Rac1 antibodies were from Cytoskeleton. Anti–phospho-cofilin (Ser3), anti–LIMK-1, anti–phospho-LIMK-1/LIMK-2 (Thr508/505), anti-PAK1, anti-PAK2, anti-PAK3, anti-PAK4, anti–phospho-PAK1/PAK2 (Thr423/402), anti–phospho-PAK4/5/6 (Ser474/Ser602/Ser560), anti–phospho Akt (Ser473), and anti–phospho-PKCδ (Thr505) antibodies were from Cell Signaling Technology. Antiactin depolymerizing factor (ADF)/destrin antibody was from Sigma-Aldrich. Rac/Cdc42 activation assay kit was from Cell Biolabs. All other materials were obtained as reported previously.7 Approval was obtained from the Ethic Commission of the Medical Faculty of the University of Munich.
Measurement of Rac1 activity
Rac1 activity was measured by a Rac1 Activation Assay kit (Cell Biolabs) that uses agarose beads conjugated with the p21 binding domain (PBD) of PAK to selectively isolate and pull-down the active form of Rac/Cdc42 from cell lysates. For details see the supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Immunoprecipitation of ADF from platelets
ADF was immunoprecipitated from platelets according to the protocol described previously with slight modifications.7 Briefly, the experiments were carried out in the presence of RGDS to avoid platelet aggregation. Platelet suspensions (4 × 108/mL, 0.4 mL) were stimulated with thrombin in the presence or absence of the Rac1 inhibitor NSC23766. Samples were lysed in an equal volume of 2× immunoprecipitation lysis buffer (2% Nonidet P40 [NP40], 300 mM NaCl, 20 mM Tris, pH 7.5, 2 mM ethyleneglycoltetraacetic acid [EGTA], 2 mM ethylenediaminetetraacetic acid [EDTA], 1 tablet/10 mL complete mini-protease inhibitor, 1 tablet/10 mL phosphatase inhibitor cocktail PhosSTOP, and 0.1% sodium dodecyl sulfate [SDS]) on ice. After the lysates were clarified by centrifugation, 50% protein A–Sepharose slurry was used to preclear and then subsequently incubated overnight with anti-ADF antibody (1:40 dilution) followed by collection of immune complex using protein A–Sepharose. All steps were performed at 4°C. Proteins were separated on SDS polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting (see the supplemental methods).
Isolation of washed human platelets, measurement of ATP-secretion, aggregation, and P-selectin expression were performed as previously described (see the supplemental methods).7
Results
Rac1 activation regulates granule secretion in thrombin-stimulated platelets
Thrombin at 0.1 U/mL induced shape change and a small ATP-secretion response, and at 0.2 and 0.5 U/mL enhanced dense granule secretion and in parallel aggregation (Figure 1A bottom and top). We observed no decrease of thrombin-stimulated dense granule and α-granule secretion in the presence of the integrin αIIbβ3 blocker RGDS, indicating that secretion was independent of integrin αIIbβ3 engagement (Figure S1A and data not shown).
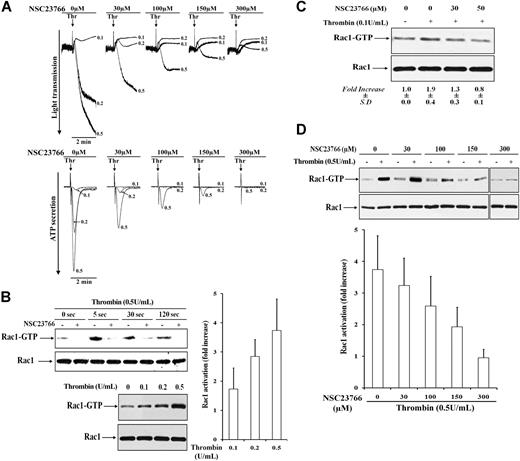
The Rac1-specific inhibitor NSC23766 blocks platelet aggregation, dense granule secretion, and Rac1 activation in platelets stimulated with thrombin. (A) Platelets untreated (0 μM) or pretreated with different concentrations of 30 to 300 μM NSC23766 for 5 minutes were stimulated with thrombin (0.1, 0.2, and 0.5 U/mL). NSC23766, dose-dependently inhibited thrombin-induced platelet aggregation (top) and dense granule secretion (bottom). Representative tracings for change in light transmission and ATP secretion (×0.05 gain) are shown, respectively. (B) Rac1 activation and inhibition by NSC23766 in thrombin-stimulated platelets. Top left, time course of Rac1 activation in 0.5 U/mL thrombin-stimulated platelets untreated (−) or pretreated with 300 μM NSC23766 (+). Platelets were stimulated in the presence of RGDS to inhibit aggregation. Representative immunoblots of pulled down Rac1-GTP and total Rac1. Bottom left, dose-dependent increase of Rac1 activation in platelets stimulated with increasing concentrations of thrombin (0.1, 0.2, and 0.5 U/mL) for 5 seconds. Representative immunoblots of pulled down Rac1-GTP and total Rac1 (left), and bar diagram exhibiting fold increase in Rac1 activation (right). Values are the mean + SD for 3 independent experiments. (C) Dose-dependent inhibition of 0.1 U/mL thrombin-induced Rac1 activation by NSC23766 (30 and 50 μM). Representative immunoblots of pulled down Rac1-GTP and total Rac1 from nonstimulated (−) and thrombin-stimulated (+) platelets. Values in mean-fold increase (± SD) obtained from 3 independent experiments are shown beneath the immunoblots. (D) Dose-dependent inhibition of 0.5 U/mL thrombin-induced Rac1 activation by 30 to 300 μM NSC23766. Top, representative immunoblots of pulled down Rac1-GTP and total Rac1 from nonstimulated (−) and thrombin-stimulated (+) platelets. Bottom, bar diagram showing inhibition of thrombin-induced Rac1 activation by NSC23766. Values are the mean + SD for 3 independent experiments.
The Rac1-specific inhibitor NSC23766 blocks platelet aggregation, dense granule secretion, and Rac1 activation in platelets stimulated with thrombin. (A) Platelets untreated (0 μM) or pretreated with different concentrations of 30 to 300 μM NSC23766 for 5 minutes were stimulated with thrombin (0.1, 0.2, and 0.5 U/mL). NSC23766, dose-dependently inhibited thrombin-induced platelet aggregation (top) and dense granule secretion (bottom). Representative tracings for change in light transmission and ATP secretion (×0.05 gain) are shown, respectively. (B) Rac1 activation and inhibition by NSC23766 in thrombin-stimulated platelets. Top left, time course of Rac1 activation in 0.5 U/mL thrombin-stimulated platelets untreated (−) or pretreated with 300 μM NSC23766 (+). Platelets were stimulated in the presence of RGDS to inhibit aggregation. Representative immunoblots of pulled down Rac1-GTP and total Rac1. Bottom left, dose-dependent increase of Rac1 activation in platelets stimulated with increasing concentrations of thrombin (0.1, 0.2, and 0.5 U/mL) for 5 seconds. Representative immunoblots of pulled down Rac1-GTP and total Rac1 (left), and bar diagram exhibiting fold increase in Rac1 activation (right). Values are the mean + SD for 3 independent experiments. (C) Dose-dependent inhibition of 0.1 U/mL thrombin-induced Rac1 activation by NSC23766 (30 and 50 μM). Representative immunoblots of pulled down Rac1-GTP and total Rac1 from nonstimulated (−) and thrombin-stimulated (+) platelets. Values in mean-fold increase (± SD) obtained from 3 independent experiments are shown beneath the immunoblots. (D) Dose-dependent inhibition of 0.5 U/mL thrombin-induced Rac1 activation by 30 to 300 μM NSC23766. Top, representative immunoblots of pulled down Rac1-GTP and total Rac1 from nonstimulated (−) and thrombin-stimulated (+) platelets. Bottom, bar diagram showing inhibition of thrombin-induced Rac1 activation by NSC23766. Values are the mean + SD for 3 independent experiments.
Human platelets contain mainly Rac1; the other isoforms of Rac (Rac2 and Rac3) were not found to be expressed in human platelets,2 a finding we could confirm (data not shown). To define the role of Rac1 in thrombin-stimulated dense granule secretion and platelet aggregation, Rac1-GTP was pulled down from human platelets stimulated with 0.1 to 0.5 U/mL thrombin. Rac1 was activated very rapidly after thrombin stimulation of platelets; the maximum was observed 5 seconds after platelet exposure to thrombin (Figure 1B left top). We observed a dose-dependent increase in Rac1 activation in platelets stimulated with 0.1 to 0.5 U/mL thrombin, which paralleled the extent of secretion from these platelets (Figure 1B left bottom and right). These results indicate that the rapid Rac1 activation might regulate secretion.
Further, we observed that the Rac1-specific inhibitor NSC2376614-16 (half maximal inhibitory concentration [IC50] = 50 μM) dose-dependently inhibited the thrombin-induced secretion and aggregation (Figure 1A bottom and top). Increasing concentrations of the Rac1 inhibitor (30-300 μM) were required to completely inhibit dense granule secretion induced by increasing thrombin concentrations (0.1-0.5 U/mL). A concentration of 30 μM NSC23766 inhibited ATP secretion induced by 0.2 U/mL thrombin, but not by 0.5 U/mL thrombin. Similarly, concentrations of 30 and 50 μM NSC23766 were sufficient to inhibit the small P-selectin expression induced by 0.1 U/mL thrombin, whereas 300 μM of NSC23766 was needed to inhibit the robust α-granule release induced by 0.5 U/mL thrombin (Table 1). Furthermore, Rac1-GTP pull-down assay shows that NSC23766 dose-dependently inhibited Rac1 activation induced by 0.1 and 0.5 U/mL thrombin (Figure 1C-D). Inhibition of Rac1 activation paralleled the inhibition of secretion; concentrations of 50 and 300 μM NSC23766 completely inhibited the rapid Rac1 activation as well as secretion induced by 0.1 and 0.5 U/mL thrombin, respectively. Inhibition of ATP-secretion by the Rac1 inhibitor was the same in the presence or absence of RGDS (supplemental Figure 1A). In contrast to NSC23766, we found that another Rac inhibitor, the substance EHT186417 (not specific for Rac1) did not inhibit Rac1 activation in thrombin-stimulated platelets. This inhibitor was used at concentrations up to 70 μM and with a preincubation time up to 1 hour (data not shown).
Differential role of Rac1 and PKC in regulating alpha-granule secretion from platelets
| Treatment . | P-selectin expression (% positive, mean ± SD, n=4) . | |||
|---|---|---|---|---|
| 0 seconds . | 15 seconds . | 180 seconds . | 300 seconds . | |
| Thr (0.5 U/mL) | 1.7 ± 1.2 | 43.6 ± 18.2 | 59.3 ± 12.4 | n.d |
| NSC23766 (300 μM) + Thr (0.5 U/mL) | 1.5 ± 1.2 | 5.2 ± 2.5 | 12.5 ± 5.2 | n.d |
| Gö6983 (2 μM) + Thr (0.5 U/mL) | 1.4 ± 0.8 | 4.0 ± 1.9 | 10.8 ± 1.8 | n.d |
| Thr (0.1 U/mL) | 2.2 ± 1.5 | 6.6 ± 1.3 | 16.0 ± 3.0 | n.d |
| NSC23766 (30 μM) + Thr (0.1 U/mL) | 1.9 ± 0.6 | 2.9 ± 0.8 | 6.6 ± 1.0 | n.d |
| NSC23766 (50 μM) + Thr (0.1 U/mL) | 1.8 ± 0.5 | 2.5 ± 1.1 | 4.4 ± 1.5 | n.d |
| PdBu (200 nM) | 1.9 ± 2.3 | 1.2 ± 0.7 | 12.2 ± 8.0 | 24.5 ± 15.5 |
| NSC23766 (300 μM) + PdBu (200 nM) | 1.3 ± 0.8 | 1.4 ± 0.7 | 9.0 ± 5.4 | 19.2 ± 7.3 |
| Gö6983 (2 μM) + PdBu (200 nM) | 1.4 ± 0.9 | 1.8 ± 0.4 | 1.9 ± 1.1 | 1.6 ± 1.0 |
| Treatment . | P-selectin expression (% positive, mean ± SD, n=4) . | |||
|---|---|---|---|---|
| 0 seconds . | 15 seconds . | 180 seconds . | 300 seconds . | |
| Thr (0.5 U/mL) | 1.7 ± 1.2 | 43.6 ± 18.2 | 59.3 ± 12.4 | n.d |
| NSC23766 (300 μM) + Thr (0.5 U/mL) | 1.5 ± 1.2 | 5.2 ± 2.5 | 12.5 ± 5.2 | n.d |
| Gö6983 (2 μM) + Thr (0.5 U/mL) | 1.4 ± 0.8 | 4.0 ± 1.9 | 10.8 ± 1.8 | n.d |
| Thr (0.1 U/mL) | 2.2 ± 1.5 | 6.6 ± 1.3 | 16.0 ± 3.0 | n.d |
| NSC23766 (30 μM) + Thr (0.1 U/mL) | 1.9 ± 0.6 | 2.9 ± 0.8 | 6.6 ± 1.0 | n.d |
| NSC23766 (50 μM) + Thr (0.1 U/mL) | 1.8 ± 0.5 | 2.5 ± 1.1 | 4.4 ± 1.5 | n.d |
| PdBu (200 nM) | 1.9 ± 2.3 | 1.2 ± 0.7 | 12.2 ± 8.0 | 24.5 ± 15.5 |
| NSC23766 (300 μM) + PdBu (200 nM) | 1.3 ± 0.8 | 1.4 ± 0.7 | 9.0 ± 5.4 | 19.2 ± 7.3 |
| Gö6983 (2 μM) + PdBu (200 nM) | 1.4 ± 0.9 | 1.8 ± 0.4 | 1.9 ± 1.1 | 1.6 ± 1.0 |
Platelets were untreated or pretreated with inhibitors and then stimulated with thrombin or PdBu. P-selectin expression was measured using the flow cytometer after collecting 10 000 platelet events.
To examine whether Rac1 has a direct role in αIIbβ3 integrin activation, 0.5 mg/mL fibrinogen was added 15 seconds before and after thrombin-stimulation of 300 μM NSC23766-treated platelets. Under both conditions, no platelet aggregation and ATP secretion was observed (data not shown and supplemental Figure 1B left). However, platelets pretreated with lower concentrations of NSC23766, where secretion was not completely blocked, showed an increase of platelet aggregation after fibrinogen addition, suggesting that secretion is required to observe aggregation of thrombin-stimulated platelets (data not shown). Indeed, in our aspirin-pretreated platelet preparation, where ADP induces reversible aggregation without secretion in the presence of fibrinogen,18 NSC23766 did not block platelet aggregation after 10 μM ADP stimulation (supplemental Figure 1B right). These results together indicate that Rac1 primarily regulates secretion and not αIIbβ3 integrin activation in stimulated platelets.
Rac1 activation regulates cofilin dephosphorylation in thrombin-stimulated platelets
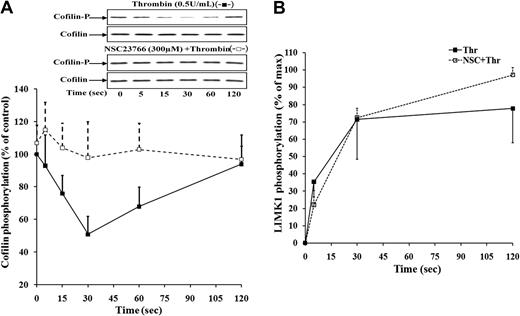
Thrombin induced a dose-dependent rapid (30 seconds after stimulation) and reversible cofilin dephosphorylation in activated platelets. Thrombin (0.1 U/mL), which induced only shape change and negligible platelet granule secretion, did not stimulate cofilin dephosphorylation in platelets (8% ± 7%, at 30 seconds). However, higher concentrations of thrombin (0.2 and 0.5 U/mL), which induced secretion and aggregation, significantly stimulated cofilin dephosphorylation at 30 seconds (36% ± 7% and 55% ± 8%, respectively; mean ± SD, n = 3, P < .04). Our previous studies showed that the rapid cofilin dephosphorylation occurring in stimulated platelets was Ca2+-dependent. The rapid cofilin dephosphorylation was not regulated by the Rho/Rho-kinase pathway and occurred upstream of secretion.8 Because Rac1 activation paralleled the cofilin dephosphorylation in stimulated platelets and is dependent on Gq-mediated Ca2+ mobilization, we wondered whether the rapid Rac1 activation might regulate cofilin dephosphorylation leading to secretion. Indeed, preincubation of platelets with NSC23766 completely blocked the thrombin-induced cofilin dephosphorylation (Figure 2A), implicating a Rac1/cofilin dephosphorylation signaling pathway in secretion.
Rac1 inhibitor NSC23766 blocks cofilin dephosphorylation but did not affect LIMK1 phosphorylation induced by thrombin. Effect of NSC23766 on cofilin (A) and LIMK-1 (Thr508; B) phosphorylation during platelet aggregation induced by 0.5 U/mL thrombin. Lysates of platelet stimulated with thrombin in the absence (-■-) or presence (-□-) of NSC23766 were immunoblotted with anti–phospho-cofilin, anti-cofilin anti–phospho-LIMK1/2, and anti-LIMK1 antibodies, and changes were estimated densitometrically. Graphic representation of the results. Values are the mean + SD or mean − SD for 3 independent experiments. Inset, representative immunoblots of cofilin phosphorylation.
Rac1 inhibitor NSC23766 blocks cofilin dephosphorylation but did not affect LIMK1 phosphorylation induced by thrombin. Effect of NSC23766 on cofilin (A) and LIMK-1 (Thr508; B) phosphorylation during platelet aggregation induced by 0.5 U/mL thrombin. Lysates of platelet stimulated with thrombin in the absence (-■-) or presence (-□-) of NSC23766 were immunoblotted with anti–phospho-cofilin, anti-cofilin anti–phospho-LIMK1/2, and anti-LIMK1 antibodies, and changes were estimated densitometrically. Graphic representation of the results. Values are the mean + SD or mean − SD for 3 independent experiments. Inset, representative immunoblots of cofilin phosphorylation.
ADF, a homologous protein of cofilin, fulfills the same functions as cofilin, and it is also phosphorylated at Ser3. Therefore, we explored whether ADF is present in platelets, and whether it might be also dephosphorylated after thrombin stimulation of platelets. We found that ADF is also present in human platelets as shown by immunoprecipitation and immunoblotting experiments with a specific anti-ADF antibody (supplemental Figure 2A). Because ADF and cofilin exhibit approximately 70% homology in their primary sequence, we probed the specificity of the anti-ADF and anti-cofilin antibodies. Anti-ADF did not recognize recombinant his-tagged cofilin and his-tagged cofilin-green fluorescent protein (GFP; supplemental Figure 2B). Also vice versa, the anti-cofilin antibody did not recognize ADF in anti-ADF immunoprecipitates of platelet lysates (supplemental Figure 2A lane 2, third immunoblot). Interestingly, we achieved to separate platelet cofilin and ADF on 18% SDS-PAGE. ADF had a slightly higher electrophoretic mobility than cofilin (supplemental Figure 2A). Also the anti–phospho-cofilin antibody did not detect phospho-ADF in platelets; it only detected phospho-cofilin. The most likely explanation is that the anti–phospho-cofilin antibody does not recognize phospho-ADF. The other possibility, that ADF is not phosphorylated in platelets, is unlikely, because studies of the platelet phospho-proteome have identified phospho-ADF in platelets.19,20
We further found that thrombin-induced LIMK1 phosphorylation was not affected by Rac1 inhibition (Figure 2B), strengthening our previous conclusions that LIMK1 is activated by the Rho/Rho-kinase pathway and not by Rac1 in stimulated platelets.7,8 This result further suggests that inhibition of cofilin dephosphorylation was not due to increased LIMK1 activation in the presence of NSC23766. In contrast to Rac1 activation, thrombin-induced phosphorylation of the Rho-kinase target MYPT was not inhibited by NSC23766 (supplemental Figure 1B), supporting the specificity of NSC23766 for Rac1 inhibition.
Because ADP can activate Rac1,21 we further analyzed the possible role of ADP secreted from dense granule in Rac1-dependent cofilin dephosphorylation. However, we found that the P2Y12 receptor antagonist AR-C69931MX did not inhibit dense granule secretion (Table 2) induced by 0.5 U/mL thrombin, indicating that P2Y12 receptor might not have a significant role for thrombin-induced secretion under these conditions. Moreover, AR-C69931MX and the P2Y1 receptor antagonists MRS-2179, were unable to inhibit cofilin dephosphorylation when applied alone (data not shown and Table 2) or in combination (data not shown) before thrombin-stimulation of platelets, indicating that these purinergic receptors are not involved in cofilin dephosphorylation during secretion induced by thrombin.
Role of PKC, calcineurin, PI3-kinase and P2Y12 receptor in secretion and cofilin dephosphorylation in thrombin-activated platelets
| Inhibitors (conc.) . | Targeted proteins . | ATP secretion, % of control ± SD . | Inhibition of cofilin dephosphorylation . |
|---|---|---|---|
| DMSO | None | 100 ± 0 | No |
| Wortmannin (100 nM) | PI3-kinases | 100 ± 19 | No |
| Gö6976 (1 μM) | PKCα, βIand μ | 93 ± 8 | No |
| Gö6983 (2 μM) | PKCα, β, γ, δ and ξ | 2 ± 2 | No |
| CAID-11R (20 μM) | Calcineurin | 10 ± 6 | Yes |
| AR-C69931MX (2 μM) | P2Y12 | 91 ± 10 | No |
| Inhibitors (conc.) . | Targeted proteins . | ATP secretion, % of control ± SD . | Inhibition of cofilin dephosphorylation . |
|---|---|---|---|
| DMSO | None | 100 ± 0 | No |
| Wortmannin (100 nM) | PI3-kinases | 100 ± 19 | No |
| Gö6976 (1 μM) | PKCα, βIand μ | 93 ± 8 | No |
| Gö6983 (2 μM) | PKCα, β, γ, δ and ξ | 2 ± 2 | No |
| CAID-11R (20 μM) | Calcineurin | 10 ± 6 | Yes |
| AR-C69931MX (2 μM) | P2Y12 | 91 ± 10 | No |
Platelets were untreated or pretreated with inhibitors at 37°C for 20 minutes and then stimulated with thrombin (0.5 U/ml). ATP-secretion was measured using the Lumi-aggregometer, and cofilin dephosphorylation 30 seconds after thrombin stimulation was analyzed by immunoblotting as described in “Methods.”
conc. indicates concentration.
Identification of PAK isoforms in platelets
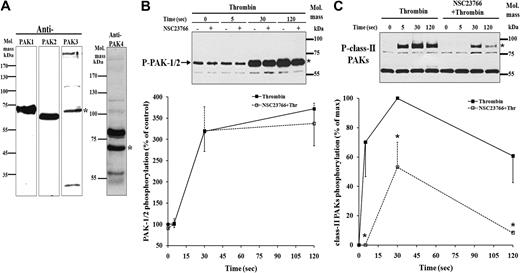
We further investigated the signaling pathway for Rac1 activation to cofilin dephosphorylation. Among targets of Rac1, PAKs are known to be phosphorylated and activated after binding to Rac1-GTP. The PAKs are classified into 2 classes, the first discovered PAK1, PAK2, and PAK3 belong to class I, and the newly discovered PAK4, PAK5, and PAK6 belong to class II.22 Using specific antibodies against the isoforms of PAK, we found that platelets express class I PAKs (PAK1/2/3) as well as PAK4, which belongs to class II PAKs (Figure 3A). We could detect PAK1, PAK2, and PAK3 as single bands migrating according to their predicted molecular masses of approximately 60, 58, and 62 kDa, respectively (www.expasy.org). The anti-PAK4 antibody detected 2 bands in platelets of approximately 68 kDa (asterisk in Figure 3A; predicted mass of PAK4) and approximately 80 kDa (unknown). We could not detect a specific band for PAK5 and PAK6 using the commercially available anti-PAK5 and anti-PAK6 antibodies, respectively. Either these PAK-isoforms are not present, or the antibodies used lack specificity and sensitivity (data not shown).
Identification of PAK isoforms in platelets and inhibition of PAK4/5/6 phosphorylation but not the PAK1/2 phosphorylation by NSC23766. (A) Identification of PAK isoforms in platelets. Resting platelets were lysed and immunoblotted using specific anti-PAK1, anti-PAK2, anti-PAK3, and anti-PAK4 antibodies. Immunoblots showing PAK1, PAK2, PAK3, and PAK4 at their approximate molecular masses (*) in platelets. (B) Effect of 300 μM NSC23766 on PAK1/2 phosphorylation. Platelets stimulated with 0.5 U/mL thrombin in the absence (-■-) or in the presence of NSC23766 (-□-) were immunoblotted with anti–phospho-PAK1/2 antibody. Top, representative immunoblot of PAK1/2 phosphorylation (*). Bottom, graphic representation of PAK1/2 phosphorylation (between 55 and 75 kDa). Values are the mean + SD or mean − SD for 3 independent experiments. (C) Inhibition of class II-PAKs phosphorylation by 300 μM NSC23766. Platelets stimulated with thrombin in the absence (-■-) or in the presence of NSC23766 (-□-) were immunoblotted with anti–phospho-PAK4/5/6 antibody. Top, representative immunoblot of class II PAKs phosphorylation (*). Bottom, graphic representation of class II PAKs phosphorylation (between 75 and 100 kDa). Values are the mean + SD or mean − SD for 3 independent experiments. * denotes statistical significance (P < .001) with respect to nontreated sample.
Identification of PAK isoforms in platelets and inhibition of PAK4/5/6 phosphorylation but not the PAK1/2 phosphorylation by NSC23766. (A) Identification of PAK isoforms in platelets. Resting platelets were lysed and immunoblotted using specific anti-PAK1, anti-PAK2, anti-PAK3, and anti-PAK4 antibodies. Immunoblots showing PAK1, PAK2, PAK3, and PAK4 at their approximate molecular masses (*) in platelets. (B) Effect of 300 μM NSC23766 on PAK1/2 phosphorylation. Platelets stimulated with 0.5 U/mL thrombin in the absence (-■-) or in the presence of NSC23766 (-□-) were immunoblotted with anti–phospho-PAK1/2 antibody. Top, representative immunoblot of PAK1/2 phosphorylation (*). Bottom, graphic representation of PAK1/2 phosphorylation (between 55 and 75 kDa). Values are the mean + SD or mean − SD for 3 independent experiments. (C) Inhibition of class II-PAKs phosphorylation by 300 μM NSC23766. Platelets stimulated with thrombin in the absence (-■-) or in the presence of NSC23766 (-□-) were immunoblotted with anti–phospho-PAK4/5/6 antibody. Top, representative immunoblot of class II PAKs phosphorylation (*). Bottom, graphic representation of class II PAKs phosphorylation (between 75 and 100 kDa). Values are the mean + SD or mean − SD for 3 independent experiments. * denotes statistical significance (P < .001) with respect to nontreated sample.
Rac1 regulates class II PAKs but not class I PAKs in platelets
Phosphorylation of Thr423 and Thr402 in PAK1 and PAK2, respectively, and of Ser474, Ser602, and Ser560 in PAK4, PAK5, and PAK6, respectively, is critical for their activation.22 Using specific antibodies against phosphorylated forms of class I (PAK1/2) and class II (PAK4/5/6),23 we found that in thrombin-stimulated platelets, phosphorylation of class I and class II PAKs is differentially regulated. Class II PAKs phosphorylation was very rapid and similar to that observed for Rac1 activation (5 seconds after thrombin stimulation), whereas PAK1/2 phosphorylation was slower (Figure 3B-C). A significant increased phosphorylation of PAK1/2 was observed only 30 seconds after stimulation of platelets with thrombin. To analyze which PAKs are activated by Rac1 stimulation, we immunoblotted lysates of thrombin-stimulated platelets pretreated and untreated with NSC23766. Preincubation of platelets with NSC23766 completely blocked the rapid class II PAKs phosphorylation at 5 seconds and significantly inhibited the phosphorylation at 30 and 120 seconds after thrombin stimulation of platelets (Figure 3C). However, PAK1/2 phosphorylation in thrombin-stimulated platelets was unaffected by NSC23766 (Figure 3B). These results indicate that Rac1 regulates class II PAKs, but not PAK1/2 phosphorylation, and therefore, class II PAKs may be involved in Rac1-mediated cofilin dephosphorylation.
Calcineurin activates Rac1/class II PAKs leading to cofilin dephosphorylation and secretion
Previously, we found that cofilin is dephosphorylated in a Ca2+-dependent manner during platelet stimulation with LPA,8 and the cofilin phosphatase slingshot has been shown to interact directly and to be activated by the Ca2+-dependent phosphatases calcineurin.24 Therefore, we pretreated platelets with the calcineurin autoinhibitory domain (CAID) coupled to an arginine-rich polypeptide (11R) that has been shown to inhibit calcineurin in neuronal cells.25 A similar approach of inserting peptide successfully into platelets has been carried out using a peptide coupled with 9 arginine polypeptide (R9).26 The CAID-11R peptide completely blocked thrombin-induced cofilin dephosphorylation and secretion (Figure 4A and Table 2). The peptide CAID (without 11R domain) and a control peptide targeted for nuclear factor activated transcription (NFAT-11R) did not inhibit cofilin dephosphorylation induced by thrombin (Figure 4A). These results suggest a role of calcineurin in regulating cofilin dephosphorylation leading to secretion from platelets.
Effect of calcineurin inhibition on cofilin dephosphorylation, Rac1 activation, and class II PAKs phosphorylation in thrombin-stimulated platelets. (A) Inhibition of cofilin dephosphorylation by the calcineurin inhibitory peptide CAID-11R and not by the control peptides CAID and NFAT-11R in thrombin-stimulated platelets. Platelet samples pretreated with CAID (-■-), CAID-11R (-□-), or NFAT-11R (-▴-; each 20 μM) and stimulated with 0.5 U/mL thrombin were immunoblotted with anti-cofilin and anti–phospho-cofilin antibodies. Top, representative immunoblots for cofilin phosphorylation. Bottom, graphic representation of the results. Values are the mean + SD or mean − SD of 3 independent experiments. (B) Inhibition of Rac1 activation by 20 μM CAID-11R. Rac1-GTP was pulled down from platelets untreated (−) or pretreated with CAID-11R (+) and then stimulated with 0.5 U/mL thrombin for 5 seconds. Representative immunoblots of pulled down Rac1-GTP and total Rac1. (C) Inhibition of class II PAKs phosphorylation by CAID-11R. Platelets stimulated with thrombin in the absence (-■-) or in the presence of CAID-11R (-□-) were immunoblotted with anti–phospho-PAK4/5/6 antibody. Top, representative immunoblots of PAK4/5/6 phosphorylation. Bottom, graphic representation of PAK4/5/6 phosphorylation. Values are the mean + SD or mean − SD for 3 independent experiments. * denotes statistical significance (P < .001) with respect to nontreated sample.
Effect of calcineurin inhibition on cofilin dephosphorylation, Rac1 activation, and class II PAKs phosphorylation in thrombin-stimulated platelets. (A) Inhibition of cofilin dephosphorylation by the calcineurin inhibitory peptide CAID-11R and not by the control peptides CAID and NFAT-11R in thrombin-stimulated platelets. Platelet samples pretreated with CAID (-■-), CAID-11R (-□-), or NFAT-11R (-▴-; each 20 μM) and stimulated with 0.5 U/mL thrombin were immunoblotted with anti-cofilin and anti–phospho-cofilin antibodies. Top, representative immunoblots for cofilin phosphorylation. Bottom, graphic representation of the results. Values are the mean + SD or mean − SD of 3 independent experiments. (B) Inhibition of Rac1 activation by 20 μM CAID-11R. Rac1-GTP was pulled down from platelets untreated (−) or pretreated with CAID-11R (+) and then stimulated with 0.5 U/mL thrombin for 5 seconds. Representative immunoblots of pulled down Rac1-GTP and total Rac1. (C) Inhibition of class II PAKs phosphorylation by CAID-11R. Platelets stimulated with thrombin in the absence (-■-) or in the presence of CAID-11R (-□-) were immunoblotted with anti–phospho-PAK4/5/6 antibody. Top, representative immunoblots of PAK4/5/6 phosphorylation. Bottom, graphic representation of PAK4/5/6 phosphorylation. Values are the mean + SD or mean − SD for 3 independent experiments. * denotes statistical significance (P < .001) with respect to nontreated sample.
We further analyzed how calcineurin might be regulating cofilin dephosphorylation in platelets. We found that thrombin-induced rapid Rac1 activation was completely blocked (1.06- ± 0.27-fold) by CAID-11R (Figure 4B). Class II PAKs phosphorylation was completely inhibited at 5 seconds and significantly reduced at 30 and 120 seconds after platelet stimulation in the presence of CAID-11R (Figure 4C). These results indicate that calcineurin-mediated cofilin dephosphorylation involves activation of a Rac1/class II PAKs signaling pathway.
Rac1-mediated cofilin dephosphorylation does not involve activation of PI3-kinase and PKC
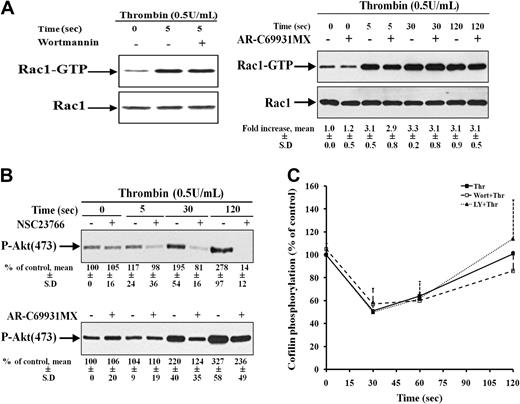
In platelets, several PI3-kinases are expressed and play a role in thrombin-induced aggregation.27 PI3-kinase activation could be downstream as well as upstream of Rac1.28 To analyze the crosstalk between Rac1 and PI3-kinase, further experiments were performed. Preincubation of platelets with wortmannin did not block Rac1 activation (Figure 5A top). However, the Rac1 inhibitor NSC23766 showed a complete inhibition of Akt (Ser473) phosphorylation (substrate of PI3-kinase) in thrombin-stimulated platelets (Figure 5B top). Previous studies demonstrated that thrombin promotes PI3K/Akt signaling indirectly through stimulation of the Gi pathway by secreted ADP.29 Indeed, the P2Y12 receptor antagonist AR-C69931MX partially inhibited PI3-kinase activation but not Rac1 activation. These results indicate that in thrombin-stimulated platelets, PI3-kinase activation is independently regulated by Rac1, and P2Y12 activation by ADP secreted from dense granules (Figure 5A-B right).
PI3-kinase is downstream of Rac1 activation, but it is not involved in cofilin dephosphorylation. (A) Left, Rac1 is upstream to PI3-kinase in thrombin-stimulated platelets. Rac1-GTP from resting platelets (lane 1) and platelets stimulated with thrombin for 5 seconds that were previously nontreated (lane 2) or pretreated with 100 nM wortmannin (lane 3) was pulled down and immunoblotted with anti-Rac1 antibody. Representative immunoblots showing Rac1 activation. Right, Rac1 activity is independent of P2Y12 receptor activation. Rac1-GTP from resting platelets and platelets stimulated with thrombin for 5 seconds that were previously nontreated or pretreated with 2 μM AR-C69931MX, alternate lanes. Values are the mean ± SD for 4 independent experiments. (B) Inhibition of Akt (Ser473) phosphorylation by 300 μM NSC23766 (top) and 2 μM AR-C69931MX (bottom). Lysates of platelets stimulated with thrombin for 0, 30, and 120 seconds in the absence or presence of NSC23766 and AR-C69931MX (alternate lanes) were immunoblotted with anti–phospho (Ser473)–Akt antibody. Values are the mean ± SD for 4 independent experiments. (C) No inhibition of cofilin dephosphorylation by 100 nM wortmannin and 40 μM LY294002 in thrombin-stimulated platelets. Platelets lysates were immunoblotted with anti–phospho-cofilin antibody. Graphical representation of the cofilin phosphorylation in nontreated platelets (-■-) and platelets pretreated with wortmannin (-□-) and LY294002 (-▴-) during platelet stimulation with thrombin. Values are the mean + SD for 3 independent experiments.
PI3-kinase is downstream of Rac1 activation, but it is not involved in cofilin dephosphorylation. (A) Left, Rac1 is upstream to PI3-kinase in thrombin-stimulated platelets. Rac1-GTP from resting platelets (lane 1) and platelets stimulated with thrombin for 5 seconds that were previously nontreated (lane 2) or pretreated with 100 nM wortmannin (lane 3) was pulled down and immunoblotted with anti-Rac1 antibody. Representative immunoblots showing Rac1 activation. Right, Rac1 activity is independent of P2Y12 receptor activation. Rac1-GTP from resting platelets and platelets stimulated with thrombin for 5 seconds that were previously nontreated or pretreated with 2 μM AR-C69931MX, alternate lanes. Values are the mean ± SD for 4 independent experiments. (B) Inhibition of Akt (Ser473) phosphorylation by 300 μM NSC23766 (top) and 2 μM AR-C69931MX (bottom). Lysates of platelets stimulated with thrombin for 0, 30, and 120 seconds in the absence or presence of NSC23766 and AR-C69931MX (alternate lanes) were immunoblotted with anti–phospho (Ser473)–Akt antibody. Values are the mean ± SD for 4 independent experiments. (C) No inhibition of cofilin dephosphorylation by 100 nM wortmannin and 40 μM LY294002 in thrombin-stimulated platelets. Platelets lysates were immunoblotted with anti–phospho-cofilin antibody. Graphical representation of the cofilin phosphorylation in nontreated platelets (-■-) and platelets pretreated with wortmannin (-□-) and LY294002 (-▴-) during platelet stimulation with thrombin. Values are the mean + SD for 3 independent experiments.
Because these results show that Rac1 is upstream of PI3-kinases, the possibility exists that PI3-kinases might also contribute in regulating cofilin dephosphorylation and secretion in thrombin-stimulated platelets. To analyze the role of PI3-kinase in regulating cofilin dephosphorylation in platelets, 2 structurally different inhibitors of PI3-kinases, wortmannin and LY294002 were used. Pretreatment of platelets with 100 nM wortmannin and 40 μM LY294002 resulted in a reduced and reversible platelet aggregation of platelets, but did not inhibit ATP secretion of platelets stimulated with thrombin (Table 2 and data not shown). Both inhibitors completely blocked PI3-kinase activation as measured by the inhibition of Akt phosphorylation at Ser473 (data not shown). However, neither Wortmannin nor LY294002 were able to inhibit class II PAKs phosphorylation and cofilin dephosphorylation in thrombin-stimulated platelets (data not shown and Figure 5C), indicating that PI3-kinases are not involved in Rac1/class II PAKs-mediated cofilin dephosphorylation and secretion.
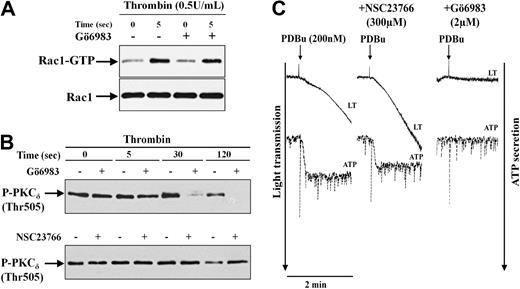
PKC has been shown to regulate secretion in platelets stimulated with various agonists,30 and in neutrophils, PKC has been shown to trigger cofilin dephosphorylation.31 To investigate the role of PKC in thrombin-stimulated cofilin dephosphorylation and dense granule secretion, we used 2 different PKC inhibitors Gö6976 and Gö6983. Gö6976 which targets PKCα, βI,and μ, did not inhibit, whereas Gö6983, which inhibits PKCα, β, γ, δ, and ξ, completely blocked thrombin-induced ATP secretion, suggesting that not the PKCα,β, and μ, but other PKCs (PKCδ, ξ, or γ) are involved in regulating dense granule secretion from platelets (Table 2). Furthermore, inhibition of thrombin-induced P-selectin expression by Gö6983 confirms the role of these PKCs in platelet granule secretion (Table 1).
PKC has been shown to phosphorylate Rac1-GEF Tiam1 and activate Rac1 in fibroblasts.32 It might be possible that inhibition of secretion by Gö6983 is due to inhibition of Rac1 activation. However, we found that Gö6983 did not affect Rac1 activation in platelets stimulated with thrombin (Figure 6A). Moreover, none of the PKCs inhibitors (Gö6976 and Gö6983) were able to inhibit cofilin dephosphorylation induced by thrombin (Table 2), indicating that PKC is not involved in the Rac1-mediated cofilin dephosphorylation that regulates dense granule secretion.
Independent activation of Rac1-mediated and PKC-mediated pathways mediating secretion induced by thrombin. (A) No inhibition of Rac1 activation by the PKC inhibitor Gö6983 (2 μM) in thrombin-stimulated platelets. Rac1-GTP was pulled down from untreated platelets (−) and platelets pretreated with Gö6983 (+) and then stimulated with 0.5 U/mL thrombin. Representative immunoblots of pulled down Rac1-GTP and total Rac1. (B) Inhibition of PKCδ (Thr505) phosphorylation by Gö6983, but not by the Rac1 inhibitor NSC23766. Platelet lysates of thrombin-stimulated platelets untreated or pretreated with Gö6983 (top) or NSC23766 (bottom) were immunoblotted with anti–phospho-PKCδ (Thr505) antibody. Representative immunoblots for PKCδ phosphorylation. (C) PDBu-induced secretion is independent of Rac1 activation. Platelets untreated (left) or pretreated with 300 μM NSC23766 or 2 μM Gö6983 were stimulated with 200 nM PDBu. Platelet aggregation and ATP secretion was monitored in lumi-aggregometer. Representative tracings for change in light transmission and ATP secretion (×0.5 gain) are shown.
Independent activation of Rac1-mediated and PKC-mediated pathways mediating secretion induced by thrombin. (A) No inhibition of Rac1 activation by the PKC inhibitor Gö6983 (2 μM) in thrombin-stimulated platelets. Rac1-GTP was pulled down from untreated platelets (−) and platelets pretreated with Gö6983 (+) and then stimulated with 0.5 U/mL thrombin. Representative immunoblots of pulled down Rac1-GTP and total Rac1. (B) Inhibition of PKCδ (Thr505) phosphorylation by Gö6983, but not by the Rac1 inhibitor NSC23766. Platelet lysates of thrombin-stimulated platelets untreated or pretreated with Gö6983 (top) or NSC23766 (bottom) were immunoblotted with anti–phospho-PKCδ (Thr505) antibody. Representative immunoblots for PKCδ phosphorylation. (C) PDBu-induced secretion is independent of Rac1 activation. Platelets untreated (left) or pretreated with 300 μM NSC23766 or 2 μM Gö6983 were stimulated with 200 nM PDBu. Platelet aggregation and ATP secretion was monitored in lumi-aggregometer. Representative tracings for change in light transmission and ATP secretion (×0.5 gain) are shown.
The increase in phosphorylation of PKCδ at Thr505 position has been proposed to be essential for dense granule secretion induced by protease-activated receptor (PAR) agonists in platelets.30 We found that PKCδ phosphorylation was increased significantly only at 30 seconds after stimulation with thrombin (157% ± 28% of control). Gö6983, which inhibited thrombin-induced secretion, inhibited the phosphorylation of PKCδ (at 30 and 120 seconds) supporting the involvement of PKCδ in secretion (Figure 6B). The Rac1 inhibitor NSC23766, which also inhibited thrombin-induced secretion, did not inhibit PKCδ phosphorylation (136% ± 33% of control) 30 seconds after thrombin stimulation, indicating that Rac1 and PKCδ independently regulate dense granule secretion induced by thrombin. Furthermore, Rac1 was not activated (1.1- ± 0.14-fold increase) at 30 seconds after addition of the PKC activator PDBu (200 nM). This concentration of PDBu induced ATP secretion, P-selectin expression, aggregation, and PKCδ (Thr505) phosphorylation that were completely blocked by Gö6983, but was not inhibited by the Rac1 inhibitor NSC23766 (Figure 6C, Table 1, and data not shown). These results indicate that the PKC-mediated and Rac1-mediated signaling pathways independently regulate secretion in thrombin-activated platelets.
Discussion
Our study shows that the negative regulation of cofilin by the Rac/PAK pathway that has been described in many cell types is not operative in platelets. In contrast, we show that Rac1 activation leads to cofilin dephosphorylation (activation) and platelet secretion. This novel signaling pathway was further delineated: it involves a calcineurin-mediated and Rac1-mediated activation of class II PAKs, but not activation of class I PAKs (PAK1/2/3). This pathway was independent of the PKC (PKCδ-Thr505) pathway; however, both signaling pathways seem to be required for secretion. We further demonstrate that PI3-kinase activation is also downstream of Rac1, but PI3-kinases are not involved in mediating cofilin dephosphorylation and secretion in thrombin-stimulated platelets.
Unlike other cells of hematopoietic origin, where Rac2 is the major isoform, human platelets express predominantly the Rac1 isoform.2 In support, we could only find the expression of Rac1, but not of Rac2 or Rac3 isoforms in human platelets. We found that Rac1 in human platelets is rapidly and maximally activated after 5 seconds of stimulation with thrombin. Rac1 activation increased with increasing concentrations of thrombin. NSC23766 dose-dependently inhibited Rac1 activation stimulated with 0.1 and 0.5 U/mL thrombin. NSC23766 is a specific Rac1 inhibitor with IC50 of 50 μM that has been extensively used to block Rac1 activation in many in vivo and in vitro studies. Moreover, this inhibitor does not affect other Rho-GTPases (Cdc42 and RhoA) activity in platelets and other cells.13-16 Confirming the specificity of NSC23766, we found that this compound did not interfere with Rho/Rho-kinase-mediated signaling pathways, such as MYPT1 and LIMK1 phosphorylation in thrombin-stimulated platelets.
It is likely that Rac1 activation regulates secretion in thrombin-stimulated platelets for 3 reasons: First, Rac1 activation paralleled the extent of secretion. Second, Rac1 activation occurred very rapidly; the maximum was observed within 5 seconds of platelet stimulation with thrombin. Third, NSC23766 dose-dependently inhibited both Rac1 activation and platelet granule secretion, in parallel. NSC23766 did not block shape change, but it inhibited platelet aggregation due to inhibition of secretion. The lack of inhibition of ADP-induced aggregation by the Rac1 inhibitor further confirms that Rac1 primarily regulates secretion but not aggregation. In agreement with the study by Akbar et al13 we found that the inhibitory effect of NSC23766 on secretion and aggregation induced by thrombin could be overcome by increasing the concentration of thrombin. This could be explained by thrombin concentrations-dependent increase in Rac1 activation surpassing the inhibition by low concentrations of NSC23766. Indeed, higher concentrations of NSC23766 were able to block Rac1 activation completely and also secretion and aggregation of platelets stimulated with higher concentrations of thrombin.
In mice platelets, the results regarding the role of Rac1 in thrombin-induced aggregation are controversial.2,13,33 Although all studies used conditional Rac1 knockout mice, one showed impaired thrombin-induced aggregation in Rac1 null platelets in accordance with our study in human platelets.13 In contrast, the 2 other studies reported that thrombin-induced secretion and aggregation was not affected in Rac1-deficient mice platelets; Rac1 was found to be involved only in glycoprotein VI (GPVI)–mediated platelet activation.2,33
The PAKs have been identified as downstream effectors of Rac1 in cells. So far 6 PAK isoforms belonging to class I (PAK1/2/3) and class II (PAK4/5/6) have been described. We studied systematically the expression of PAK isoforms in platelets by immunoblotting. We observed not only the expression of class I PAKs (PAK1/2/3) but also of PAK4 belonging to the class II PAK. The antibodies against PAK5 and PAK6 failed to detect any specific bands in platelets; we cannot rule out, however, that platelets also express these isoforms of class II PAK.
Interaction of Rac-GTP or Cdc42-GTP with class I PAKs leads to autophosphorylation of PAKs at a conserved threonine residue (Thr423 in PAK1 and Thr402 in PAK2) that is necessary for their activation.22 In agreement with previous observations, we found an increased phosphorylation of PAK1/2 in thrombin-stimulated platelets.34,35 However, the time course of PAK1/2 phosphorylation did not correlate with the rapid Rac1 activation, and PAK1/2 phosphorylation was unaffected by NSC23766, indicating that class I PAKs are not regulated by Rac1. By carefully analyzing the study by Vidal et al,35 we found that the lethal toxin from Clostridium sordelli, which inhibits Rac1 and not Cdc42, was not able to block PAK1/2 activity, further suggesting that class I PAKs are not regulated by Rac1 activation in thrombin-stimulated platelets. In contrast to thrombin, collagen has been shown to induce Rac1-dependent PAK1/2 phosphorylation in platelets.13,36 Moreover, Rac1 activation in platelets stimulated by thrombin and collagen seems also to be regulated by different mechanisms: PI3-kinase was found to be involved in collagen-induced, but not in thrombin-induced, Rac1 activation (present study).11 Together it appears that Rac1 regulates PAK1/2 phosphorylation differently in platelets, and this is based on the signaling pathways upstream of Rac1 activation.
Because class 1 PAKs are not regulated by Rac1 in thrombin-stimulated platelets, we analyzed the phosphorylation of class II PAKs. Similar to class I PAKs, class II PAKs are also activated by autophosphorylation (of a conserved serine residue: Ser474 in PAK4), but the regulatory mechanism is poorly understood. Although all members of class II PAKs have been shown to interact with Rac-GTP or Cdc42-GTP, only PAK5 autophosphorylation is suggested to be controlled by this interaction.23,37 It is unknown, how class II PAKs are regulated in activated platelets. We found that class II PAKs phosphorylation was as rapid as Rac1 activation, showing a maximum at 5 seconds after platelet stimulation with thrombin, and that it was completely inhibited by NSC23766, indicating that class II PAKs activation is regulated at this time point entirely by Rac1. Due to the lack of specific immunoprecipitating antibodies, we could not determine which of the class II PAKs (PAK4/5/6) is regulated by Rac1 activation in human platelets. However, based on an increased phosphorylation of a protein with a mass of approximately 90 kDa detected by the anti–phospho-PAK4/5/6 antibody, which recognizes specifically the phosphorylated class II PAKs23,38 (Figure 3C), we suggest that PAK5 (MW, 90 kDa) could be a target downstream of Rac1-GTP.
Rac1-activation has been reported to mediate secretion by regulating the actin cytoskeleton reorganization in bovine chromaffin cells.39 Several studies in adrenal chromaffin cells and parotid acinar cells also linked cofilin activation with Ca2+-triggered exocytosis and amylase exocytosis, respectively.40,41 Previously, we found that LPA-induced cofilin dephosphorylation and dense granule secretion was blocked by the intracellular Ca2+-chelator BAPTA-AM.8 Because Rac activation is dependent on prior Ca2+-mobilization,11,12 we hypothesized that Rac1 activation might be linked to cofilin dephosphorylation. Indeed, in this study, we found that stimulation of Rac1 leads to cofilin activation and dense granule secretion in platelets exposed to thrombin. In support of our results, a recent study has shown cofilin dephosphorylation after transfecting constitutive active Rac1 into keratinocytes.42
Our results with the Ca2+/calmodulin-dependent phosphatase calcineurin inhibitor show that calcineurin regulates Rac1 activity in platelets. Similarly, calcineurin has been shown to regulate activation of the Rac2 isoform in human neutrophils.43 Our further results with the calcineurin inhibitor point to a critical role of Rac1/class II PAKs activation in cofilin dephosphorylation and secretion (Table 2). In support, independent overexpression of PAK1 and ADF in chromaffin cells showed similar enhancement of agonist-induced secretion from these cells.39 In other cells, calcineurin as well as PAK4 have been shown to interact directly with slingshot1 (SSH1), thereby either activating or inactivating this cofilin phosphatase, respectively.24,44 Our findings in platelets do not fit with these results, because they show that the (unknown) cofilin phosphatase is positively regulated by a calcineurin/Rac1/class II PAK pathway. It might be possible that chronophin, a cofilin phosphatase other than SSH family phosphatase,45 is involved in dephosphorylating cofilin through this signaling pathway in platelets.
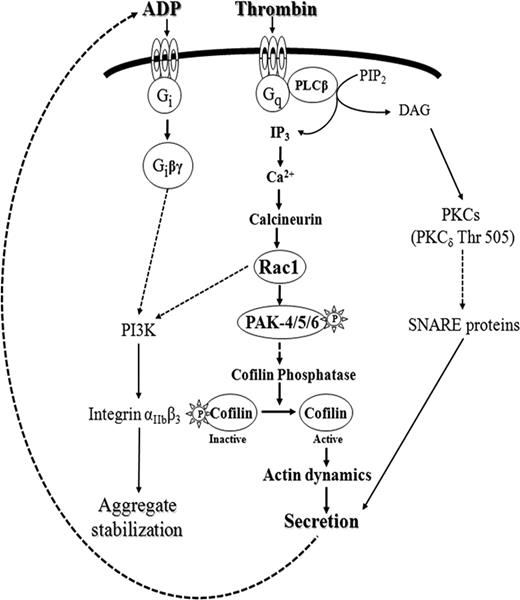
We also studied whether Rac1 might have other targets than PAK that could regulate cofilin dephosphorylation in stimulated platelets. Because in thrombin-stimulated platelets the PI3-kinase inhibitor wortmannin showed no effect on Rac1 activation,11 which was confirmed by our study, PI3-kinases are clearly downstream of Rac1. Indeed, we observed that Akt phosphorylation was inhibited by NSC23766, indicating for the first time that Rac1 activation stimulates PI3-kinases in thrombin-stimulated platelets. So far, it is not clear how Rac1 can regulate PI3-kinase activity. It is controversial whether Rac1 regulates PI3-kinase activity by direct interaction with the p85 regulatory subunit or not.28 In insulin-stimulated cells, PI3-kinase has been shown to stimulate SSH1 activity, which was related to the processes of membrane protrusions.46 Therefore, PI3-kinase activation could be a second link between Rac1 activation and cofilin dephosphorylation in platelets. However, neither Wortmannin nor LY294002 was able to inhibit class II PAK activation and cofilin dephosphorylation, suggesting that PI3-kinase activation is not involved in cofilin dephosphorylation in thrombin-stimulated platelets. Therefore, we identified a bifurcating signaling pathway downstream of Rac1 in stimulated platelets, the activation of PI3-kinases and class II PAKs. Activation of class II PAKs and cofilin leads to secretion, whereas PI3-kinases through integrin activation might stabilize platelet aggregates as observed previously47,48 (scheme in Figure 7).
Delineation of the Ca2+-dependent signaling pathway mediating secretion in thrombin stimulated human platelets: calcineurin→Rac1→class II PAKs activation→cofilin dephosphorylation (bold, center). The PKC-dependent pathway (right) regulates secretion independently of Rac1-mediated pathway and through different target proteins. The second Rac1–stimulated pathway identified in this study activates PI3-kinases (left), which, through αIIbβ3 integrin activation, is involved in aggregate stabilization. PI3-kinase activity is partially regulated by secreted ADP through P2Y12 receptor activation, which is independent of Rac1-mediated PI3-kinase activation.
Delineation of the Ca2+-dependent signaling pathway mediating secretion in thrombin stimulated human platelets: calcineurin→Rac1→class II PAKs activation→cofilin dephosphorylation (bold, center). The PKC-dependent pathway (right) regulates secretion independently of Rac1-mediated pathway and through different target proteins. The second Rac1–stimulated pathway identified in this study activates PI3-kinases (left), which, through αIIbβ3 integrin activation, is involved in aggregate stabilization. PI3-kinase activity is partially regulated by secreted ADP through P2Y12 receptor activation, which is independent of Rac1-mediated PI3-kinase activation.
PKC is known to play a central role in dense granule secretion in platelets induced by different agonists, and in neutrophils, PKC-dependent and -independent pathways have been suggested to trigger cofilin dephosphorylation.31 We found, however, that the PKC inhibitor Gö6983, which inhibited secretion and PKCδ (Thr505) phosphorylation, had no effect on Rac1 activation and cofilin dephosphorylation (Figure 6A and Table 2). Furthermore, Rac1 inhibition did not block thrombin-induced PKCδ phosphorylation as well as PDBu-induced secretion and aggregation. These results indicate that activation of PKC (such as PKCδ)30 might regulate secretion independently of the Rac1/class II PAKs/cofilin dephosphorylation pathway identified in the present study (Figure 7). In platelets, PKC has been shown to have multiple targets, like Munc-18, syntaxin-4, and CDCrel1, which belong to soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins and their chaperones, known to be part of the secretory machinery (exocytotic core complex) in platelets (reviewed in Flaumenhaft et al49 ).
We previously observed a rapid increased association of cofilin with F-actin, which correlated with rapid cofilin dephosphorylation in thrombin-stimulated platelets.7 The changes in cofilin activity brought by Rac1 activation might be an important regulator for modulating the actin cytoskeleton leading to secretion (Figure 7). Cofilin dephosphorylation allows cofilin to bind to F-actin. Cofilin acting at local sites within the platelet might enhance secretion due to its dual activity: cofilin as an actin severing and depolymerizing protein could help to release the constraints postured by the actin cytoskeleton for secretion, and cofilin by increasing actin filament formation might guide the dense granules and help their fusion with open canalicular system (OCS) membranes required for secretion. Supporting the latter, recently an in vitro study has shown that actin polymerization is needed for vacuole membrane fusion.50
In this study, we also tried to analyze the phosphorylation of ADF, because it performs identical functions of severing and depolymerizing actin filaments as cofilin. We could detect ADF in human platelets supporting 2 previous proteomic studies that identified phosphorylated ADF in resting platelets.19,20 However, we could not study the kinetics of ADF-dephosphorylation, because the anti–phospho-cofilin antibody did not detect phospho-ADF, and anti–phospho-ADF antibodies are not available. Interestingly, phosphoproteome studies showed that both cofilin and ADF are dephosphorylated after platelet stimulation with thrombin receptor-activating peptide (TRAP) or thrombin.19,20 Thus it might well be possible that ADF is also dephosphorylated as cofilin through an identical signaling pathway in thrombin-stimulated platelets.
In conclusion, we delineated a Ca2+-dependent pathway mediating dense granule secretion in human platelets; it involves activation of the Ca2+-dependent phosphatase calcineurin and Rac1. Rac1 then stimulates class II PAKs (PAK4/5/6) mediating cofilin dephosphorylation by activating an unknown cofilin phosphatase. We found that both the Rac1 and PKC pathways are required for regulating dense granule secretion induced by thrombin, however, these 2 pathways function independently of each other. We further show that PI3-kinases are activated downstream of Rac1, but that they are neither involved in cofilin dephosphorylation nor secretion in thrombin-stimulated platelets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nicole Wilke for her technical assistance in this study.
This study was supported by the August-Lenz-Stiftung, Friedrich-Bauer-Stiftung, and Deutsche Forschungsgemeinschaft (Si 274/11; and the Graduate Program Vascular Biology in Medicine GRK 438 [D.P. and P.G.]).
Authorship
Contribution: D.P. is the major and primary contributor of this study and designed and performed the experiments, analyzed data and interpreted results, and wrote the first draft of the paper; P.G. designed the research, interpreted results, and assisted in paper preparation (discussion); S.D. performed some experiments and assisted in paper preparation; and W.S. accompanied the study from its beginning in discussing, focusing, and designing the experiments and in writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfgang Siess, Institut für Prophylaxe und Epidemiologie der Kreislaufkrankheiten, Klinikum Innenstadt, Universität München, Pettenkoferstr 9, D 80336 München, Germany; e-mail: wsiess@med.uni-muenchen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal