The daunting task of dissolving solid mineral iron under physiologic conditions to mine ferritin iron is now shown to follow 2 different cellular pathways selectively targeted by different iron chelators.
Mineralized ferritin in human cells is distributed between the cytoplasm, mitochondria, and in the lysosomes with hemosiderin (denatured ferritin). Clinically familiar serum ferritin, reporting on iron overload or iron deficiency, is a tiny fraction of body ferritin. In the cells, ferritins are nature's nanomachines with the critical role of taming iron/oxygen chemistry. The embryonic lethality of ferritin gene deletion in mice1 reflects the importance of the ferritin iron concentrates as precursors in the synthesis of iron cofactors such as heme. In addition, ferritins coordinately regulated with other antioxidant response proteins, such as thioredoxin reductase and NADPH quinone reductase by bach1 through DNA–antioxidant response element promoters,2 reestablish normal redox conditions in phase II of oxidative stress by removing ferrous ions and oxygen from the cytoplasm. The ferritin family is ancient and found in aerobic and anaerobic single-celled organisms (archea and bacteria), animals, and plants. Ferritins have a complex structure and function that depends on (1) a central nanocavity where iron minerals grow, (2) protein catalytic sites related to diiron oxygenases where mineral formation is initiated, and (3) ion channels with gated pores related to other ion channel proteins where ferrous ions enter and exit the protein cage.3
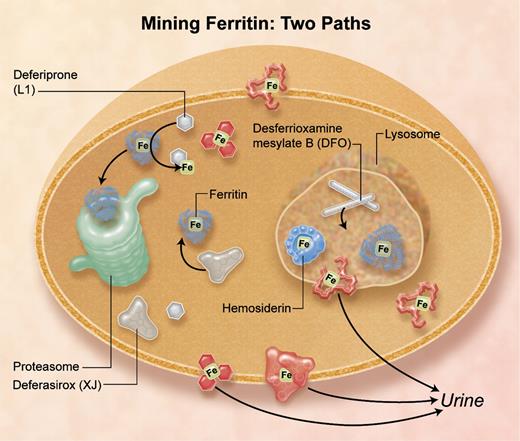
Iron chelators that enter cells by different pathways remove iron from different subcellular compartments: deferasirox and deferiprone target cytosolic ferritin iron, and desferrioxamine mesylate targets lysosomal ferritin iron increased by stimulated autophagy, and damaged ferritin (hemosiderin) iron.4 Professional illustration by A. Y. Chen.
Iron chelators that enter cells by different pathways remove iron from different subcellular compartments: deferasirox and deferiprone target cytosolic ferritin iron, and desferrioxamine mesylate targets lysosomal ferritin iron increased by stimulated autophagy, and damaged ferritin (hemosiderin) iron.4 Professional illustration by A. Y. Chen.
In this issue of Blood, De Domenico and colleagues demonstrate the selective targeting of desferrioxamine, deferiprone, and deferasirox to ferritin iron in different cellular compartments4 (see figure). Identification of the cytoplasmic compartment for recovering iron from ferritin, followed by turnover of the protein in proteasomes, was identified by the same group,5 while the lysosomal sites where ferritin proteins turn over before the iron is pumped out of the lysosome have been studied by many groups.6 Now, the Utah Group shows that deferiprone (L1) plus desferiox (XJ) target cytosolic ferritin iron, but desferrioxamine mesylate (Desferal; DFO), which is endocytosed and stimulates autophagy of ferritin to lysosomes, targets the iron in lysosomes after the iron is exposed by degrading ferritin protein cages.4 Whereas all 3 chelators form stable Fe3+ complexes with 3-dimensional shapes, DFO is distinctive. It is linear without metal whereas L1 and XJ have aromatic structures with or without metal. The lipophilicity of L1 and XJ likely contributes to the ability of the chelators to cross cell membranes into the cytoplasm, with and without iron, and to the efficacy of oral administration.
DFO without metal, which enters cells by endocytosis, selectively induced ferritin entry into lysosomes, indicated by the association of ferritin turnover with DFO-dependent increases in LCB3 accumulation on lysosomes; L1 + XJ had no effect.4 LBC3 is a derivative of LC3, a protein associated with increased autophagy and lysosomal protein turnover. However, inhibition of chaperone-mediated autophagy (CMA) using cells deficient in LAMP1 and -2, or of the TOR pathway (target of rapamycin) of macroautophagy using rapamycin, had no effect on the DFO-induced lysosomal turnover of ferritin.4 Thus, the mechanism of DFO-induced ferritin accumulation in lysosomes is distinctive and may reflect specific pathways used by pathogens carrying siderophores such as DFO, or other molecules with similar functions in the host/pathogen battle over iron.
Chelators compete for ferrous ions released from ferritin minerals with the ferritin protein active sites,3 with the “labile iron pool,” and with carrier proteins such as mitoferrin frataxin and PCBP1.1 Cells are full of reductants that could dissolve the ferric ferritin mineral, thus ferritin protein pores are closed most of the time, which contributes to the slow rates of iron chelation.7 Because DFO, XJ, and L1 bind ferric iron more stably than ferric iron, as soon as iron oxidation occurs yielding ferric-chelator complexes, competition by ferrous sites ceases. (Whether or not oxy radicals are released during oxidation of less stable ferrous to more stable ferric-chelator complexes is not well understood.)
During iron overload, ferritin is damaged and hemosiderin increases because of the increased numbers of oxidoreductase cycles with the ferrous and oxygen substrates. The increased ferritin iron content reflects a mismatch between iron homeostatic mechanisms that increase ferritin protein synthesis1,8 and cell iron burdens. When hemosiderin is low, XJ + L1 may be preferred chelators while later, as hemosiderin forms, DFO might be preferred. The challenge is to understand more about natural regulators of ferritin subcellular distribution and to design chelators that can mine ferritin iron wherever it is in the cell.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal