Abstract
Abstract 1317
Poster Board I-333
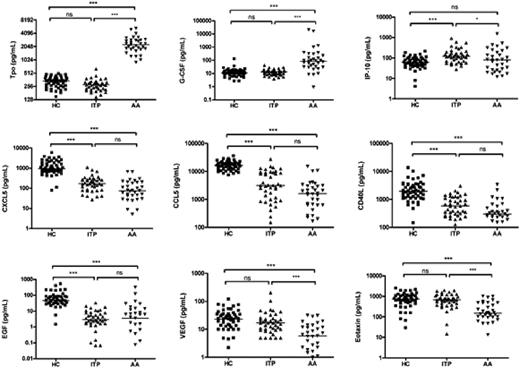
Thrombocytopenia occurs in aplastic anemia (AA) as a result of hematopoietic failure, in which decreased or absent megakaryocytes are observed in a hypocellular bone marrow. In immune thrombocytopenia (ITP), thrombocytopenia primarily occurs secondary to peripheral destruction and marrow cellularity is normal with megakaryocytes numerically normal or increased. In both AA and ITP, platelet function is normal and the disturbance in hemostasis is the result of the diminution in the platelet count. We recently reported that high plasma concentrations of Tpo and G-CSF, in combination with low levels of CD40L, CXCL5, CCL5, CXCL11, EGF, VEGF, and eotaxin, served as a cytokine signature profile for AA when compared to MDS or healthy controls (Blood 2008;112:380-381). In 8 patients with severe AA who responded to immunosuppressive therapy, the levels of CD40L, CXCL5, EGF, and CCL5 in plasma correlated with platelet recovery, as compared with absolute neutrophil count and hemoglobin recovery. We hypothesized that the plasma levels of CD40L, CXCL5, EGF, and CCL5 reflected platelets or their precursor megakaryocytes. We measured these cytokines in the plasma of 41 ITP patients using the Luminex assay, for comparison with 33 untreated AA patients and 52 healthy controls (Figure 1). Results were assessed by the Kruskal-Willis one-way analysis of variance statistic. ITP patients showed similar low levels of circulating CCL5, CD40L, CXCL5, and EGF as did AA patients, when compared to healthy controls. These low plasma levels of cytokines/chemokines in AA and ITP may not reflect inflammation but rather the common feature of deficiency of platelets. Soluble CD40L appears to be a platelet agonist and may promote coagulation; CCL5-deficient mice model demonstrate that CCL5 blockade compromises systemic immune responses, delays macrophage-mediated viral clearance and impairs normal T cell function; CXCL5 attracts leukocytes and activates platelets; and EGF upregulates tissue factor and acts as a procoagulant. Levels of Tpo, G-CSF, VEGF, and eotaxin were not statistically different between ITP and healthy controls, but the levels of these cytokines were different between AA patients and healthy controls, indicating that Tpo levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. High Tpo, and G-CSF levels in AA represent a compensatory physiologic mechanism operating to counter impaired blood hematopoiesis. In addition, ITP patients showed higher levels of IP-10 than both AA patients and healthy controls, suggesting the involvement of interferon-gamma related responses in ITP. We compared cytokine gene expression in platelets, differentiated CD61+ cells (megakaryocytes), and CD61- cells (non-megakaryocytes) from bone marrow CD34+ cells of healthy controls by real-time PCR. Platelets and megakaryocytes showed higher mRNA expression of CXCL5 (440 and 16.3-fold), CD40L (9.6 and 51.9-fold), EGF (171 and 112-fold) and CCL5 (526 and 27.7-fold) than did CD61- cells (non-megakaryocytes), respectively. Together, our study provides evidence that platelets as well as megakaryocytes appear to be sources of CXCL5, CCL5, EGF, and CD40L; these cell types may contribute to the physiological levels of these cytokines. Similarities in cytokine levels between AA and ITP may reflect the autoimmune destruction of targets at varying stages of differentiation. Measurement of platelet-associated cytokines allows inferences concerning pathophysiology in quantitative platelet disorders.
Comparison of plasma cytokine levels among patients with immune thrombocytopenia (ITP), aplastic anemia (AA), and healthy controls (HC). Cytokine levels in the plasma samples from 41 ITP, 33 untreated AA patients and 52 HC were measured using Luminex assay. The bars represent median values. ***, p < .001; *, p < .05; ns, not significant (Kruskall-Wallis). Tpo, Thrombopoietin; G-CSF, granulocyte colony-stimulating factor; IP-10, Interferon-inducible protein-10; CXCL5, chemokine (C-X-C motif) ligand 5; CCL5, chemokine (C-C motif) ligand 5; CD40L, CD40 ligand; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor.
Comparison of plasma cytokine levels among patients with immune thrombocytopenia (ITP), aplastic anemia (AA), and healthy controls (HC). Cytokine levels in the plasma samples from 41 ITP, 33 untreated AA patients and 52 HC were measured using Luminex assay. The bars represent median values. ***, p < .001; *, p < .05; ns, not significant (Kruskall-Wallis). Tpo, Thrombopoietin; G-CSF, granulocyte colony-stimulating factor; IP-10, Interferon-inducible protein-10; CXCL5, chemokine (C-X-C motif) ligand 5; CCL5, chemokine (C-C motif) ligand 5; CD40L, CD40 ligand; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal