Abstract
Abstract 1393
Poster Board I-415
Chronic lymphocytic leukemia (CLL) is the most prevalent form of adult leukemia. Patients with CLL have a long life expectancy and little attention has been paid to the symptom burden that accompanies this disease. We, therefore, wanted to explore the reliability and validity of the M. D. Anderson Symptom Inventory (MDASI) in measuring the symptom burden of patients with CLL. The MDASI is an established, reliable and validated tool for assessing cancer-related symptoms regardless of therapy or specific cancer diagnosis.1 The MDASI measures the most common symptoms across most cancer types and treatments: 13 items assess symptom severity at its worst in the last 24 hours and 6 items assess symptom-related interference in the last 24 hours, all rated on a 0–10 numeric scale. These 19 symptom and interference items comprise the “core” MDASI.
We enrolled 126 consecutive patients with CLL being followed at our outpatient center. Patients eligible for this study were required to be speak English and have a pathological diagnosis of CLL. A clinical nurse conducted interviews, had patients complete self-administered MDASI, and collected information from patients' medical record.
Sixty-three % of the patients were male, 95% were white non-Hispanic, the mean age was 59 years (range 29-79) with 27% of them age 65 years or older. Eighty-three % of the patients had Rai stage 0-2, and 56% were untreated.
Factor analysis of the MDASI items resulted in a three-factor solution (physical symptoms, psychological symptoms, and gastrointestinal symptoms), which satisfied criteria for interpretability and model fit in a confirmatory setting. Cronbach coefficient alphas for the MDASI were 0.89 for the symptom severity subscale and 0.94 for the interference subscale. Convergent validity showed that the two MDASI subscales were significantly correlated with similar subscales of the SF-12 (12-Item Short-Form Health Survey).
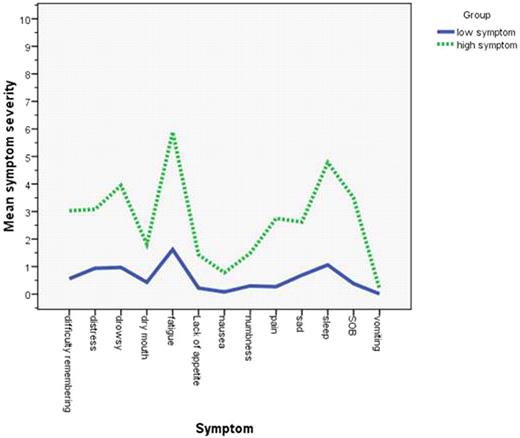
The most severe patient-reported MDASI symptoms were fatigue, disturbed sleep, drowsiness, distress, and difficulty remembering. Patients with poorer performance status (ECOG PS 2 vs. 1 vs. 0) reported significantly higher severity for both core MDASI and total interferences (all P < .001). Patients with higher Rai staging [3-4 vs 0-2] reported significantly higher severity on core symptoms and interferences (P<.01), especially on fatigue, nausea, sleep, shortness of breath, drowsiness (All P<.05), and lack of appetite (P<.001). Older patients (aged 65 years and older) had significantly higher fatigue, shortness of breath, and lack of appetite [P<.001], but lower sadness. We observed that female reported significantly higher pain and sadness (All <.05). Patients with more prior chemotherapy [1 or more vs. 0 cycles chemotherapy) reported significantly higher symptom severity for core items on MDASI [P=.013] and higher symptom interferences [P=.001], especial on fatigue, shortness of breath, and drowsiness (All P<.01). Sixty-one percent of the sample rated at least one symptom as moderate to severe (≥5 on the MDASI's 0–10 scale). In cluster analysis, approximately 30% of patients belonged to the high-symptom group (Fig 1: symptom severity by items on MDASI for both high and low symptom group). The most burdens from symptoms were interference of working, enjoyment of life and activity.
In conclusion, our data analysis showed that the MDASI is a reliable and valid tool for assessing symptom severity and interference with daily functioning in patients with CLL. The MDASI symptom profile demonstrated that almost one-third of patients had significantly higher symptom severity. This validated MDASI provides a useful tool for measuring symptom burden and monitor symptom control in patients with CLL.
Patient reported symptom severity (0-10 scale) on MDASI: majority of patient (70%) had multiple low symptom profile
Patient reported symptom severity (0-10 scale) on MDASI: majority of patient (70%) had multiple low symptom profile
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal