Abstract
Abstract 3829
Poster Board III-765
A large proportion of patients (pts) with myelodysplastic syndromes (MDS) become dependent on red blood cell (RBC) transfusions, escalating the risk of transfusional hemosiderosis and associated adverse effects. The US03 study was designed to evaluate the long-term efficacy and safety of the oral iron chelator, deferasirox, administered once-daily in pts with lower-risk MDS. 173 pts at 45 centers in the US and Canada enrolled in the 1-year core phase of the study and 83 pts participated in the 2-year extension phase. We now present data from pts who have completed 2 years of deferasirox treatment.
US03/E is an ongoing, Phase II, open-label, 3-year trial in pts with Low- or Int-1 IPSS-risk MDS. Eligible pts had transfusional iron overload (serum ferritin ≥1000 μg/L and >20 units of RBC transfusions) with serum creatinine (SCr) ≤2-fold the upper limit of normal (ULN). Initial deferasirox dose was 20 mg/kg/day, which could be increased up to 40 mg/kg/day based on tolerability and response criteria. Serum ferritin and creatinine were monitored monthly.
83 pts entered the extension study; mean age was 68 years (range 21–90) with 41 men and 42 women. IPSS risk groups were Low in 23 (28%) pts and Int-1 in 60 (72%). Mean serum ferritin level at the beginning of the extension phase was 2496 μg/L (range 546–7770); mean lifetime number of transfusions was 83.6 (range 20–364) and the mean duration of transfusion therapy was 4.6 (2–12) years. 18 (22%) pts were receiving growth factors (8 darbepoetin, 5 G-CSF, 4 epoetin alpha and 1 tranexamic acid), and 13 pts were receiving other MDS specific therapies (5 decitabine, 4 azacitidine, 3 lenalidomide, and 1 hydroxyurea). The calculated creatinine clearance at extension study entry was normal (>80 mL/min) in 20 (25%) pts and abnormal in 59 (75%), including mild renal insufficiency (51–80 mL/min) in 41 (52%) pts, moderate (30–50 mL/min) in 16 (20%) and severe in 2 (3%).
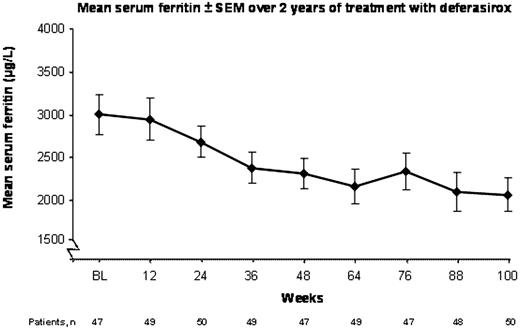
At the time of this analysis, 54 pts completed 2 years of treatment (1 year in the core and one year in the extension portion of the study). Over 24 months, the mean dose of deferasirox was 22.8 mg/kg/day and the mean transfusion rate was 4 units/month. Serum ferritin results are available from 50 pts who have received deferasirox for 100 weeks (Figure). The mean serum ferritin level decreased significantly from study baseline: 3002 to 2069 μg/L at 100 weeks (Δ=933 μg/L; P<0.001, signed rank test).
Six (7%) pts experienced hematological improvement according to IWG 2000 criteria. Three patients experienced an erythroid response (major, n=2; minor n=1); only one who achieved a minor response received MDS treatment with darbepoetin. Others included major platelet response (n=1; not receiving MDS treatment), major neutrophil response (n=1; not receiving MDS treatment) and one combined major platelet and neutrophil response while receiving G-CSF and decitabine treatment.
Of 83 pts, 35 (42%) discontinued from the study: 8 (9.6%) due to abnormal laboratory values (increase of creatinine 6, thrombocytopenia 1, and neutropenia and thrombocytopenia 1), 8 (9.6%) due to adverse events (gastrointestinal symptoms 3, Clostridium dificile infection 1, renal dysfunction 1, cardiac symptoms 1, unknown 1), 11 (13.2%) due to serious AEs with an outcome of death (none of the deaths were related to deferasirox) and 8 (9.6%) due to MDS progression/AML. Of 67 pts with normal baseline SCr, 24 (30%) had an increase in SCr values above the ULN on at least two occasions (3.0 mg/dL max SCr). Of 16 pts with abnormal baseline SCr, one had an increase to above the ULN on at least two occasions. New onset of grades 3 and 4 thrombocytopenia and neutropenia occurred in 15 (18%) and 29 (35%) pts, respectively.
In pts with lower-risk MDS and iron overload, deferasirox significantly reduced serum ferritin over 2 years. Deferasirox was generally well tolerated over 2 years. The final year of this study will continue to assess the long-term safety and efficacy of deferasirox in these lower-risk MDS pts with iron overload.
Raza:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Esposito:Novartis Pharmaceuticals: Employment. Martinez-Lopez:Novartis Pharmaceuticals: Employment. Paley:Novartis Pharmaceuticals: Employment, Equity Ownership. Besa:Novartis: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal