Abstract
Abstract 1839
Poster Board I-865
Bortezomib (BZ) is a chemotherapeutic agent approved for the treatment of multiple myeloma (MM). BZ acts through proteasome inhibition, inducing significant ER stress and ideally resulting in cell death. Unfortunately, nearly 20% of MM patients are primarily resistant to BZ treatment and responses to BZ are difficult to predict based on the currently available clinical, cytogenetic and genomic biomarkers. Our function hypothesis is that extracellular metabolites have a greater potential to be found in circulating biofluids as biomarkers. As a result we used a metabolite ‘footprinting’ approach in cell growth media to examine the metabolic consequences of BZ treatment using eight human MM cell lines, three of which have been determined to be less sensitive to BZ treatment than the others with a 10 fold difference in their IC50 at 24 hours (5 nM vs 50 nM). Our aims were 1) to establish whether analysis of growth media was suitable for monitoring metabolic changes and 2) to determine specific biopatterns of BZ resistance.
Eight MM cell lines (MM1S, MM1R, INA6, U266, RPMI8266, OPM2, KMS11 and PCL1) were cultured under standard conditions without (control group, 10% FBS) or with bortezomib added (10nM). Media samples were taken for metabolic analysis at 6 and 24 hours for a total of 32 media profiles. Metabolite profiling was accomplished using gas chromatography mass spectrometry (GC-MS) and nuclear magnetic resonance spectroscopy (NMR). GC-MS data was analysed using AMDIS (NIST), and NMR data using Chenomx NMR Suite. Significant metabolites were identified using multivariate regression analysis by supervised projection methods (two-way orthogonal partial least squares discriminant analysis, O2PLS-DA) using SIMCA-P (Umetrics).
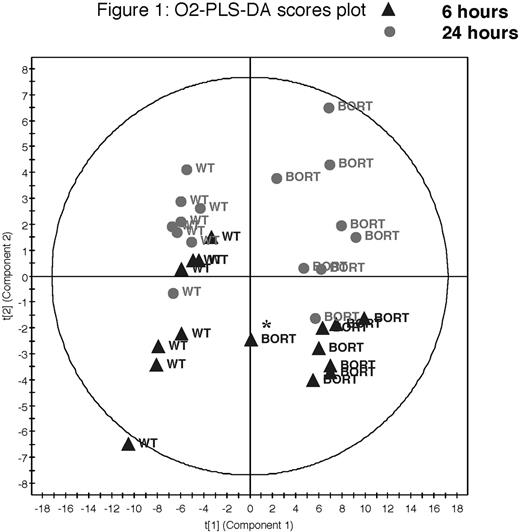
An average of 756 chemical or metabolite components per sample were profiled, which was reduced to a subset of 116 unique features that were shared in at least 75% of samples. An initial O2PLS-DA model was successfully built from the GC-MS feature set using both growth time (p=0.03) and BZ status (p=6.9e-13) in the Y-matrix. Figure 1 shows a scores plot, where each point represents a single sample, and the position is calculated as a combination of the underlying metabolite concentrations. Changes in cell growth were consistent with the known uptake of carbohydrate substrates and elimination of various amino acids and waste products such as lactate. The remarkable metabolic difference between BZ-treated and untreated cells resulted from reduced energy-related metabolites such as citric acid cycle intermediates and sugars, with a concomitant increase in selected amino acids. Intriguingly, the BZ-insensitive cell cultures exhibit overall metabolic phenotypes much more similar to the BZ-sensitive cultures than to the untreated group, with the exception of a single sample after 6 hours (denoted with an asterisk in Figure 1) which showed an averaged profile. To further probe the phenomenon of BZ resistance, the treated group was analyzed independently, with the 37 most influential components providing discriminating ability between the BZ-insensitive and BZ-sensitive cells (p= 0.04) in an OPLS-DA model.
We conclude that metabolite footprinting is a reliable and robust method for monitoring metabolic events for both cell growth and BZ treatment. Furthermore, BZ-insensitivity is accompanied by a notable shift from carbohydrate metabolism to fatty acid metabolism, while the overall metabolic phenotype remains very similar in both BZ-sensitive and insensitive strains in the presence of the drug. This result suggests that BZ function remains largely intact in both sensitive and insensitive cell lines, and resistance is conferred through alternate mechanisms with measureable metabolic endpoints. Success in measuring extracellular metabolites also supports the notion of serum-accessible biomarkers or biopatterns of BZ resistance. The unique genetic instability underlying each cell line may provide a further avenue for characterizing resistance mechanisms along with analysis of various intracellular components.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal