Abstract
Abstract 2590
Poster Board II-566
Chronic myelogenous leukemia (CML) is caused by a consistent genetic abnormality, termed the Philadelphia chromosome (Ph). It results in the production of BCR-ABL fusion protein, a constitutively active tyrosine kinase. Imatinib mesylate (IM, Gleevec®), the first generation tyrosine kinase inhibitor (TKI), has revolutionized therapy for CML patients. However, resistance for IM develops in a significant proportion of cases, and is predominantly mediated by single point mutations within the BCR-ABL kinase domain. Second generation TKIs such as dasatinib (Sprycel®) and nilotinib (Tasigna®) represent viable alternatives for IM-resistant or intolerant CML patients. Each mutated BCR-ABL has different sensitivity to those TKIs. Thus, it is significantly important to detect early the existence of BCR-ABL mutations and their specificities in treating Ph+ leukemias.
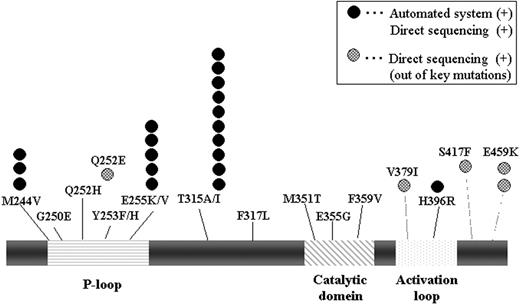
We have developed a novel automated method that has high sensitivity to detect a few copies of mutation sequences that are mixed in many copies of normal sequences. This method consists of PCR amplification step and Tm (melting temperature) analysis step that uses a quenching probe. And we have already shown that this system has clinical efficacy in JAK2V617F mutation that is one of the genetic hallmarks of chronic myeloproliferative diseases. (Tanaka R, et al. Leuk Res, 2008). When a whole blood sample or a purified DNA sample reacts with reagents, PCR and Tm analysis automatically processed in the same tube, and whole procedure finishes in approximately 1 hour. The detection of mutation is extremely accurate because the quenching probe is designed perfectly matched for mutated sequence. As Tm value of mutation sequence is higher than that of normal one, it is easy to detect the existence of mutation from the Tm analysis data. We have constructed the probes for 14 mutations concerned for IM-resistance (M244V, G250E, Q252H, Y253F, Y253H, E255V, E255K, T315I, T315A, F317L, M351T, E355G, F359V, and H396R). Considering the clinical significance of T315I mutation, which renders resistance to all currently available TKIs, we refined this method to higher sensitivity for detecting T315I mutation.
First, we analyzed the sensitivity of this system on BCR-ABL. In dilution assays using wt and mutated plasmid, the system reliably quantified the mutation in a population containing as few as 3.0% mutant. Moreover, for T315I setting, we successfully detected as few as 0.3% (30 copies from 10,000 copies) mutations by a higher-sensitive assay. Next, we examined the clinical samples. Each sample was also examined by direct sequencing in comparison to our method. Kinase domain mutations were identified in 24 of the 50 (48%) patients. Our automated analysis was enabled to detect mutations in 19 patients, including p-loop mutations (G250E: n=3; E255K: n= 5), IM-binding domain mutations (T315I: n=10), and an activation-loop mutation (H396R: n=1). And all the positive cases (19 of 19) showed a concordance with the result of direct sequencing. On the other hand, 5 cases were detected just by direct sequencing, but all that cases were out of our setting mutations (Q252E, V379I, S417F, E459K). Impressively, in one case, only higher-sensitivity assay could reveal T315I mutation, although it was detected as a wild type both by direct sequence and our usual method. It suggests that the higher-sensitive system could detect low amount of T315I mutation in the earlier stage of disease.
In conclusion, sensitivity of our system (3%) is significantly greater than that of direct sequencing (15 – 25%), and results can be obtained within one hour. By the serial monitoring, it is demonstrated the availability of the higher-sensitive analysis (0.3%) to detect T315I mutation. This rapid and accurate detection of clinically significant mutations enables us to contribute to better clinical practice in treating Ph+ leukemia patients, such as in selecting alternative strategies of IM dose escalation, second generation TKIs, or allogeneic stem cell transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal