Abstract
The interaction between the hormone hepcidin and the iron exporter ferroportin (Fpn) regulates plasma iron concentrations. Hepcidin binds to Fpn and induces its internalization and degradation, resulting in decreased iron efflux from cells into plasma. Fpn mutations in N144, Y64N, and C326 residue cause autosomal dominant disease with parenchymal iron overload, apparently due to the resistance of mutant Fpn to hepcidin-mediated internalization. To define the mechanism of resistance, we generated human Fpn constructs bearing the pathogenic mutations. The mutants localized to the cell surface and exported iron normally, but were partially or completely resistant to hepcidin-mediated internalization and continued to export iron despite the presence of hepcidin. The primary defect with exofacial C326 substitutions was the loss of hepcidin binding, which resulted in the most severe phenotype. The thiol form of C326 was essential for interaction with hepcidin, suggesting that C326-SH homology is located in or near the binding site of hepcidin. In contrast, N144 and Y64 residues were not required for hepcidin binding, but their mutations impaired the subsequent internalization of the ligand-receptor complex. Our observations explain why the mutations in C326 Fpn residue produce a severe form of hemochromatosis with iron overload at an early age.
Introduction
Hereditary hemochromatosis is a disease characterized by high transferrin saturation and parenchymal iron overload. Most forms are caused by autosomal-recessive mutations in HFE, transferrin receptor 2, hemojuvelin, or hepcidin. These mutations lead to deficiency of the iron-regulatory hormone hepcidin. Rarely, hereditary hemochromatosis is caused by autosomal-dominant mutations in the hepcidin receptor ferroportin (Fpn).1 Fpn is the only known cellular iron exporter in vertebrates and is expressed on all tissues that handle major iron flows, including absorptive enterocytes, iron-recycling macrophages, and iron-storing hepatocytes.2 The rate of iron efflux from these cells is a major determinant of iron concentration in plasma and is directly proportional to the number of Fpn molecules on the cell surface. Furthermore, by controlling the absorption of dietary iron, Fpn expression on the basolateral membranes of enterocytes ultimately determines total body iron. The concentration of cell surface Fpn is regulated mainly by the interaction with its ligand hepcidin. Hepcidin binding to Fpn results in internalization and degradation of the ligand-receptor complex and leads to decreased iron efflux from cells into plasma.3 The hepcidin-Fpn interaction is critical for normal iron homeostasis and underlies the pathogenesis of iron disorders, including not only hereditary hemochromatosis, but also anemia of inflammation and iron-loading anemias.4
Although the complete loss of Fpn is embryonic lethal in zebrafish and mice,2,5 some heterozygous missense mutations in the Fpn gene result in an autosomal-dominant form of iron overload disease called Fpn disease or type IV hemochromatosis.6 The disease encompasses at least 2 different phenotypes related to different types of Fpn mutations. The loss-of-function mutations cause decreased cellular iron export because the mutant protein is retained inside the cell or has impaired iron-exporting function.7-10 As a result, iron accumulates in macrophages, leading to very high ferritin levels, but the patients have normal-to-low transferrin saturation, and a tendency to develop anemia particularly when phlebotomized.6 It is not yet clear whether this condition impacts health or requires treatment. A less common group, the gain-of-function mutations, prevent hepcidin-mediated internalization and degradation of Fpn, resulting in increased iron efflux into plasma.8,11 Such patients develop a phenotype similar to classical hemochromatosis caused by hepcidin deficiency, with increased duodenal iron absorption, high transferrin saturation, and iron deposition in hepatic parenchyma and other tissues.12-17 The most severe form with an early age of onset is associated with substitutions in C326 residue (C326S/Y),13,17 whereas a milder hemochromatosis phenotype was observed with substitutions at N144 (N144D/T/H)12,14,15 and Y64 (Y64N)16 residues. A letter published in this issue of Blood reports that serum and urinary hepcidin levels in patients carrying C326S mutation are at the upper limit of the normal range or above, confirming that this form of hemochromatosis is hepcidin-resistant.18
In this study, we investigated the mechanism of hepcidin resistance in C326, N144, and Y64 Fpn mutants.
Methods
Constructs
Human Fpn sequence was amplified from human liver cDNA using primers, as follows: 5′-ccagcggggtaccctagtgtcatgacc-3′ and 5′-ggatccggggcccacaacagatgtatttgc-3′. The fragment was cloned into pGFPN3 (BD Clontech) using ApaI and KpnI restriction sites. The sequence of the cloned Fpn was verified to be free of any errors. Protein expression was confirmed by Western blot analysis using anti–green fluorescent protein (GFP) antibody (ab6556; Abcam), as previously described.3 N144D, N144T, C326S, C326T, Y64N, C205S, C205T, C205S/N144D, and C205/Y64N mutations were introduced into human Fpn-GFP using the QuikChange Mutagenesis kit (Stratagene). The coding sequence of the mutant plasmids was fully sequenced to confirm the presence of only the desired mutations.
Transient transfection of cells
HEK293T cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, gentamicin, and ciprofloxacin. During transfection experiments, 20 μM ferric ammonium citrate (FAC) was added to the medium. Cells were transiently transfected with wild-type (wt) and mutant Fpn constructs using Lipofectamine 2000 (Invitrogen) or Amaxa V nucleofection kit (Amaxa), according to the manufacturer's instructions. After transfection, cells were incubated at 37°C for 24 hours before analysis. Transfection efficiency and protein expression levels of wt and mutant Fpn-GFP were assessed by Western blotting using anti-GFP antibody.
Cells were visualized with an epifluorescence microscope (Nikon Eclipse) with a 40× objective. Images were acquired with a Spot camera and software (Diagnostic Instrumentsz).
Ferritin measurement
Twenty-four hours after transfection, cells were treated with 5 μg/mL hepcidin (Bachem) or its solvent for 24 hours. Cellular protein was extracted and ferritin levels were determined using the Spectro Ferritin enzyme-linked immunosorbent assay (ELISA) kit (Ramco Laboratories), according to the manufacturer's instructions. The values were normalized for the total protein concentration in each sample, as determined by the bicinchoninic acid assay (Pierce).
125I-hepcidin internalization assay
125I-hepcidin was prepared, as described3 (specific activity ∼107 cpm/μg), and 106 cpm was added to transiently transfected cells for 1 hour at 37°C. Cells were washed with phosphate-buffered saline (PBS) to remove unbound radioactive hepcidin and centrifuged for 2 minutes at 12 000g through a silicone oil layer (Nyosil M25; Nye). The radioactivity in cell pellets was determined by gamma counting. The data were normalized to the amount of radioactivity in wt Fpn-GFP cells, with the radioactivity of untransfected cells subtracted as background for each point.
Fluorescent hepcidin preparation
Texas Red-X succinimidyl ester (Molecular Probes) was added to 200 μg hepcidin (Bachem) resuspended in PBS with sodium bicarbonate and stirred for 1 hour at room temperature (RT). Labeled hepcidin was purified on a C18 Sep-Pak cartridge (Waters) equilibrated in 0.1% trifluoroacetic acid, and eluted with 60% acetonitrile. Hepcidin was further purified by reverse-phase high-performance liquid chromatography on Vydac C18 column (218TP510; Waters) equilibrated with 0.1% trifluoroacetic acid.3
Inhibition of receptor internalization
Ponasterone-inducible Fpn-GFP cell line3 was pretreated for 15 minutes with 80 μM dynasore (Sigma-Aldrich), a cell-permeable, reversible inhibitor of dynamin GTPase activity. Hepcidin (1 μg/mL) was added for another 45 minutes, and localization of Fpn-GFP was assessed by epifluorescent microscopy. Hepcidin binding was assayed by adding 106 cpm of 125I-hepcidin for 45 minutes after dynasore pretreatment and measuring cell-associated radioactivity, as described previously.
Alternatively, inducible Fpn-GFP cells were transfected with wt or K44A dynamin-hemagglutinin (HA) expression constructs (pcDNA3.1(−)HA-Dyn1 wt or K44A; ATCC). Fpn-GFP localization and 125I-hepcidin binding were determined as before. Dynamin expression was verified by Western blotting with anti-HA antibody (3F10; Roche).
Autoradiography of 125I-hepcidin-Fpn binding
Cells were transfected with wt and mutant Fpn-GFP for 24 hours, and 125I-hepcidin (106 cpm) was added for 15 minutes at 37°C. Cells were then either left untreated or treated with cross-linkers disuccinimidyl suberate (DSS, 5 mM final concentration; Pierce) or succinimidyl-[N-maleimidopropionamido]-2 ethyleneglycol ester (NHS-PEO2-maleimide, 2 mM final concentration; Pierce) for 30 minutes at RT. To rule out the possibility that the mutations may sufficiently weaken the hepcidin binding to cause it to dissociate during washing, the cross-linkers were added to cells in the presence of hepcidin. The reaction was quenched with 1 M Tris for 15 minutes. Protein extracts were immunoprecipitated using anti-GFP antibody (ab6556) and protein A agarose (Roche). The immunoprecipitated samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by autoradiography.3 The experiment was repeated 5 times with similar results.
Alkylation and thiol isomerase inhibition
Twenty-four hours after transfection with wt and mutant Fpn-GFP, cells were treated with nonpermeable thiol modifiers (1-3 mM dithio-bis-2-nitrobenzoic acid (DTNB), 0.5-2 mM iodoacetamide, and 1-4 mM maleimide-PEO2-biotin; Pierce), with the thiol isomerase inhibitor bacitracin (0.5-2.5 mM; Pierce), and with thiol isomerase-neutralizing antibodies (anti–protein disulfide isomerase mouse mAb; Affinity BioReagents) for 30 minutes at 37°C. The reagents were then washed off, 125I-hepcidin (106 cpm) was added for 1 hour at 37°C, and 125I-hepcidin internalization was assessed, as described above.
Cell surface biotinylation
Twenty-four hours after transfection with wt and mutant Fpn-GFP, cells were treated with 50 μM nonpermeable biotinylation reagent maleimide-polyethylene glycol (PEG)2-biotin (Pierce) for 30 minutes at 4°C. The reagent was washed off, and total protein was isolated and immunoprecipitated with anti-GFP antibody, separated by SDS-PAGE, and blotted using avidin–horseradish peroxidase (HRPO; Pierce) for detection of biotinylated species.
Results
Cellular localization and iron-exporting function are not impaired in N144D/T, Y64N, and C326S/T Fpn mutants
Human wt Fpn was cloned into a GFP-expression vector to generate a Fpn-GFP fusion protein, with GFP at the C terminus of Fpn. Using site-directed mutagenesis, we introduced mutations N144D, N144T, Y64N, and C326S previously reported to cause a hepcidin resistance phenotype. We also generated a new Fpn C326 mutant, in which Cys was substituted with a similar amino acid Thr, to verify that the behavior of the mutant Fpn is due to the loss of Cys rather than the presence of a specific substituted amino acid. Another disease-causing substitution of the same Cys residue, C326Y, was described as hepcidin resistant in other in vitro studies.11
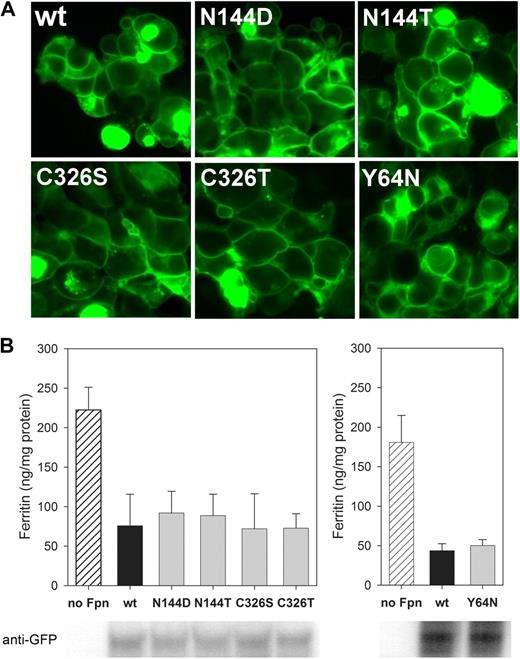
Transient transfection of plasmids into HEK293T cells demonstrated that all mutants localized to the cell surface similarly to wt Fpn-GFP (Figure 1A). To assess the iron-exporting function, we measured the intracellular iron levels as reflected by the cytosolic iron storage protein ferritin. Incubation of untransfected cells with FAC increased ferritin levels, whereas transient transfection of wt, N144D/T, Y64N, and C326S/T Fpn prevented ferritin accumulation (P < .001 by t test for wt and mutant Fpn compared with untransfected cells, but differences were not significant between wt and each mutant Fpn; Figure 1B). Thus, these substitutions did not affect the iron-exporting function.
Cellular localization and iron-exporting function are not impaired in N144D/T, Y64N, and C326S/T Fpn mutants. (A) Localization of mutants is similar to wt Fpn: HEK293T cells were transiently transfected with human wt Fpn-GFP or with indicated Fpn mutants for 24 hours and imaged by epifluorescence microscopy. (B) Iron export of mutants is similar to wt Fpn: control untransfected HEK293T cells or HEK293T cells transiently transfected with human wt or mutant Fpn-GFP constructs were incubated for 24 hours in the presence of 20 μM FAC. Total cellular protein was isolated, and ferritin content was determined by ELISA. Results are presented as the mean and SD of 3 or 4 separate experiments. Bottom panel illustrates similar expression levels of wt and mutant Fpn-GFP constructs as assessed by Western blotting using anti-GFP antibody. Y64N mutant is presented in a different graph because its ferritin ELISA and GFP Western blotting were performed separately from the analyses of the other mutants.
Cellular localization and iron-exporting function are not impaired in N144D/T, Y64N, and C326S/T Fpn mutants. (A) Localization of mutants is similar to wt Fpn: HEK293T cells were transiently transfected with human wt Fpn-GFP or with indicated Fpn mutants for 24 hours and imaged by epifluorescence microscopy. (B) Iron export of mutants is similar to wt Fpn: control untransfected HEK293T cells or HEK293T cells transiently transfected with human wt or mutant Fpn-GFP constructs were incubated for 24 hours in the presence of 20 μM FAC. Total cellular protein was isolated, and ferritin content was determined by ELISA. Results are presented as the mean and SD of 3 or 4 separate experiments. Bottom panel illustrates similar expression levels of wt and mutant Fpn-GFP constructs as assessed by Western blotting using anti-GFP antibody. Y64N mutant is presented in a different graph because its ferritin ELISA and GFP Western blotting were performed separately from the analyses of the other mutants.
N144D/T, Y64N, and C326S/T Fpn mutants are resistant to hepcidin-mediated internalization and inhibition of iron export
When the cellular localization of mutants after hepcidin treatment was compared with wt Fpn by microscopy (1-24 hours), N144D/T, Y64N, and C326S/T mutants showed less or no internalization (data not shown). These findings are in agreement with previous reports that substitutions N144D/H8,9,11 and C326Y11 rendered Fpn partially or completely resistant to hepcidin-mediated internalization.
Importantly, iron export from cells transfected with mutant Fpn continued even in the presence of hepcidin. Measurement of cellular ferritin levels after a 24-hour hepcidin treatment showed that in comparison with iron retention in wt Fpn-GFP cells, there was only partial or no iron retention in mutant cells (by t test, P < .001 for all mutants compared with wt Fpn, except Y64N, where P = .002; Figure 2).
Hepcidin is less effective in inhibiting iron export in N144D/T, Y64N, and C326S/T mutants than in wt Fpn. HEK293T cells were transiently transfected with human wt or mutant Fpn-GFP constructs and incubated for 24 hours in the presence of 20 μM FAC. Hepcidin (5 μg/mL) was added for 24 hours, and intracellular ferritin concentration was determined by ELISA. n = 7 for all mutants except Y64N, where n = 4. For each separate experiment, results were expressed as the relative change in ferritin, according to the formula relFx = [Fx − Fwt]/[Fno Fpn − Fwt], where Fwt is ferritin concentration in the wt Fpn sample, Fno Fpn in the untransfected cells, and Fx in any given sample. For each sample, ferritin content was expressed as a fraction of the ferritin content of the positive control (wt cells treated with hepcidin) according to the formula 100 × [relFx/relFwt+hepc]. P < .001 for all mutants in comparison with wt Fpn-GFP, except for Y64N, where P = .002.
Hepcidin is less effective in inhibiting iron export in N144D/T, Y64N, and C326S/T mutants than in wt Fpn. HEK293T cells were transiently transfected with human wt or mutant Fpn-GFP constructs and incubated for 24 hours in the presence of 20 μM FAC. Hepcidin (5 μg/mL) was added for 24 hours, and intracellular ferritin concentration was determined by ELISA. n = 7 for all mutants except Y64N, where n = 4. For each separate experiment, results were expressed as the relative change in ferritin, according to the formula relFx = [Fx − Fwt]/[Fno Fpn − Fwt], where Fwt is ferritin concentration in the wt Fpn sample, Fno Fpn in the untransfected cells, and Fx in any given sample. For each sample, ferritin content was expressed as a fraction of the ferritin content of the positive control (wt cells treated with hepcidin) according to the formula 100 × [relFx/relFwt+hepc]. P < .001 for all mutants in comparison with wt Fpn-GFP, except for Y64N, where P = .002.
Internalization of the ligand hepcidin is also impaired in N144D/T, Y64N, and C326S/T Fpn mutants
Uptake of radiolabeled hepcidin by Fpn-expressing cells in vitro3 and its accumulation in Fpn-rich organs after injection in mice19 suggested that hepcidin is internalized together with Fpn.
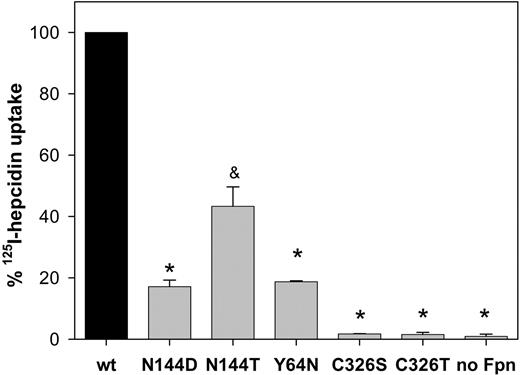
We measured the uptake of radiolabeled hepcidin by cells transiently transfected with wt or mutant Fpn (Figure 3). One hour after addition of 125I-hepcidin, uptake by N144D/T or Y64N mutants was only 20% to 40% of that in the wt Fpn cells (by t test, P < .001 for N144D and Y64N, and P = .009 for N144T). C326S/T cells did not internalize radiolabeled hepcidin and were indistinguishable from the negative control cells expressing only GFP (P < .001 compared with wt Fpn-GFP).
Impaired uptake of radiolabeled hepcidin by N144D/T, Y64N, and C326S/T Fpn mutants. HEK293T cells were transiently transfected with wt or mutant Fpn-GFP constructs for 24 hours, then incubated with 125I-hepcidin for 1 hour at 37°C and washed. Cells were centrifuged through silicone oil, and the amount of 125I-hepcidin in cell pellet was determined by gamma counting. The data were normalized to the amount of radioactivity bound to wt Fpn-GFP cells, and the amount of radioactivity in untransfected cells was subtracted as background for each point. The bars represent the average of 3 replicates with standard deviations. * P < .001 and & P = .009, compared with wt Fpn-GFP.
Impaired uptake of radiolabeled hepcidin by N144D/T, Y64N, and C326S/T Fpn mutants. HEK293T cells were transiently transfected with wt or mutant Fpn-GFP constructs for 24 hours, then incubated with 125I-hepcidin for 1 hour at 37°C and washed. Cells were centrifuged through silicone oil, and the amount of 125I-hepcidin in cell pellet was determined by gamma counting. The data were normalized to the amount of radioactivity bound to wt Fpn-GFP cells, and the amount of radioactivity in untransfected cells was subtracted as background for each point. The bars represent the average of 3 replicates with standard deviations. * P < .001 and & P = .009, compared with wt Fpn-GFP.
To document the location of cell-associated hepcidin, we labeled hepcidin with Texas Red. The modified hepcidin retained its bioactivity and internalized wt Fpn-GFP (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The green and red fluorescence was colocalized, indicating that hepcidin is internalized together with Fpn. The red and green signals were completely separable because there was no red signal in Fpn-GFP cells treated with unlabeled hepcidin (supplemental Figure 1), and no green signal in cells expressing Fpn-HA and treated with Texas Red–labeled hepcidin (data not shown). In contrast to the internalization of wt Fpn, when Texas Red–labeled hepcidin was added to HEK293T cells transfected with N144D/T and C326S/T Fpn mutants, the internalization of Texas Red–hepcidin was absent.
Hepcidin binding to Fpn is independent of Fpn internalization
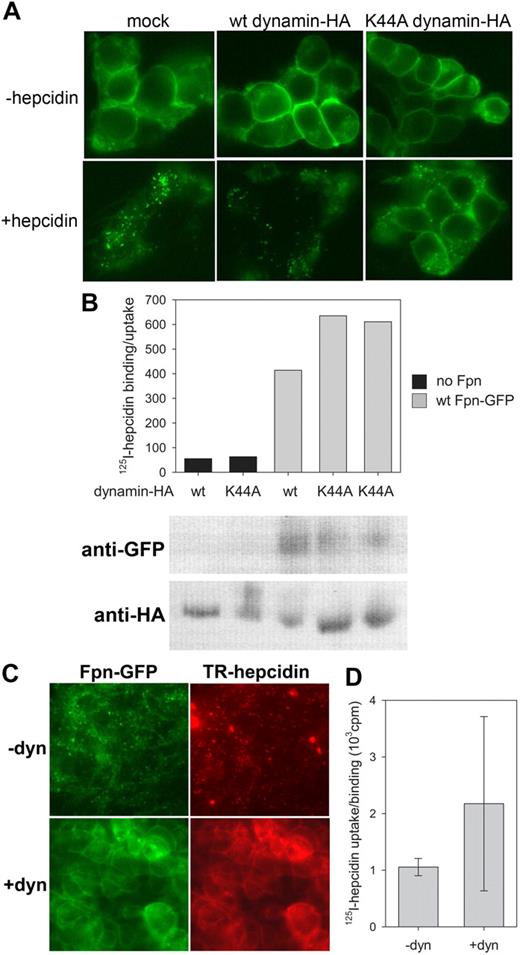
Radiolabeled hepcidin can be cross-linked to Fpn-GFP, indicating physical interaction between the ligand and the receptor,3 but it was unknown whether the internalization of hepcidin-Fpn complex was necessary to stabilize their interaction. Fpn was shown to be internalized by clathrin/dynamin-dependent endocytosis.20 To prevent internalization after hepcidin binding, we either treated cells with dynasore, a reversible inhibitor of dynamin-mediated internalization, or transfected cells with dominant-negative K44A dynamin mutant lacking GTPase activity. Whereas both treatments prevented internalization of Fpn-GFP after hepcidin addition, they did not affect binding of 125I-hepcidin (Figure 4), indicating that hepcidin binding to Fpn is independent of the internalization of the ligand-receptor complex.
Hepcidin binding to Fpn does not depend on Fpn internalization. (A) Ponasterone-inducible Fpn-GFP cells were transfected with wt dynamin-HA or dominant-negative K44A mutant for 24 hours. Hepcidin (1 μg/mL) was added for 1 hour, and cells were imaged by epifluorescence microscopy. (B) Cells were transfected as in (A), 125I-hepcidin was added for 1 hour at 37°C, and cell-associated radioactivity was determined by gamma counting. The same samples were analyzed by Western blotting to assess the expression levels of Fpn-GFP and wt or K44A dynamin-HA. (C) Ponasterone-inducible Fpn-GFP cells were treated with 80 μM dynasore for 15 minutes, followed by 45 minutes of 1 μg/mL Texas Red–labeled hepcidin, and imaged by epifluorescence microscopy. (D) Cells were treated with dynasore as in (C), 125I-hepcidin was added for 45 minutes at 37°C, and cell-associated radioactivity was measured in a gamma counter. Hepcidin binding to cells treated with dynasore was not lower than to control cells (P = .4 by Mann-Whitney test).
Hepcidin binding to Fpn does not depend on Fpn internalization. (A) Ponasterone-inducible Fpn-GFP cells were transfected with wt dynamin-HA or dominant-negative K44A mutant for 24 hours. Hepcidin (1 μg/mL) was added for 1 hour, and cells were imaged by epifluorescence microscopy. (B) Cells were transfected as in (A), 125I-hepcidin was added for 1 hour at 37°C, and cell-associated radioactivity was determined by gamma counting. The same samples were analyzed by Western blotting to assess the expression levels of Fpn-GFP and wt or K44A dynamin-HA. (C) Ponasterone-inducible Fpn-GFP cells were treated with 80 μM dynasore for 15 minutes, followed by 45 minutes of 1 μg/mL Texas Red–labeled hepcidin, and imaged by epifluorescence microscopy. (D) Cells were treated with dynasore as in (C), 125I-hepcidin was added for 45 minutes at 37°C, and cell-associated radioactivity was measured in a gamma counter. Hepcidin binding to cells treated with dynasore was not lower than to control cells (P = .4 by Mann-Whitney test).
Fpn C326 residue is essential for hepcidin binding
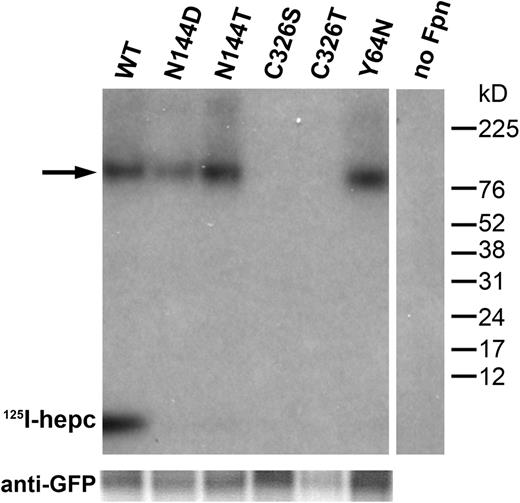
To assess the specific effect of N144, Y64, and C326 mutations on hepcidin binding to Fpn, we added radiolabeled hepcidin to cells transiently transfected with wt or mutant Fpn-GFP constructs. The samples were analyzed by SDS-PAGE and autoradiography. In wt Fpn-GFP cells, 125I-hepcidin remained bound to Fpn through the immunoprecipitation with anti-GFP, and comigrated with wt Fpn-GFP on the SDS-PAGE (Figure 5), in which the expected size of the Fpn-GFP-hepcidin complex is ∼100 kDa). This complex could be dissociated when a reducing agent (dithiothreitol or β-mercaptoethanol) was added to the samples (data not shown), suggesting that hepcidin-Fpn interaction involves a disulfide exchange. Dissociation by reducing agent could be prevented when hepcidin was cross-linked to Fpn using DSS, an amine-reactive homobifunctional cross-linker, or NHS-PEO2-maleimide, an amine-to-sulfhydryl heterobifunctional cross-linker.
C326, but not N144 or Y64, is necessary for hepcidin binding. HEK293T cells were transiently transfected with wt or mutant Fpn-GFP constructs for 24 hours, and incubated with 125I-hepcidin for 1 hour at 37°C. Protein was isolated, immunoprecipitated using anti-GFP antibody, and analyzed by SDS-PAGE and autoradiography. The size of the Fpn-GFP fusion protein is 97 kDa, and hepcidin is 2.8 kDa. → indicates the FpnGFP/hepcidin complex. Bottom panel shows Western blot of the same samples probed with anti-GFP antibody as a loading control.
C326, but not N144 or Y64, is necessary for hepcidin binding. HEK293T cells were transiently transfected with wt or mutant Fpn-GFP constructs for 24 hours, and incubated with 125I-hepcidin for 1 hour at 37°C. Protein was isolated, immunoprecipitated using anti-GFP antibody, and analyzed by SDS-PAGE and autoradiography. The size of the Fpn-GFP fusion protein is 97 kDa, and hepcidin is 2.8 kDa. → indicates the FpnGFP/hepcidin complex. Bottom panel shows Western blot of the same samples probed with anti-GFP antibody as a loading control.
Interestingly, when not cross-linked, a fraction of 125I-hepcidin dissociated from Fpn-GFP during SDS-PAGE even when no reducing agent was added, and migrated as free 125I-hepcidin on autoradiography. If disulfide exchange indeed occurs when hepcidin binds to Fpn, our result indicates that the disulfide bond can be destabilized after internalization, possibly in lysosomes. Apart from the disulfide bond, other interactions must exist that preserve the hepcidin-Fpn complex during immunoprecipitation with anti-GFP, but are sensitive to later disruption by nonreducing SDS-PAGE.
No radiolabeled hepcidin was detected on autoradiography in C326S/T samples, indicating that mutations in this residue completely ablated hepcidin binding to Fpn. In contrast, N144D/T and Y64N mutations did not appear to interfere with hepcidin binding because the 100-kDa 125I-hepcidin/Fpn-GFP complex was indistinguishable from the wt sample. However, no free 125I-hepcidin was observed on the autoradiogram (Figure 5) most likely because of the absence of internalization and subsequent destabilization of the hepcidin-Fpn complex. The sum of the signals from 100-kDa complex and free 125I-hepcidin agrees with measurements of cell-associated radioactivity in Figure 3, which showed that N144D/T and Y64N cells had less than 40% of the wt cell counts.
Thiols are required for Fpn-hepcidin binding
Liu et al9 showed that C326 and C205 residues were present on the cell surface as free thiols. To evaluate the importance of the free thiols for hepcidin-Fpn binding, we treated wt Fpn-GFP cells with nonpermeable thiol-modifying reagents iodoacetamide, DTNB and PEO2-maleimide, and assessed the uptake of 125I-hepcidin. Blocking of thiols on the cell surface drastically reduced 125I-hepcidin uptake (P < .001 compared with wt Fpn-GFP by t test; Figure 6) confirming that −SH groups are required for hepcidin-Fpn interaction.
Thiols are required for hepcidin binding to Fpn, but disulfide bond rearrangement is not. HEK293T cells were transiently transfected with wt Fpn-GFP for 24 hours, then incubated with nonpermeable thiol modifiers (DTNB, iodoacetamide [IA], PEO2-maleimide [PM], shown as [GRAPHIC024]) or thiol isomerase inhibitor bacitracin (▨). After 30 minutes, 125I-hepcidin was added for 1 hour at 37°C and cell-associated radioactivity was determined by gamma counting. The data were normalized to the amount of radioactivity bound to wt Fpn-GFP cells not treated with any modifiers, whereas the amount of radioactivity in untransfected cells was subtracted as background for each point. The bars represent the average of 3 replicates with standard deviations. *P < .001.
Thiols are required for hepcidin binding to Fpn, but disulfide bond rearrangement is not. HEK293T cells were transiently transfected with wt Fpn-GFP for 24 hours, then incubated with nonpermeable thiol modifiers (DTNB, iodoacetamide [IA], PEO2-maleimide [PM], shown as [GRAPHIC024]) or thiol isomerase inhibitor bacitracin (▨). After 30 minutes, 125I-hepcidin was added for 1 hour at 37°C and cell-associated radioactivity was determined by gamma counting. The data were normalized to the amount of radioactivity bound to wt Fpn-GFP cells not treated with any modifiers, whereas the amount of radioactivity in untransfected cells was subtracted as background for each point. The bars represent the average of 3 replicates with standard deviations. *P < .001.
In some receptors, free extracellular thiols participate in conformational rearrangements that are catalyzed by bacitracin-sensitive protein thiol isomerases.21 In Fpn, a second extracellular9 cysteine residue C205 may be in a position to interact with C326. We therefore examined whether hepcidin binding to Fpn initiates the enzymatic formation or rearrangement of a disulfide bond that involves the cell-surface cysteine(s). We treated wt Fpn-GFP cells with bacitracin or thiol isomerase neutralizing antibodies, and measured hepcidin uptake. Neither bacitracin (Figure 6) nor neutralizing antibodies (data not shown) affected hepcidin uptake, indicating that isomerase-catalyzed disulfide bond exchange is not required for hepcidin-Fpn interaction.
Fpn residue C205 is not required for hepcidin binding
Previous experiments showed the importance of the C326 residue and free thiols in hepcidin-Fpn binding. Because 2 free cysteines, C205 and C326, were identified in the extracellular domains of Fpn, we assessed the role of C205 in hepcidin-Fpn binding. No human disease involving this residue has been reported. We generated C205S and C205T Fpn-GFP mutants and transfected them into HEK293T cells. The mutant proteins localized to the cell surface and were fully functional as iron exporters (data not shown). We next examined the uptake of 125I-hepcidin, as well as the cross-linking of 125I-hepcidin using the amine-to-sulfhydryl heterobifunctional cross-linker, NHS-PEO2-maleimide. In contrast to the severity of C326S/T mutations, C205S/T mutations had a much smaller effect on the cross-linking of radiolabeled hepcidin to Fpn (supplementalFigure 2A) or internalization of the radiolabeled hepcidin (no significant difference compared with wt; supplemental Figure 2B), indicating that C205 is not critical for hepcidin-Fpn binding.
N144 and Y64 mutations do not alter the cell surface accessibility of C326-SH residue
Autoradiography of hepcidin-FpnGFP complexes (Figure 5) suggested that N144 and Y64 mutations cause hepcidin resistance by impairing internalization, but not binding. To verify that the key contact residue C326-SH remained accessible in these mutants, we performed cell surface biotinylation with a SH-reactive nonpermeable biotinylation reagent in cells expressing N144 and Y64 mutations. Because the biotinylation of C205-SH, Fpn's other exofacial free thiol, could mask the changes in C326-SH biotinylation, we generated Fpn-GFP double mutants C205S/N144D and C205S/Y64N. HEK293T cells were transfected with wt and mutant constructs and treated with maleimide-PEG2-biotin. Total cellular protein was immunoprecipitated with anti-GFP and analyzed by SDS-PAGE using avidin-HRPO to detect biotinylated Fpn-GFP (Figure 7). In comparison with wt or C205S Fpn-GFP, there was no significant difference in biotinylation of double mutants N144D/C205S and Y64N/C205S, indicating that N144 and Y64 substitutions did not alter the cell surface accessibility of C326-SH residue to the biotinylation reagent.
Hepcidin resistance of N144 and Y64 substitutions is not caused by altered accessibility of the C326-SH residue. HEK293T cells were transiently transfected with wt or mutant Fpn-GFP constructs for 24 hours, then incubated in the presence of sulfhydryl-reactive nonpermeable biotinylation reagent for 30 minutes at 4°C. Total cellular protein was isolated and immunoprecipitated with anti-GFP antibody, and samples were analyzed by Western blotting using avidin-HRPO (left panel) or anti-GFP antibody (right panel).
Hepcidin resistance of N144 and Y64 substitutions is not caused by altered accessibility of the C326-SH residue. HEK293T cells were transiently transfected with wt or mutant Fpn-GFP constructs for 24 hours, then incubated in the presence of sulfhydryl-reactive nonpermeable biotinylation reagent for 30 minutes at 4°C. Total cellular protein was isolated and immunoprecipitated with anti-GFP antibody, and samples were analyzed by Western blotting using avidin-HRPO (left panel) or anti-GFP antibody (right panel).
Discussion
Fpn disease is caused by heterozygous mutations in the SLC40A1 gene encoding the iron exporter Fpn. The disease has varied phenotypic manifestations, because different types of mutations have distinct effects on Fpn function. The most common phenotype characterized by iron loading of macrophages and normal transferrin saturation results from mutations causing mislocalization of Fpn (eg V162del) or the loss of iron export function (eg, N174I).7-10,22 The less common phenotype with parenchymal iron loading similar to classical hemochromatosis results from mutations that cause a gain of function, because they interfere with hepcidin-mediated internalization and degradation of Fpn (N144D/T/H, C326S/Y, Y64N).8,11-17 Some mutations (eg, A77D) appear to cause a mixed phenotype, with both parenchymal and macrophage iron deposition, and normal to high transferrin saturation.23,24 In vitro, A77D mutation was reported to cause Fpn mislocalization and a loss of function,7,25 but additional studies are needed to explain the development of the phenotype. C326 substitutions appear to cause the most severe disease with an early age of onset, similar to juvenile hemochromatosis. In the present study, we analyzed the mechanism of hepcidin resistance in N144, Y64, and C326 Fpn mutants.
N144H/T, Y64N, and C326Y mutations were previously studied in vitro and reported to export iron normally,7-9 but partially or fully resisted internalization by hepcidin.8,11 We had previously documented impaired hepcidin binding to N144H.8 In the current study, we generated human Fpn-GFP constructs bearing N144D or T mutations, Y64N, and C326S or T. Of note, C326T substitution was not based on a known human mutation, but was made to evaluate the effects of a conservative Cys substitution. In vitro, all mutants showed membrane localization and iron export similar to that of the wt Fpn-GFP, but the effect of hepcidin was impaired. In wt Fpn cells, binding of hepcidin resulted in Fpn and hepcidin internalization, and the inhibition of cellular iron export. In cells transfected with mutant constructs, internalization of Fpn and hepcidin (radiolabeled or Texas Red–conjugated) was impaired, and hepcidin was less effective at inhibiting iron export.
Comparison of hepcidin binding with wt and mutant Fpn by autoradiography (Figure 5) indicated that the mechanism of hepcidin resistance was not the same for all mutations. C326 substitutions affected the binding of hepcidin to Fpn, because no 125I-hepcidin was associated with C326 mutants. The lack of binding was not a consequence of the lack of internalization because the binding step is independent of internalization, as shown by normal binding of hepcidin to the cells with defective dynamin-dependent internalization. Thus, we conclude that C326 residue is essential for hepcidin binding. In all tests, C326 substitutions exhibited the most severe effect in agreement with the severity of the clinical phenotype. N144 and Y64 mutations did not impair hepcidin binding, and did not decrease the cell surface localization of C326-SH, but prevented the internalization of the complex. Both of these residues are thought to be located in the transmembrane segments of Fpn and could participate in ligand-induced conformational change that initiates internalization. The exact role of N144 and Y64 residues in this process remains to be determined.
C326, the critical residue for hepcidin-Fpn binding, is a thiol located on the cell surface.9 In this study, we showed that extracellular thiols are required for hepcidin binding, because the alkylation of cell surface cysteines with nonpermeable sulfhydryl-reactive reagents prevented hepcidin binding to wt Fpn. C326 is not the only reduced cysteine located on the cell surface,9 because C205 was also shown to be accessible to nonpermeable alkylators. However, we showed that C205 thiol was not essential for hepcidin binding because mutations in this residue interfered much less with hepcidin uptake or cross-linking than C326 mutations. Given the importance of C326-SH, we hypothesized that hepcidin binding to Fpn may involve extracellular disulfide bond formation or rearrangement, such as between the 2 Fpn cell surface cysteines, or between a cell surface cysteine and an existing disulfide bond in Fpn, or between a cell surface cysteine and an existing disulfide bond in hepcidin. Inhibition of thiol isomerase, enzyme involved in extracellular disulfide exchange, had no effect on hepcidin-Fpn interaction. However, because hepcidin-Fpn complex stability was sensitive to reducing agents, it is possible that hepcidin-Fpn binding involves a nonenzymatic disulfide rearrangement. Our results indicate that C326 is directly involved in hepcidin binding to Fpn, most likely as one of the contact residues.
In the companion article (Sham et al), we measured hepcidin levels in the family carrying C326S mutation. At the time of the measurement, the patients have already been undergoing phlebotomy and their iron stores were no longer increased. With the exception of one patient who was truly iron deficient and had hepcidin levels in the low normal range, the other 6 patients had serum and urinary hepcidin concentrations in the high normal range or above. Despite that, patients' transferrin saturation was greatly elevated (70%-93%; 51% in the iron-deficient patient). These in vivo data further confirm that not only hepcidin deficiency, but also hepcidin resistance can cause hereditary hemochromatosis with parenchymal iron-loading phenotype.
In hepcidin-resistant and hepcidin-deficient hemochromatosis, iron overload results from increased Fpn activity, leading to increased and dysregulated absorption of dietary iron. As a result, plasma transferrin is transiently or persistently oversaturated, and the excess iron is distributed among the various tissues. Iron-recycling macrophages are spared because their iron uptake, which is predominantly in the form of phagocytosed erythrocytes, remains largely unchanged. Moreover, Fpn hyperactivity causes decreased iron retention, resulting in the obligate transfer of macrophage iron into other cell types. In contrast, hepatocytes massively accumulate iron.17 At first glance, this may appear puzzling because hepatocytes have also been described to express Fpn,2,26 and Fpn-mediated iron export is regulated by hepcidin in primary hepatocytes (T.G. and E. Valore, unpublished data, January 2008). As with macrophages and enterocytes, hepcidin deficiency or resistance to its effects would be expected to increase iron efflux from hepatocytes. However, for all cell types, iron accumulation depends on their relative rates of iron uptake and release. As hepatocytes avidly take up nontransferrin bound iron,27 they become overloaded even if their iron export is increased.
In conclusion, we showed that resistance of Fpn to hepcidin-mediated internalization and degradation can be caused either by the loss of hepcidin binding, as in substitutions of C326, or by impaired internalization, as in substitutions of N144 and Y64 residues. The profound loss of hepcidin binding in C326 mutations leads to a disease-manifesting parenchymal iron overload at an early age, resembling juvenile hemochromatosis17 caused by severe hepcidin deficiency. This condition is distinct from the macrophage iron-loading syndrome caused by the intracellular mislocalization of other Fpn mutants with resulting Fpn deficiency on cell membranes. The nomenclature of Fpn diseases should be revised to reflect the dissimilar molecular, pathophysiologic, and clinical consequences of different Fpn mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Bo Qiao for technical assistance.
This work was supported by National Institutes of Health grants KO1 DK0753 (to E.N.), RO1 DK065029 (to T.G.), and DK 070947 (to J.K.) and by the Will Rogers Fund (to T.G.).
National Institutes of Health
Authorship
Contribution: A.F. performed research, analyzed data, and wrote the paper; G.C.P. performed research and analyzed data; Y.P. performed research; I.D. and J.K. provided reagents and wrote the paper; T.G. designed research, analyzed data, and wrote the paper; and E.N. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Elizabeta Nemeth, University of California Department of Medicine, 10833 LeConte Avenue, CHS 37-131, Los Angeles, CA 90095; e-mail: enemeth@mednet.ucla.edu.

![Figure 2. Hepcidin is less effective in inhibiting iron export in N144D/T, Y64N, and C326S/T mutants than in wt Fpn. HEK293T cells were transiently transfected with human wt or mutant Fpn-GFP constructs and incubated for 24 hours in the presence of 20 μM FAC. Hepcidin (5 μg/mL) was added for 24 hours, and intracellular ferritin concentration was determined by ELISA. n = 7 for all mutants except Y64N, where n = 4. For each separate experiment, results were expressed as the relative change in ferritin, according to the formula relFx = [Fx − Fwt]/[Fno Fpn − Fwt], where Fwt is ferritin concentration in the wt Fpn sample, Fno Fpn in the untransfected cells, and Fx in any given sample. For each sample, ferritin content was expressed as a fraction of the ferritin content of the positive control (wt cells treated with hepcidin) according to the formula 100 × [relFx/relFwt+hepc]. P < .001 for all mutants in comparison with wt Fpn-GFP, except for Y64N, where P = .002.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/2/10.1182_blood-2008-03-146134/4/m_zh89990937770002.jpeg?Expires=1770653051&Signature=zDMpgiAFhvRNnJjgfA-K5M3ZLWtVKNy8S5b4ejqdKn0lk4bIBKc9ZN7yn7T3Iq~dxqjn4dwUhbdsHQChx10ujsOxSaDXXPT4xxwKGO8mOdVifeW7kKtaZSn8RbDEmGfgGACdb3h6QJu84~TXsqpBkZ2u2u94wblg4Vau7quqz9uQcmVs9Ta9dImz5Acc5Jntj1vfubXzOeZCBKQejQC7de0vFr0~SWmtYEtEulNpOAIrexE5ibMdCsJ1SA18yKSkINDdn4~~UnMNP4t4QYHKZyOm7gLCOS9W9RhNOZbdlNdPIKl45S5YYYPh9UH6SikKAvNX~JZZwcTPuZOlund8DQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Thiols are required for hepcidin binding to Fpn, but disulfide bond rearrangement is not. HEK293T cells were transiently transfected with wt Fpn-GFP for 24 hours, then incubated with nonpermeable thiol modifiers (DTNB, iodoacetamide [IA], PEO2-maleimide [PM], shown as [GRAPHIC024]) or thiol isomerase inhibitor bacitracin (▨). After 30 minutes, 125I-hepcidin was added for 1 hour at 37°C and cell-associated radioactivity was determined by gamma counting. The data were normalized to the amount of radioactivity bound to wt Fpn-GFP cells not treated with any modifiers, whereas the amount of radioactivity in untransfected cells was subtracted as background for each point. The bars represent the average of 3 replicates with standard deviations. *P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/2/10.1182_blood-2008-03-146134/4/m_zh89990937770006.jpeg?Expires=1770653051&Signature=10bHflTRDJ0OFDIBYqtBr3FPWT7qD3BN2ZX8XD3kkv9pJNXuY4pdhWlbLWjiY40087eMPy3TZt4p0IuR1NqpYb3VLpZFpC5GdTzjz6nAqpeVD0id2UvmMsk9x7v~kOqZlAsH63pWSujEnAQ5RElL8mCgLEINZZ~JjwuFN8eQTpSY9TtdmpkPubphTdMJt9V6S5lq7zUWU5Wl3-J3dUVEFcVUK6ZY0lIdRaT7SEypJhPqij~MFMZ39BysF9yUcKrA7sTzezsU3W~bwpONMyijASIE5AKqBTGB9ibqxDzLUgLA-UuQQ3q6jpk0~VLxug3VqKmI32WBQAgWZZw5Vcy3Vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
