Abstract
Abstract 2363
Poster Board II-340
Clinical trials report that FR as initial therapy in symptomatic CLL produces an overall response rate of 90% and significantly improves progression-free survival (PFS) and overall survival (OS) compared retrospectively to F alone [Byrd Blood 2003, 2005] and that FCR (F, cyclophosphamide and R) improves response rates and PFS compared to FC alone [Hallek ASH 2008]. FR has been the standard initial therapy for CLL/SLL in British Columbia (BC) (population = 4.2 × 106) since 2004. We wished to determine if, in an unselected community based population, the same results could be achieved with FR therapy as those seen in highly selected clinical trial populations.
The BC Cancer Agency (BCCA) Lymphoid Cancer Database was searched to identify pts who received FR as initial therapy for CLL/SLL as per BCCA guidelines (F 40mg/m2/day orally D1-5 or 25mg/m2/day IV D1-5 [adjusted for renal function] and R 375mg/m2 IV D1, q 28 days for 4-8 cycles). Pts were excluded if they had more than one hematologic malignancy (n=2). OS was calculated from date of FR to date of death or last follow-up. Treatment-free survival (TFS) was calculated from date of FR to date of next treatment or death from toxicity. Factors present at initiation of treatment were analyzed for impact on outcomes.
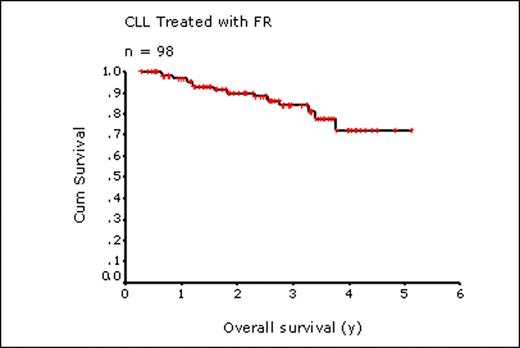
98 pts were identified who received FR from 2004-2009 as initial therapy for CLL (83%) or SLL (17%). Median age at FR was 62 y (range, 42 – 86 y). 36% had Rai stage 3 or 4 and 13% had ECOG performance status 2-3. Median lymphocyte count was 30 × 109/L (range 0.6-282 × 109/L) and LDH was elevated in 34% (29/86 with available levels). Nearly half of all pts tested were positive for CD38 (34 of 69 pts, 49%) and ZAP 70 expression (10 of 22 pts, 45%). FISH was performed in 35 pts: 13q deletion, 21 (60%); 11q deletion, 5 (14%); 17p deletion, 10 (29%); trisomy 12, 11 (31%). Most pts (65%) were observed prior to FR for a median of 28 months (range, 3 – 172 mos). Common indications for treatment included symptomatic lymphadenopathy (n=36), cytopenias (n=28), fatigue (n=23), and constitutional symptoms (n=19). Pts underwent a median of 6 cycles of FR (range, 1-8). Treatment was discontinued due to toxicity in 13 pts (13%) (cytopenias, 5; febrile neutropenia, 3; infection, 2; pulmonary disease, 1; ITP, 1; AIHA, 1) and due to progression in 4 pts (4%). Hospitalization during therapy was required in 13 pts (13%) (febrile neutropenia, 5; documented infection, 2; elective splenectomy, 2; pulmonary disease, 2; ITP, 1; rituximab administration, 1). At a median follow up of living pts of 2.4 y, 31 pts (32%) have received additional therapy after FR including CVP in 11 pts (+R, 8); chlorambucil, 7 (+ R, 1; +prednisone, 1); FR, 5; cyclophosphamide, 5 (+prednisone, 3; +vincristine, 1); CHOP, 3 (+R, 2). At time of analysis (June 2009), 86 pts were alive; 14 died, 12 from progressive CLL and 2 from toxicity of FR (severe infection, 1; interstitial lung disease, 1). For the entire 98 pts, the 2 and 4 y OS were 90% and 72%, respectively (median not reached); 2 and 4 y TFS were 69% and 54%, respectively (median 4.0 y). Factors at treatment contributing to OS in univariate analysis included elevated LDH (P=0.004), Hb <110 g/L (P=0.03), and the presence of deletion 17p by FISH (P=0.04) or CD38 expression (>15% of CD19+ cells) by immunophenotyping (P=0.04). TFS was significantly worse in those with Hb <110 g/L (P=0.003), Rai stage 3-4 (P=0.008) or lymphocyte count >150 × 109/L (P=0.0013) while cytogenetic abnormalities and immunophenotyping had no effect on TFS. Age ≥ 60 y (n=60) or age ≥ 70 y (n=26) had no effect on either OS or TFS.
In this non-selected community based population of pts with CLL/SLL, including many over 60 y (61%) and 70 y (27%) of age, our results show that initial treatment with FR leads to excellent OS and TFS, consistent with clinical trial results: 2 y OS, this study 90%; FR Byrd [Blood 2005] 93%; FCR Hallek [ASH 2008] 91%; 2 y TFS, this study 69%; FR Byrd, 2 y PFS 67%; FCR Hallek 2 y PFS 77%. FR can be safely and successfully given to community based pts, irrespective of age, for first-line therapy for CLL/SLL with OS and TFS similar to that achieved in clinical trial settings.
Connors:Roche Canada: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal