Abstract
Abstract 3421
Poster Board III-309
While the prognosis of patients with MM has been drastically improved with the introduction of novel agents into the treatment armamentarium, relapse management remains a difficult task, especially in the post-transplant and high-risk MM setting, as defined by the presence of cytogenetic abnormalities (CA) and gene expression profiling (GEP).
We have explored the efficacy and safety of SB in 95 patients with MM. The regimen comprised BCNU at 300mg/m2 on day 1, etoposide 200mg/m2 on days 1-4; cytarabine 400mg/m2 on days 1-5; melphalan 140mg/m2 on day 5 plus: bortezomib 1.0-1.3mg/m2 on days 1+4, thalidomide 100-200mg on days 1-5, dexamethasone 20-40mg days 1-5, cisplatin 10-12.5mg/m2/d by continuous infusion on days 1-5, rapamycin 3mg on day 1 and 1mg on days 2-5; followed by autotransplant (AT) on day 6.
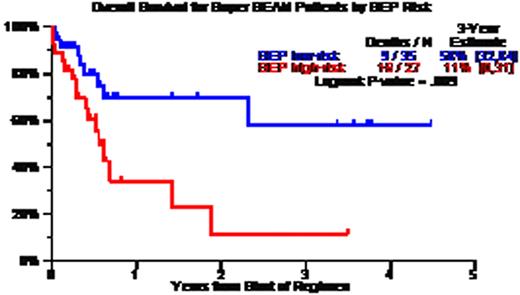
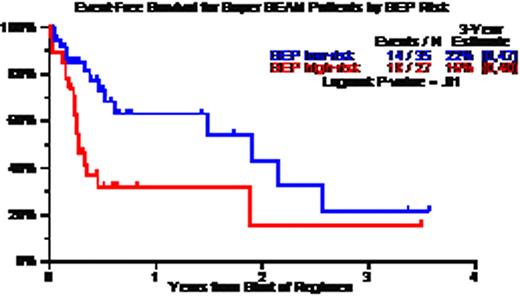
Patient characteristics included age >=65, 19%; LDH>ULN, 43%; CA, 67%; GEP high-risk, 44%; GEP MF, 15%; GEP delTP53, 22%; prior AT, 75% including 46% with 2AT and 12% with 3 AT. CR and near-CR status was documented in 50%; TRM occurred within 100 days in 6%. Three-year estimates of overall survival (OS) and event-free survival (EFS) were 34% and 18%; OS/EFS were superior: (a) without CA – 60%/50% versus 15%/5% with CA (p=0.003/0.0005); (b) with GEP low-risk – 55%/20% versus 10%/15% with high-risk MM (p=0.009/0.01); (c) with GEP Hyperdiploidy and Low Bone disease molecular subgroups – 70%/35% versus 20%/10% in the remainder (p=0,001/0.002); and (d) absence of progression prior to SB – 52%/35% versus 16%/5% for the remainder (p=0.002/0.0006). Univariately significant adverse variables for OS and EFS included low albumin, B2M>=3.5mg/L, CA, GEP high-risk and relapse just prior to SB, of which B2M, CA and pre-SB survived on multivariate analysis for the 78 patients with all variables; among the 57 with GEP data, OS was inferior with GEP high-risk MM (HR=3.55, p=0.007) whereas EFS was inferior with high LDH (HR=3.44, p=0.004), CA (HR=2.79, p=0.02) and pre-SB relapse (HR=2.94, p=0.035).
We conclude that SB is a safe salvage regimen that was well-tolerated for the majority in the outpatient setting. Although beneficial to patients lacking CA and GEP high-risk status, the poor outcome also with SB in the presence of these adverse features emphasize the urgent need to discover agents/strategies effective in this setting.
van Rhee:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal