Abstract
Abstract 3751
Poster Board III-687
Limited-stage non-Hodgkin's lymphomas with bulky mass are known to have a poor prognosis as like to those with advanced disease. There are very limited data to compare the results of chemotherapy alone with those of combined modality treatment. However, defining local failure at bulky sites as one component of relapse, radioimmuotherapy (RIT) appears to reduce failure rates at sites of bulky disease. We assessed the clinical efficacy and toxicity of 6th R-CHOP chemotherapy and followed by ibritumomab tuixetan (Zevalin¢ç) consolidation in patients with bulky diffuse large B cell lymphoma (DLBCL).
This prospective, multi-center phase II clinical trial included the patients following as: i) patients with newly diagnosed CD20+ DLBCL ii) patients with stage I/II and bulky disease defined by maximum diameter greater than 10 cm or any mediastinal mass exceeding 1/3 the maximum trans-thoracic diameter (more than 8 cm) iii) patients that achieved a complete (CR) or partial response (PR) after 6th R-CHOP chemotherapy iv) aged over 18 years and ECOG performance status 0-2. Conventional, single dose of ibritumomab tuixetan (0.4 mCi/kg) was administered after 6th R-CHOP. Clinical response was evaluated after completion of 3rd and 6th R-CHOP and at 8 and 20 weeks after RIT, and assessed according to revised International Workshop Criteria.
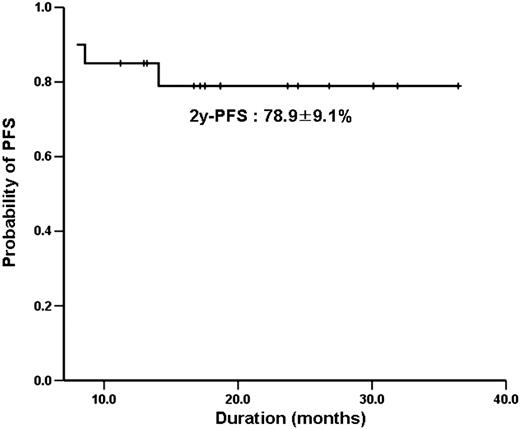
Twenty patients were treated with ibritumomab tuixetan as consolidation. Median age was 45.5 years and median size of bulky mass was 10.0 × 6.6 at diagnosis. 5 patients (35%) presented with high-intermediate or high risk by IPI and 4 patients underwent debulking operation. After a median of 17.5 months follow-up, the overall response rate was 95%, with a 80% CR and 15% PR. Two patients with PR after R-CHOP chemotherapy improved to CR after consolidation. However, four patients (20%) experienced a relapse with one of them progressed within 1 month after RIT. 2-year probability of progression-free survival (PFS) was 78.9 ±9.1%. The adverse events were mainly hematologic toxicities (more than grade3); neutropenia (60%) and thrombocytopenia (35%). Only one patient experienced a febrile neutropenia.
Consolidation treatment with Ibritumomab tuixetan resulted in a favorable response with tolerable toxicity in patients with bulky DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal