Abstract
Abstract 2271
Poster Board II-248
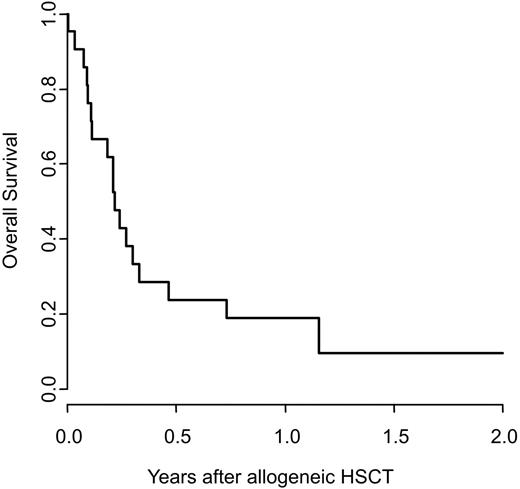
Relapsed/refractory acute myeloid leukemia (AML) has a poor prognosis and the therapeutic options are limited. Myeloablative allogeneic hematopoietic stem cell transplantation (HSCT) may be used, however its outcome is poor compared to patients treated in remission. Recently, high-dose melphalan (HDM) with autologous stem cell support has shown promising activity in patients with relapsed AML. Few data exist on the impact of HDM re-induction in AML patients planned for allo-HSCT. We analyzed the outcome of 23 patients with highly advanced relapsed and/or refractory AML re-induced with HDM followed by myeloablative allo-HSCT. The re-induction regimen consisted of a single dose of melphalan 200mg/m2 i.v. on day -1. Where possible, stem cell support was given (group 1; n=12): patients for whom a previously harvested autologous hematopoietic stem cell product was available received autologous HSCT on day 0 (n=3). Patients with an HLA-identical sibling donor were allocated to tandem allo-HSCT and received a first CD34-selected graft from their sibling donor (n=8). One patient relapsing after haploidentical HSCT and scheduled for unrelated HSCT was provided with stem cell support from his previous haploidentical donor. All other patients, planned for allo-HSCT from a matched unrelated, mismatched related or cord blood donor had HDM without stem cell support (group 2; n=11). Both groups were prospectively scheduled to undergo a second conditioning regimen 2-4 weeks after HDM consisting of cyclophosphamide/busulfan or cyclophosphamide/12 Gy total body irradiation +/- etoposide followed by allo-HSCT. The primary endpoints were blast clearance and the number of patients proceeding to allo-HSCT. Secondary endpoints were the toxicity and feasibility of HDM and final outcome. From March 2005 to April 2009, 12 males and 11 females aged 21 to 69 years (median 39) with advanced AML were included. Seven were in primary induction failure, 7 in untreated relapse, 4 were refractory to salvage chemotherapy and 5 were in second relapse after successful salvaging. Nine patients relapsed after a previous HSCT, seven after allo, two after autologous HSCT. Eighteen patients (78%) achieved complete remission, defined as absence of blasts in the bone marrow, peripheral blood or extramedullary sites. The four remaining patients had substantial reduction of the initial leukemia burden warranting treatment continuation. None of the patients died within 25 days of HDM. Twenty patients (87%) went on to the second phase of the planned protocol, to full conditioning and allo-HSCT (9 HLA-identical sibling, 2 haploidentical donor, 9 unrelated donor). Three patients went off continuation, 1 due to progressive disease, 2 due to severe toxicity (1 combined cytomegalovirus and respiratory syncytial virus pneumonitis; 1 obstructive lung disease). There was no difference between patients with (group 1) or without (group 2) stem cell support after HDM regarding mucositis grade 3 and 4 (63% versus 54%; p=0.66), other organ toxicity or overall survival (OS) after the second HSCT (76 versus 87 days, median; p= 0.74). Of the patients proceeding to allogeneic HSCT, 2 (10%) were alive and in remission at last follow-up (Figure). Seven patients (35%) had died from TRM (infection n=2, bleeding n=2, veno-occlusive disease n=2, interstitial pneumonitis n=1) at a median of 32 days after allo-HSCT. Eleven patients (55%) relapsed at a median of 84 days (range 42-1019) after allo-HSCT. Median progression free survival (PFS) was 74 days and median OS 79 days after HSCT. Both patients alive and well had had a 1st CR of more than one year's duration. In conclusion, our study demonstrates that HDM is effective in achieving complete blast elimination in patients with highly advanced relapsed/refractory AML. However – compared to historical controls of patients directly transplanted for relapsed/refractory AML – leukemia burden reduction with HDM did not translate into improved overall survival after HSCT.
Off Label Use: This presentation includes results of the off-label use of melphalan as a re-induction regimen for AML. Gratwohl:Amgen: Research Funding; Bristol Myers Squibb: Research Funding; Novartis: Advisory Board, Research Funding; Roche: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal