Abstract
Abstract 2685
Poster Board II-661
Although standard therapies cure most adolescents and young adults with Burkitt's lymphoma/leukemia (BL), older patients (pts) have an inferior prognosis with an estimated 1-year survival of 50%. The inferior outcome is attributable to both insufficient efficacy and excess toxicity. Cyclophosphamide (Cy) has long been recognized to be arguably the most active agent in BL. Prior work at our institution showed that high-dose Cy, equivalent to transplantation doses, could be given without stem cell rescue with minimal toxicity even in older pts.
A phase II trial for pts age ≥ 30, based on intensive Cy and incorporating rituximab but no anthracycline, was developed with a primary endpoint of 1-year overall survival. Entry requirements included newly diagnosed BL or atypical BL; any performance status (PS); HIV negative; and no significant cardiac dysfunction. Renal failure, even if necessitating dialysis, was permitted if it was acute. Treatment consisted of 3 cycles, with successive cycles beginning on day 15 or when ANC was ≥ 500/μL. Cycles 1 and 2 consisted of Cy 1500 mg/m2 IV day 1; vincristine 1.4 mg/m2 (2 mg cap) day 1; prednisone 100 mg days 1-5; rituximab 375 mg/m2 IV days 1 and 8; methotrexate 3 g/m2 IV day 8 with leucovorin rescue; cytarabine 100 mg intrathecally days 1, 4, and 11; and filgrastim. Cycle 3 consisted of rituximab 375 mg/m2 IV day 1; high-dose Cy (50 mg/kg IV days 2, 3, 4, and 5) with uroprotection; filgrastim; and rituximab 375 mg/m2 IV weekly for 4 weeks once ANC was ≥ 1000/μL. Eligibility for cycle 3 included ECOG PS < 4; no disease progression or uncontrolled meningeal disease; not on dialysis; and transaminases ' 5X upper limit of normal.
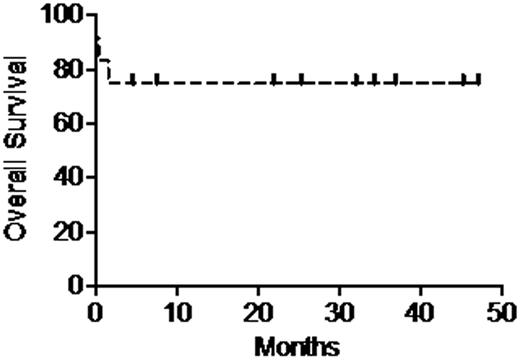
A prespecified interim analysis of the first 12 of a planned 20 evaluable pts is presented. Diagnosis was BL in 8 and atypical BL/unclassifiable high-grade lymphoma with features intermediate between BL and diffuse large B-cell lymphoma in 4. Median age was 56 (range 34 – 75), 8/12 (67%) had Ann Arbor stage III/IV disease, and all were high-risk by Magrath's criteria. PS ranged from 0 to 4. Two pts received hemodialysis on presentation. For all pts, actuarial event-free survival and overall survival (Figure) are 66% and 75%, respectively, at both 1 year and 2 years after treatment initiation. Three pts died during cycle 1: tumor lysis syndrome on day 1, neutropenic sepsis on day 8, multiorgan failure on day 46 after respiratory arrest on day 20. All of the other 9 pts completed protocol therapy: 8 (89%) achieved anatomic CR/CRu as well as a complete metabolic response by PET, and are event-free at a median of 29 months (range < 1 – 44 months) after therapy completion. The remaining pt had residual marrow disease followed by progression and is in remission 1 year after myeloablative allogeneic BMT. Adverse events in these 9 pts included 7 neutropenic fevers; 1 non-neutropenic bacteremia; and 1 self-limited episode of pericarditis with rapid atrial fibrillation. Grade 3 peripheral neuropathy was limited to 2 pts. The planned dose intensity was achievable: median time to cycle 2 was 15 days (14 – 21), and median time from start of cycle 1 to start of cycle 3 was 31 days (28 – 35). Median time to neutrophil recovery after the last dose of Cy was 16 days (10 – 21); median time to platelets ≥ 20,000/μL, without transfusion in the preceding week, was 22 days (0 – 30). Early stopping criteria for response or all-cause mortality have not been met.
A very short regimen based on intensive Cy without anthracycline produces a high rate of durable CR's in older, poorer-risk pts with BL or atypical BL.
Kasamon:Genentech: Research Funding. Swinnen:Genentech: Consultancy, Research Funding; Enzon: Consultancy; Abbot: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal