Abstract
Abstract 2554
Poster Board II-531
Hydroxyurea induces fetal hemoglobin (HbF), improves laboratory parameters, and reduces acute clinical complications of sickle cell anemia (SCA), but its long-term efficacy and safety remain incompletely defined. One long-term safety concern is that hydroxyurea may elicit DNA alterations via genotoxic damage. During normal erythropoiesis, red blood cells (RBC) extrude their nucleus as they develop into functional reticulocytes. Occasionally, membrane bound DNA remains in the cell after erythrocyte maturation and these inclusion bodies are known as micronuclei (MN) or Howell-Jolly Bodies. MN-containing reticulocytes are formed at higher frequency upon exposure to genotoxic agents. Patients with SCA have increased basal MN production while also having decreased MN clearance due to diminished splenic filtrative function. In a previous small cross sectional study, we showed that hydroxyurea exposure further increases MN production in SCA patients. To better address this long-term safety issue of hydroxyurea, we evaluated MN production and clearance both in a large cross-sectional and prospective study of children with SCA on hydroxyurea therapy.
A high-throughput flow cytometric technique was used to detect and quantitate MN within circulating erythrocyte subpopulations. After written informed consent, venous blood samples were collected from children with SCA enrolled in the Hydroxyurea Study of Long-term Effects (HUSTLE, ClinicalTrials.gov NCT00305175). A total of 105 subjects had at least 1 MN measurement, including 37 subjects with serial measurements at baseline and at follow-up time points up to 24 months of hydroxyurea exposure. MN were quantified in both reticulocytes (MN-CD71(+)) and mature RBC (MN-RBC), and then tested for associations with individual subject laboratory and clinical data.
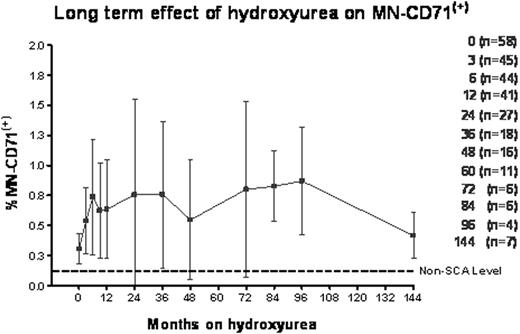
In cross-sectional analysis of 293 samples from 105 children with SCA and a median of 2 years hydroxyurea exposure (range 3 months – 12 years), hydroxyurea therapy significantly lowered %CD71(+) reticulocytes (mean fold reduction 0.53 ± 0.44, p < 0.001). Compared to baseline values, hydroxyurea treatment increased levels of MN in both reticulocytes (%MN-CD71(+), mean fold increase 1.80 ± 0.91, p < 0.05) and mature RBC (%MN-RBC, mean fold increase 1.89 ± 1.39, p<0.01). The increase in MN-CD71(+) was evident by 6 months of hydroxyurea treatment, but did not significantly escalate further with up to 12 years of continued drug exposure (Fig. 1).
To prospectively determine the genotoxic effect of hydroxyurea, serial measurements over 2 years were performed on 37 patients. After 9 months on hydroxyurea therapy all subjects were on a stable maximum tolerated dose (MTD, average 25.1 mg/kg/day); 15 of 37 children had > 3.0 fold increase in %MN-CD71(+) while 22 of 37 had < 3.0 fold increase (Mean 3.68 ± 0.65 vs. 1.52 ± 0.52, p < 0.001). The observed inter-individual variation was associated with the predicted laboratory effects of hydroxyurea; increases in %MN-CD71(+) were positively correlated with MTD values of HbF (r2=0.22, p=0.005), mean corpuscular volume (r2=0.35, p=0.002), and mean corpuscular hemoglobin (r2=0.29, p=0.006) but negatively correlated with absolute neutrophil count (r2=0.14, p=0.02) and bilirubin levels (r2=0.20, p=0.008). There were no significant associations between %MN-CD71(+) and gender, age, or hydroxyurea dosage although %MN-RBC clearance decreased with age.
A highly sensitive and quantitative flow cytometric technique can detect circulating MN-containing erythrocytes and this technique may be used to assess the in vivo genotoxic effect of any drug. Children with SCA have high basal MN production that is probably related to the degree of erythropoiesis in these patients. Hydroxyurea therapy was associated with genotoxicity but with substantial inter-patient variability in hydroxyurea induced %MN-CD71(+) levels. The increases in %MN-CD71(+) are observed within 6 months of starting hydroxyurea therapy but persist at the same level in patients with up to 12 years of continued drug exposure. Correlations between increased %MN-CD71(+) and predicted hydroxyurea effects on other laboratory parameters suggest that hydroxyurea induces measurable genotoxicity that may be related to individual patient sensitivity and efficacy of hydroxyurea within the bone marrow. These patients will be monitored further to confirm that hydroxyurea does not pose any long term safety issues.
Off Label Use: Off label use of hydroxyurea in children with sickle cell anemia. Avlasevich:Litron Laboratories: Employment. Dertinger:Litron Laboratories: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal