Abstract
Abstract 429
Patients with MM who have progressed after multiple novel agents have limited treatment options. Pomalidomide (CC4047) is the newest immunomodulatory (IMiD) agent that has demonstrated response rates of 63% in a Phase 2 trial of Pom/dex in patients with relapsed MM (in press, JCO). To better define the response rate in this group, we treated an additional cohort of patients who were truly resistant/refractory to lenalidomide.
To be eligible, patients must:be refractory to lenalidomide therapy defined as relapsing on or within 60 days of stopping lenalidomide. Pomalidomide was given orally 2 mg daily on days 1–28 of a 28-day cycle. Dexamethasone was given orally at a dose of 40 mg daily on days 1, 8, 15 and 22 of each cycle. Response was assessed by the International Myeloma Working Group Uniform Response criteria. All patients received aspirin 325 mg daily as prophylaxis against DVT. Pom/dex regimen would be declared promising if we observed at least 4 confirmed responses out of 34 patients based on a single stage Fleming design.
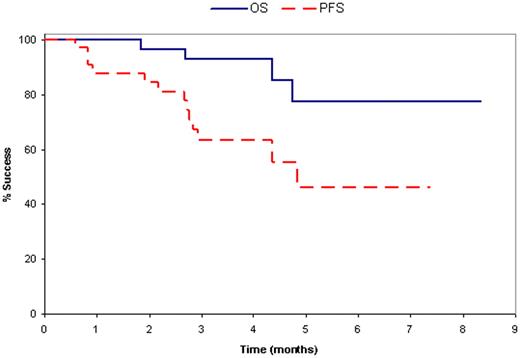
34 patients (23 male and 11 female) resistant/refractory to lenalidomide were enrolled. The median age was 62 years (range, 39-77). The median time from diagnosis to enrollment was 62 months (range 8-179). 12 patients had high risk molecular markers including loss of 17p (6 pts), t(4;14) (1 pt) and del 13 by cytogenetics (5 pts). 70% had ≥3 prior regimens including 38% with ≥5 prior regimens; 56% had prior thalidomide, 59% had prior bortezomib, and 68% had prior SCT. Toxicity (at least possibly related to treatment) was mild and consisted primarily of myelosuppression: grade 3/4 neutropenia (21%); grade 3 thrombocytopenia (9%); and grade 3 anemia (12%). Grade 3/4 non-hematologic toxicities occurred in 24%: pneumonia (3%), non-infectious pneumonitis (3%), hyperglycemia (3%), edema(3%), fatigue(9%) skin rash(3%). Neuropathy occurred in 18% (12% grade 1; 6% grade 2). No thromboembolic events have occurred so far. Nine (26%) patients were confirmed responders (all PR), surpassing the threshold for a positive trial, and 18 (53%) had SD. With a median follow-up of 4 months (range:0-8), 65% remain progression free; 88% remain alive; and 12% have died, all due to progressive MM.
Pom/dex is active and well tolerated in this heavily pre-treated population of lenalidomide-refractory MM patients. The majority of patients have not progressed and objective responses are seen in 26%. This study confirms that pomalidomide has non-cross resistance with lenalidomide and offers benefit for patients who have relapsed after other novel therapies.
Lacy:Celgene: Research Funding. Gertz:celgene: Honoraria; millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kumar:celgene: Honoraria; millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bergsagel:Genentech, Inc.: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Merck: Research Funding. Stewart:Takeda-Millenium, Celgene, Novartis, Amgen: Consultancy; Takeda, Millenium: Research Funding; Genzyme, Celgene, Millenium, Proteolix: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal