Abstract
Abstract 3942
Poster Board III-878
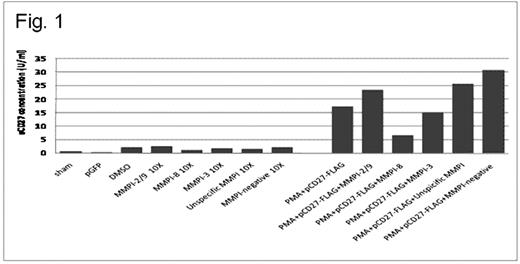
Waldenström's macroglobulinemia (WM) is characterized by bone marrow infiltration with lymphoplasmacytic cells (LPCs), and increased presence of mast cells. CD27 is a marker of memory B-cells, and is heterogeneously expressed on the cell surface of WM cells. In recent studies, we showed that the soluble CD27 (sCD27) is produced by WM LPC and is a faithful marker of disease burden in WM patients. Importantly, sCD27 triggers via CD70 the expression of CD40L on neighboring Mast Cells, which in turn supports the growth and survival of WM LPC. sCD27 therefore represents a promising target for the treatment of WM. The mechanism for sCD27 release in WM remains to be elucidated, but is likely cleaved from cell surface CD27. We therefore examined the effects of various matrix metalloproteinase (MMP) inhibitors on the production of sCD27. sCD27 levels were measured by ELISA from supernatants of cultured BCWM.1 and BL2126 B-cells transfected with a vector expressing either FLAG-tagged CD27 (pCD27-FLAG) or a control vector. PMA treatment of pCD27-FLAG transfected BCWM.1 WM and BL2126 cells led to sustained sCD27 release in cultured supernatants; in contrast, PMA treatment of control vector transfected cells showed minimal sCD27 release. Importantly, treatment of pCD27-FLAG transfected BCWM.1 WM and BL2126 cells with MMP 8 and MMP 3 inhibitors (Calbiochem) led to four-fold and two-fold decrease in sCD27 levels, respectively (Figure 1). In contrast, no significant change in sCD27 release was observed following treatment with MMP 2/9 or non-specific MMP inhibitors. These studies demonstrate that matrix metalloproteinases play an important role in sCD27 release, and provide the framework for efforts examining MMP 8 and 3 specific inhibitors in the treatment of WM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal