Abstract
Abstract 1371
Poster Board I-393
The study examines the quality of life (QOL) of pediatric thalassemia patients and their families enrolled in the Thalassemia Longitudinal Cohort (TLC) study of the NHLBI-sponsored Thalassemia Clinical Research Network, which comprises 17 centers in the US, Canada, and London, UK. The study evaluates 99 baseline responses to the Children's Health Questionnaire (CHQ) PF28, a self-administered survey filled out by the parent or guardian of patients age 5 y and older. The CHQ utilizes 12 scales that can be grouped into parental assessments of the child's physical well-being; mental, emotional and behavioral health; and the familial context. We previously showed that compared to the US population, the thalassemia population is significantly different on 7 of the 12 scales. Here we evaluate these data by patient gender, race, age, and chelator type.
The mean age of the population was 9.7 y (range 5.0-13.8 y), with 48% male, and 65% non-white. While parents assessed males to have a lower QOL than females, the only significant difference was in their assessment of the child's emotional role/behavior (school or friends). When comparing white to non-white (predominantly Asian) patients, with the exception of bodily pain, non-white thalassemia patients reported poorer PF28 scores. However, only the reported impact on parental time and emotion are significant. These variances appear to mirror the US population.
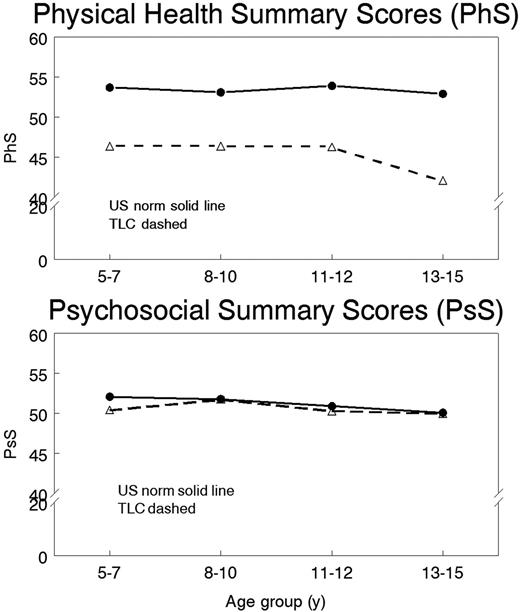
Figure 1 shows PF28 summary scores by age in thalassemia compared to the general US population. Parents evaluate their child with thalassemia with lower physical health than US norms (p<0.0001), with an apparent decline in scores in adolescents (though p=0.10 for age effect). In contrast, psychosocial scores are close to US norms (p=0.81).
Finally, when CHQ PF28 assessments are compared by chelator type (subcutaneous deferoxamine vs. oral deferasirox), parents report that children receiving deferoxamine have generally lower QOL than the rating for those receiving Deferasirox. However, the only significant differences are with perceived physical function, impact on family activities, and the overall physical summary scale.
The CHQ PF28 data provides important insight into the impact the child with thalassemia has on the family. Although psychosocial QOL is similar, children, and especially adolescents, with thalassemia have lower physical QOL, especially those on chelation with Deferoxamine. Because PF28 takes the parents' point of view, it can't be determined from these data alone whether the reported difference between parenteral and oral chelator would hold true for direct patient assessments. The gender of the child also appears to affect parental expectation of the child's QOL. These data validate the observational evidence that a child with thalassemia has a significant impact on the family.
TLC CHQ PF28 Summary Scales compared to US norms
Odame: Novartis: Consultancy, Speakers Bureau. Thompson: Novartis: Research Funding. Neufeld: Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal