Abstract
It has been suggested that mast cells might serve, under certain circumstances, as antigen-presenting cells (APCs) for T cells. However, whether cognate interactions between mast cells and class II–restricted CD4+ T cells actually occur is still an open question. We addressed this question by using peritoneal cell–derived mast cells (PCMCs) and freshly isolated peritoneal mast cells as APC models. Our results show that in vitro treatment of PCMCs with interferon-γ and interleukin-4 induced surface expression of mature major histocompatibility complex class II molecules and CD86. When interferon-γ/interleukin-4–primed PCMCs were used as APCs for CD4+ T cells, they induced activation of effector T cells but not of their naive counterparts as evidenced by CD69 up-regulation, proliferation, and cytokine production. Confocal laser scanning microscopy showed that CD4+ T cells formed immunological synapses and polarized their secretory machinery toward both antigen-loaded PCMCs and freshly isolated peritoneal mast cells. Finally, on cognate interaction with CD4+ T cells, mast cells lowered their threshold of activation via FcϵRI. Our results show that mast cells can establish cognate interactions with class II–restricted helper T cells, implying that they can actually serve as resident APCs in inflamed tissues.
Introduction
Mast cells are known to play a pivotal role in allergic hypersensitivity reaction and, more generally, in inflammation.1,2 By virtue of their strategic and widespread location in tissues, namely at host-environment interface, and of their functional characteristics, mast cells behave as sentinels of the immune system.3 Once activated, mast cells release several preformed and de novo–synthesized mediators (including histamine, proteases, leukotrienes, prostaglandins, and various cytokines and chemokines), resulting in the recruitment and activation of other immune cells.3
Several lines of evidence highlight an emerging role of mast cells in numerous steps of innate and adaptive immune responses, indicating that their contribution to immunity goes far beyond their well-known role in allergy.3-8 Functional interplay between mast cells and T cells has been suggested by studies that document colocalization of activated mast cells and T cells in inflamed tissues9,10 or involvement of mast cells in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis.11-13 Additional lines of evidence show that mast cells can contribute to the development of different T cell–associated responses by influencing the activation, the proliferation, the differentiation, and the recruitment of T cells.7,14,15 Recent in vitro studies showed that IgE-activated mast cells can enhance T-cell proliferation by a mechanism involving tumor necrosis factor α (TNF-α) secretion, cell contact, and mast cell expression of OX40L.6,7 In turn, it has been shown that activated T cells can induce in vitro histamine, TNF-α, matrix metalloproteinase-9 secretion, and interleukin 4 (IL-4) mRNA expression in mast cell subsets.16-18 Finally, mast cells have been reported to promote in vivo T-cell migration to inflammatory sites by secreting chemotactic factors, such as lymphotactin and IL-1619 and to orient Th differentiation via the production of IL-4 and histamine.20,21 Taken together these studies highlight the existence of functionally important mast cell/T-cell crosstalk and raise the question of whether mast cell/T-cell cognate interactions might occur in the course of immune responses.
Immunological synapses (ISs) are the morphologic manifestation of the cognate interactions occurring between T cells and other cells of the immune system serving as antigen-presenting cells (APCs). These specialized areas of signal transduction, formed at the T cell/APC contact site, are characterized by large scale clustering and segregation of surface molecules and intracellular signaling components.22-24 Among the different molecular rearrangements occurring at the IS, the polarization of T-cell Golgi apparatus toward the APCs for polarized secretion is a distinctive, rapid, and efficient T-cell response, occurring within minutes after T cell/APC encounter both in resting and activated CD4+ and CD8+ T cells.25-27 It is, therefore, considered, together with T-cell receptor (TCR) enrichment into the IS, a morphologic hallmark of specific TCR engagement.
To investigate whether cognate interactions could be established at the contact site between CD4+ T cells and mast cells, resulting in the formation of ISs and in T-cell polarization toward the APCs, we used, as potential APCs for ovalbumin (OVA)–specific CD4+ (OT-II) T cells, (1) a recently described cellular model (peritoneal cell–derived mast cells, PCMCs) based on the expansion in vitro, for several weeks, of murine peritoneal mast cells,28 and (2) freshly isolated peritoneal mast cells.
Our results show that, after priming with a combination of interferon-γ (IFN-γ) and IL-4, a fraction of PCMCs express mature major histocompatibility complex (MHC) class II molecules, present antigenic determinants to OT-II T cells, and elicit functional responses in effector T cells but not in their naive counterparts. We also show the formation of ISs and T-cell polarization at the contact site between T cells and mast cells (both PCMCs and freshly isolated peritoneal mast cells), thus unambiguously illustrating the formation of cognate interaction between these cells. Our results show that, although less efficiently than professional APCs, mast cells can indeed present antigens to T cells, resulting in antigen-dependent cell-cell cooperation.
Methods
Abs and cell-surface immunofluorescence
The following Abs were purchased from eBioscience: fluorescein isothiocyanate (FITC)–anti-FcϵRI (MAR-1), FITC–anti-CD40 (HM40-3), PE–anti-CD44 (IM7), FITC–anti-CD117 (ACK2), FITC–anti-CD69 (H1.2F3), FITC–anti-CD80 (16-10A1), phycoerythrin (PE)–anti-CD86 (GL-1), PE–anti-ICOSL (HK53), PE–anti-OX40L (RM134L), FITC–anti-IAb (M5/114), PE–anti-Vα2 (B20.1), anti–4-1BBL (TKS-1), FITC–anti-rat IgG, and isotype-matched control Ig. Biotinylated anti-IAb mAb (KH74) was purchased from BioLegend. In direct immunofluorescence experiments, cells were blocked with 2.4G2 hybridoma supernatant for 15 minutes at 4°C (gift from Dr Joost van Meerwijk, Inserm U563); otherwise, cells were blocked with phosphate-buffered saline (PBS) containing 10% normal goat serum and 10% normal mouse serum. Antibodies were added in PBS containing 1% bovine serum albumin (BSA) and incubated for 30 minutes at 4°C. Flow cytometric data were acquired on a BD FACScan cytometer with the use of BD CellQuest software (Version 4.0) and were analyzed with the use of FlowJo software (Version 7.2.5; TreeStar Inc).
Mice
C57BL/6 male C57BL/6 OT-II mice aged 6 to 12 weeks old and synthetic peptide corresponding to amino acid residues 323 to 339 (ISQAVHAAHAEINEAGR) of ovalbumin (OVAp) were kindly provided by Dr S. Guerder (Inserm U563). All mice used in this study were raised and housed under specific pathogen–free conditions and handled according to protocols approved by the Inserm Ethics Committee on Animal Experimentation in compliance with European Union guidelines.
Generation of PCMCs
Peritoneal cells from C57BL/6 mice were collected by peritoneal washing with 4 mL of RPMI 1640. Cells were washed and seeded in a 24-well culture plate at 106/mL in Opti-MEM supplemented with 10% FCS, 100 IU/mL penicillin, 100 μg/mL streptomycin (Invitrogen), 3% supernatant of Chinese hamster ovary transfectants secreting murine stem cell factor (a gift from Dr P. Dubreuil, Inserm U891). Twenty-four hours later, nonadherent cells were removed, and fresh culture medium was added to adherent cells. Three days later, nonadherent cells and adherent cells harvested by flushing were pelleted and resuspended (3 × 105/mL) in fresh culture medium. The same procedure was repeated twice a week. Cells were used for experiments between week 4 and week 10. PCMCs were characterized by histochemistry: cell suspensions were cytocentrifuged onto glass slides, air-dried, and stained with toluidine blue or Alcian blue followed by Safranin.29
Ex vivo peritoneal mast cells
Peritoneal cells from 20 C57BL/6 mice were collected by peritoneal washing. Nonadherent cells were washed in magnetic-activated cell sorting buffer, and CD117+ cells were positively selected by using CD117 MicroBeads (Miltenyi Biotec) according to the manufacturer's protocol. Mast cell–enriched cell population was immediately primed with 50 ng/mL IFN-γ and 10 ng/mL IL-4 for 72 hours in complete Opti-MEM and used as APCs.
Degranulation assay
Mast cell degranulation was determined by measuring the release of β-hexosaminidase. PCMCs were incubated in culture medium with or without anti–dinitrophenol (DNP) IgE (0.5 μg/mL SPE-7; Sigma-Aldrich) overnight at 37°C. The cells were then washed and distributed in 96-well flat-bottom plates at a density of 105 cells in 50 μL of Tyrode buffer. The cells were adapted to 37°C for 20 minutes and then treated with prewarmed 50 μL of the mentioned stimuli diluted in Tyrode buffer for 45 minutes at 37°C. β-Hexosaminidase release in the supernatants was measured according to Bachelet et al.30
Confocal microscopy
Effector CD4+ OT-II T cells (105) were plated with APCs (2 × 105) either unpulsed or pulsed with 10 μg/mL OVAp or 400 μg/mL ovalbumin in 96-well U-bottom plates and spun to 500 rpm to allow conjugates formation. The cells were transferred onto poly-L-lysine–coated slides and then fixed with 4% paraformaldehyde in PBS. Cells were first permeabilized and blocked in 10% normal goat serum/10% normal mouse serum in PBS containing 0.5% saponin. Cells were stained with the following primary antibodies in PBS containing 1% BSA, 0.5% saponin 45 minutes at room temperature: anti–IFN-γ (clone AN-18), anti-CD3ϵ (clone 17A2), anti-p56lck (clone 28/lck) from BD PharMingen; polyclonal anti–protein kinase C (PKC) θ (Santa Cruz Biotechnology Inc); and anti–α-tubulin (clone DM1A; Sigma-Aldrich). In some experiments, 2 μg/mL avidin-sulforhodamine 101 (highly cationic glycoprotein that selectively stains mast cell granules31,32 ; Sigma-Aldrich) was added to unambiguously identify mast cells. After washing, secondary antibodies (Alexa Fluor–conjugated; Invitrogen) were applied in PBS containing 1% BSA, 0.5% saponin. In some experiments, PCMCs were labeled with anti–mast cell tryptase polyclonal rabbit Ab (Santa Cruz Biotechnology Inc). The samples were mounted and examined with the use of a Zeiss LSM 510 or a Zeiss LSM 710 confocal microscope with a 63× Plan-Apochromat objective (1.4 oil), electronic zoom 3, as described.27 Scoring of the slides was performed in a blinded fashion by evaluating for each condition at least 100 conjugates in randomly selected fields from at least 3 independent experiments.
Results
Phenotypic and functional characterization of murine PCMCs
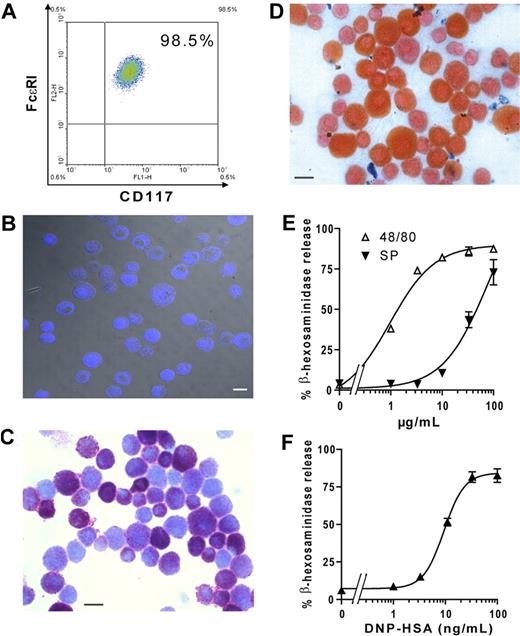
Cells obtained from peritoneal washing of C57BL/6 mice were cultured in the presence of stem cell factor to generate PCMCs as described by Malbec et al.28 After 1 month in culture, more than 98% of the cells expressed on their surface characteristic markers of mast cells, such as FcϵRI and CD117 (Figure 1A), and stained positive for mast cell tryptase (Figure 1B) as previously described for PCMCs.28 None of these cells were positive for B220 or CD11c (data not shown).
Phenotypic and functional characterization of PCMCs. (A) CD117 and FcϵRI expression on PCMC surface. The number in the top right indicates the percentage of double-positive cells. (B) Mast cell tryptase staining by indirect immunofluorescence with an Alexa 633–conjugated secondary Ab (blue). Samples were inspected with a confocal laser-scanning microscope. (C-D) Histochemical staining of PCMCs with toluidine blue (C) or Alcian blue followed by safranin (D). Bars = 10 μm. (E) Secretagogue-induced β-hexosaminidase release. PCMCs were stimulated with increasing concentrations of Substance P or compound 48/80 for 30 minutes at 37°C. (F) FcϵRI-dependent β-hexosaminidase release. PCMCs were sensitized with mouse IgE anti-DNP and challenged with DNP-HSA. The percentage of β-hexosaminidase released was plotted against the concentration of the stimulus. Tests for phenotypic and functional analysis of PCMCs were routinely performed after 3 to 5 weeks of culture. Data are from 1 representative experiment of 3.
Phenotypic and functional characterization of PCMCs. (A) CD117 and FcϵRI expression on PCMC surface. The number in the top right indicates the percentage of double-positive cells. (B) Mast cell tryptase staining by indirect immunofluorescence with an Alexa 633–conjugated secondary Ab (blue). Samples were inspected with a confocal laser-scanning microscope. (C-D) Histochemical staining of PCMCs with toluidine blue (C) or Alcian blue followed by safranin (D). Bars = 10 μm. (E) Secretagogue-induced β-hexosaminidase release. PCMCs were stimulated with increasing concentrations of Substance P or compound 48/80 for 30 minutes at 37°C. (F) FcϵRI-dependent β-hexosaminidase release. PCMCs were sensitized with mouse IgE anti-DNP and challenged with DNP-HSA. The percentage of β-hexosaminidase released was plotted against the concentration of the stimulus. Tests for phenotypic and functional analysis of PCMCs were routinely performed after 3 to 5 weeks of culture. Data are from 1 representative experiment of 3.
PCMCs were further characterized by toluidine blue staining and Alcian blue/safranin staining, a procedure commonly used to discriminate mast cells of the mucosal type (Alcian blue high, safranin low) from connective tissue—type mast cells (CTMCs; Alcian blue low, safranin high29,33 ). Toluidine blue staining for metachromatic granules was positive, with approximately 40% of the cells showing a stronger staining compared with the remaining cells (Figure 1C). Alcian blue/safranin staining showed that all PCMCs counterstained red with safranin, whereas they were either negative or faintly positive for Alcian blue, indicating that these cells were mostly of the CTMC-like phenotype (Figure 1D). Interestingly, similarly to toluidine blue staining, also safranin staining was heterogeneous: approximately 40% of the PCMCs exhibited a stronger safranin staining than did the remaining cells (Figure 1D). Taken together toluidine blue and safranin staining suggested that, among the PCMCs, approximately 40% of cells had a more mature phenotype, characterized by a higher content of mature secretory granules.
We next investigated PCMC biologic function by using a standard degranulation assay. We used 2 classes of stimuli: polycationic compounds, such as compound 48/80 and substance P, that induce degranulation specifically in CTMCs (and not in mucosal-type mast cells) or antigen (dinitrophenylated human serum albumin [DNP-HSA]) in IgE-sensitized mast cells. When PCMCs were incubated with increasing concentrations of 48/80 or substance P, they exhibited a strong and dose-dependent degranulation as detected by β-hexosaminidase release (Figure 1E). Moreover, PCMCs, previously sensitized with anti-DNP IgE, degranulated in a dose-dependent fashion when incubated with increasing concentrations of DNP-HSA (Figure 1F). Together these results indicated that PCMCs exhibit connective tissue mast cell functional properties.
Treatment with IFN-γ and IL-4 induces MHC class II molecule expression on PCMC surface
Having phenotypically and functionally characterized PCMCs, we used these cells to investigate whether they could be primed to become APCs for class II–restricted T lymphocytes.
PCMCs expressed neither surface MHC class II molecules nor costimulation molecules (Figure 2). We thus investigated whether these cells might be induced to express class II molecules by using treatments known to prime conventional APCs. Treatment of PCMCs with Toll-like receptor ligands (different lipopolysaccharide preparations from Gram-negative bacteria, peptidoglycan, poly I:C, lipoteichoic acid) added to PCMCs for 24, 48, 72, or 96 hours did not result in a detectable increase in I-Ab expression (data not shown).
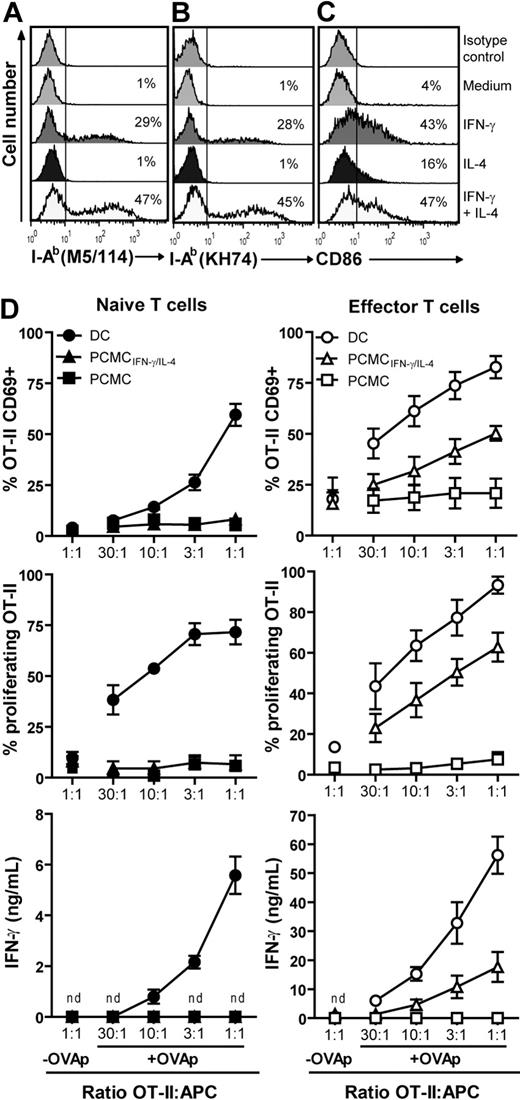
IFN-γ/IL-4–primed PCMCs express MHC class II and CD86 and induce functional responses in effector OT-II T cells but not in their naive counterparts. (A-C) PCMCs were incubated 72 hours with IFN-γ (50 ng/mL) or IL-4 (10 ng/mL) or with IFN-γ + IL-4. CD86 and I-Ab molecule expression (using the KH74 or M5/114 mAb) was analyzed by flow cytometry. The numbers inside the panels indicate the percentage of positive cells. Data are representative of at least 10 experiments. (D) APCs were pulsed or not for 16 hours with 10 μg/mL OVAp and cocultured with naive (left) or effector (right) OT-II T cells. OT-II T-cell activation was monitored by CD69 expression (after 20 hours of coculture), IFN-γ production (after 20 hours of coculture), and proliferation (after 72 hours of coculture). Results are presented as mean ± SEM of 4 independent experiments. nd indicates not detected.
IFN-γ/IL-4–primed PCMCs express MHC class II and CD86 and induce functional responses in effector OT-II T cells but not in their naive counterparts. (A-C) PCMCs were incubated 72 hours with IFN-γ (50 ng/mL) or IL-4 (10 ng/mL) or with IFN-γ + IL-4. CD86 and I-Ab molecule expression (using the KH74 or M5/114 mAb) was analyzed by flow cytometry. The numbers inside the panels indicate the percentage of positive cells. Data are representative of at least 10 experiments. (D) APCs were pulsed or not for 16 hours with 10 μg/mL OVAp and cocultured with naive (left) or effector (right) OT-II T cells. OT-II T-cell activation was monitored by CD69 expression (after 20 hours of coculture), IFN-γ production (after 20 hours of coculture), and proliferation (after 72 hours of coculture). Results are presented as mean ± SEM of 4 independent experiments. nd indicates not detected.
We next tested whether IFN-γ and IL-4 could induce I-Ab on the PCMC surface. After 72 hours of culture with 50 ng/mL IFN-γ, a significant fraction of PCMCs (20%-35%) expressed I-Ab (Figure 2A-B). Interestingly, although the addition of 10 ng/mL IL-4 alone to PCMC cultures did not affect I-Ab expression level, it increased the frequency of IFN-γ–induced MHC class II expression, resulting in 40% to 50% MHC class II+ PCMCs (Figure 2A-B). Moreover, the level of I-Ab expression per cell was also significantly increased. I-Ab expression was assessed by using 2 different monoclonal antibodies: M5/114 that recognizes mature and immature forms of MHC class II molecules and KH-74 that mainly binds mature forms of MHC class II.34 Under these conditions, although individual class II+ PCMCs expressed fairly high levels of I-Ab, approximately 50% of the PCMC population remained I-Ab negative (Figure 2A-B).
We next investigated by fluorescence-activated cell sorting (FACS) analysis whether IFN-γ plus IL-4 could induce the expression of costimulatory molecules. Untreated PCMCs were negative for several costimulation molecules: CD80, CD86, CD40, ICOSL, 4-1BB-L, and OX40L (Figure 2; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Treatment with IFN-γ plus IL-4 resulted in the up-regulation of the surface expression of CD86 (Figure 2C) but did not enhance the expression of the other costimulation molecules tested (supplemental Figure 1). To test whether the PCMC population used in this study, although expressing phenotypic and histochemical markers of mast cells, could result from the skewing of peritoneal mast cells toward monocytes, we measured the surface expression of CD11b and F4/80 before and after treatment with IFN-γ plus IL-4. This analysis showed that neither untreated PCMCs nor cytokine-treated PCMCs expressed detectable levels of CD11b and of F4/80, ruling out the contribution of monocyte-like cells to the T-cell activation we observed (supplemental Figure 2). Together the above-mentioned results indicate that a fraction of the whole PCMC population treated with IFN-γ plus IL-4 (further referred to as PCMCsIFN-γ/IL-4) develop into potential APCs.
PCMCsIFN-γ/IL-4 can serve as APCs for CD4+ T lymphocytes
To examine the antigen-presenting function of PCMCs, we investigated whether they could present the OVAp to CD4+ T cells from OT-II TCR transgenic mice. When freshly purified naive OT-II T cells where cocultured overnight with unprimed PCMCs or with PCMCsIFN-γ/IL-4 either unpulsed or pulsed with OVAp at different T cell/APC ratios, they did not undergo detectable up-regulation of the T-cell activation marker CD69. Accordingly, neither proliferation nor IFN-γ production (measured after 3 days of coculture) was detected (Figure 2D). Conversely, these cells exhibited CD69 up-regulation, proliferation, and IFN-γ production when cocultured with OVAp-pulsed mature dendritic cells (DCs; Figure 2D).
We next tested whether PCMCsIFN-γ/IL-4 could serve as APCs for effector T cells. To this end, OT-II T cells were expanded with anti-CD3/anti-CD28 coated beads for 8 to 10 days before coculture with PCMCsIFN-γ/IL-4 or mature DCs either unpulsed or pulsed with OVAp. These previously activated T cells exhibited effector T-cell phenotype CD62L−CD44high (data not shown). As shown in Figure 2D, effector OT-II T cells up-regulated CD69, proliferated and produced IFN-γ when interacting with OVAp-pulsed DCs, and, although to a lesser extent, also with OVAp-pulsed PCMCs. Similar results were obtained when IL-2 production was measured; conversely, in the same conditions, we did not detect IL-4 production by OT-II T cells (data not shown). Peptide-pulsed PCMCs not primed with IFN-γ and IL-4 were unable to induce detectable responses in the same OT-II T-cell population (Figure 2D).
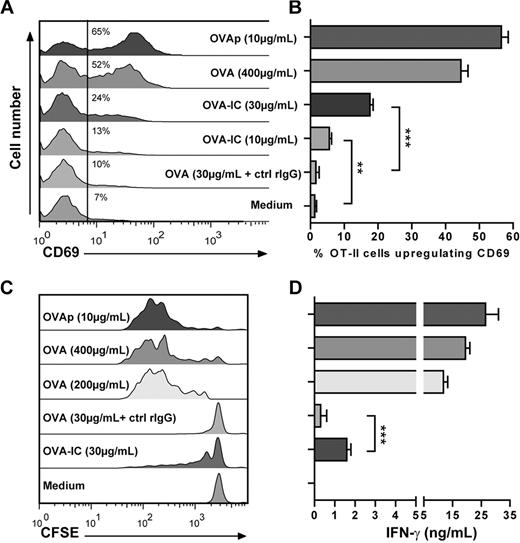
To further characterize the APC potential of PCMCs we investigated whether they could capture and process ovalbumin. PCMCsIFN-γ/IL-4 or DCs were incubated 16 hours with ovalbumin alone or with ovalbumin complexed with IgG, and their capacity to process and present ovalbumin was assessed by measuring CD69 up-regulation in effector OT-II T cells. As shown in Figure 3A and B, PCMCsIFN-γ/IL-4 incubated with a high concentration of ovalbumin (400 μg/mL) efficiently activated OT-II T cells to CD69 up-regulation, indicating that PCMCsIFN-γ/IL-4 can internalize and process native antigens. When incubated with a lower concentration of ovalbumin (30 μg/mL) in the presence of irrelevant rabbit IgG, PCMCsIFN-γ/IL-4 failed to induce CD69 up-regulation in OT-II T cells. Conversely, PCMCs were able to present the antigenic determinant to OT-II T cells when pulsed with the same concentration of ovalbumin complexed with anti–ovalbumin IgG, indicating that PCMCsIFN-γ/IL-4 can uptake antigens via an IgG-assisted mechanism. Similar results were obtained when T-cell proliferation (Figure 3C) and IFN-γ production (Figure 3D) were measured, indicating that the activation of OT-II T cells by PCMCs presenting a native antigen is not limited to CD69 up-regulation.
IFN-γ/IL-4–primed PCMCs can capture and process of protein antigen. (A-D) IFN-γ/IL-4–primed PCMCs were incubated for 16 hours with soluble OVA, OVA-IgG immune complexes (ICs), or soluble OVA in the presence of control rabbit IgG (ctrl rIgG) or OVAp. (A-B) Cells were cocultured with effector OT-II T cells for 16 hours, and CD69 up-regulation on OT-II T-cell surface was measured by FACS analysis on Vα2+ gated cells. (A) FACS profiles representative of 3 independent experiments. The numbers inside the panel indicate the percentage of positive cells. (B) Data are presented as percentage of Vα2+ gated cells up-regulating CD69 (mean ± SEM of 3 independent experiments). (C) Proliferation of T cells was monitored by carboxyfluorescein succinimidyl ester dilution after 72 hours of coculture. Panels show FACS profiles from 1 representative experiment of 3. (D) IFN-γ in supernatant was dosed by ELISA after 16 hours of coculture (mean ± SEM of 3 independent experiments). Difference between groups was evaluated by an unpaired Student t test with the GraphPad Prism software. It should be noted that DCs were more efficient than PCMCs in inducing functional responses to whole ovalbumin in OT-II T cells. Approximately 95% of T cells proliferated when cultured with DCs previously incubated with OVA (400 μg/mL) and approximately 85% of T cells proliferated when cultured with DCs previously incubated with IgG-OVA (30 μg/mL). IFN-γ production by OT-II T cells when cultured with DCs pulsed with OVA (400 μg/mL) or IgG-OVA (30 μg/mL) was approximately 50 ng/mL and approximately 10 ng/mL, respectively.
IFN-γ/IL-4–primed PCMCs can capture and process of protein antigen. (A-D) IFN-γ/IL-4–primed PCMCs were incubated for 16 hours with soluble OVA, OVA-IgG immune complexes (ICs), or soluble OVA in the presence of control rabbit IgG (ctrl rIgG) or OVAp. (A-B) Cells were cocultured with effector OT-II T cells for 16 hours, and CD69 up-regulation on OT-II T-cell surface was measured by FACS analysis on Vα2+ gated cells. (A) FACS profiles representative of 3 independent experiments. The numbers inside the panel indicate the percentage of positive cells. (B) Data are presented as percentage of Vα2+ gated cells up-regulating CD69 (mean ± SEM of 3 independent experiments). (C) Proliferation of T cells was monitored by carboxyfluorescein succinimidyl ester dilution after 72 hours of coculture. Panels show FACS profiles from 1 representative experiment of 3. (D) IFN-γ in supernatant was dosed by ELISA after 16 hours of coculture (mean ± SEM of 3 independent experiments). Difference between groups was evaluated by an unpaired Student t test with the GraphPad Prism software. It should be noted that DCs were more efficient than PCMCs in inducing functional responses to whole ovalbumin in OT-II T cells. Approximately 95% of T cells proliferated when cultured with DCs previously incubated with OVA (400 μg/mL) and approximately 85% of T cells proliferated when cultured with DCs previously incubated with IgG-OVA (30 μg/mL). IFN-γ production by OT-II T cells when cultured with DCs pulsed with OVA (400 μg/mL) or IgG-OVA (30 μg/mL) was approximately 50 ng/mL and approximately 10 ng/mL, respectively.
These results show that PCMCsIFN-γ/IL-4 can capture and process native antigens and can present antigenic peptides to effector CD4+ T lymphocytes, leading to cytokine production and proliferation.
ISs and T-cell polarization responses at the mast cell/T-cell contact site
Taken together the above-mentioned results show that IFN-γ/IL-4–primed PCMCs can activate CD4+ T cells in an antigen-dependent fashion. These observations do not formally rule out the possibility that bystander professional APCs (possibly present as contaminants of T-cell purification) could uptake antigenic determinants and in turn present them to T cells.35
To address this point we aimed to directly visualize T cell/PCMC cognate interactions. In a first round of experiments we measured the formation of conjugates between OT-II T cells and PCMCsIFN-γ/IL-4 by FACS analysis. As shown in supplemental Figure 3, OT-II T cells formed stable conjugates with PCMCsIFN-γ/IL-4 (lasting up to 1 hour). Conjugate formation was more pronounced with antigen-pulsed PCMCs than with unpulsed PCMCsIFN-γ/IL-4 (supplemental Figure 3).
We next investigated by confocal microscopy the capacity of OT-II T cells to form ISs with PCMCs. To this end, OT-II T cells were cocultured with PCMCsIFN-γ/IL-4 for 20 minutes and stained with anti–phosphotyrosine (PTyr) antibodies to detected PTyr staining (an early marker of signaling at the IS27 ) and with Alexa 488–labeled phalloidin (to detect F-actin). Confocal microscopy analysis showed that OT-II T cells interacting with ovalbumin-pulsed PCMCsIFN-γ/IL-4 exhibited F-actin (∼ 37% of scored conjugates) and PTyr enrichment (∼ 22% of scored conjugates) at the synaptic area (supplemental Figure 4).
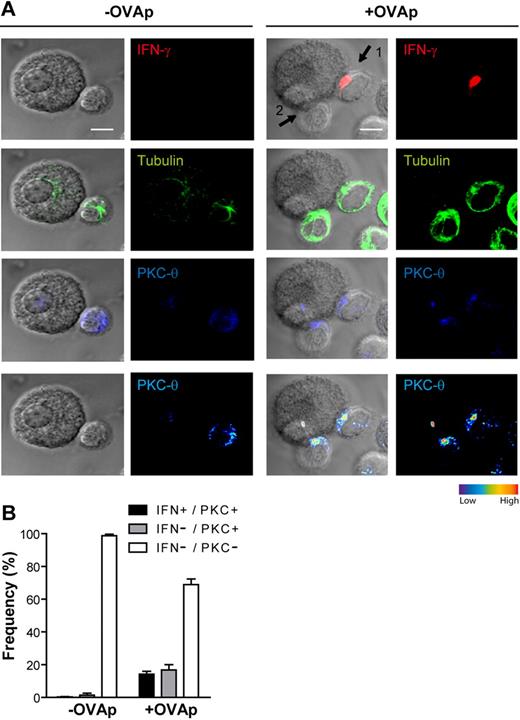
To further characterize T-cell activation during interaction with PCMCs, compared with the activation induced by professional APCs, we focused on later steps of the TCR-mediated signaling cascade. OT-II T cells were cocultured with PCMCsIFN-γ/IL-4 or mature DCs (either unpulsed or OVAp-pulsed) for 2.5 hours; cells were fixed, permeabilized, and stained with anti-PKCθ, anti-tubulin, and anti–IFN-γ antibodies to detect sustained signaling at the ISs and polarization of the T-cell secretory machinery toward the APCs. As shown in supplemental Figure 5, T cells interacting with OVAp-pulsed DCs exhibited enrichment of PKCθ (∼ 60% of scored conjugates) and polarization of IFN-γ secretion (∼ 40% of scored conjugates) toward the APCs. Interestingly, also at the T cell/PCMC contact site a significant number of conjugated T cells enriched PKCθ and polarized IFN-γ toward PCMCs (∼ 33% enriched PKCθ and ∼ 16% enriched PKCθ and polarized IFN-γ of 300 total scored T cells from 3 independent experiments, Figure 4). The above-mentioned results show that, among OT-II T cells exhibiting an enrichment of PKCθ at the IS, a limited fraction only polarized IFN-γ toward PCMCs. This observation can be explained by the fact that the T-cell lines were expanded in nonpolarizing conditions; thus, only a fraction of T cells was of the Th1 phenotype. In some experiments, to better show the establishment of functional ISs between mast cells and T cells, cell conjugates were simultaneously stained with anti-PKCθ, anti–IFN-γ, and avidin-sulforhodamine 101 (which stains mast cell granules31,32 ). This triple staining unambiguously showed T cell/mast cell cognate interactions (supplemental Figure 6). Similar results were obtained when mast cell tryptase staining was used to identify PCMCs (data not show).
CD4+ T cells polarize their secretory machinery toward IFN-γ/IL-4–primed PCMCs. IFN-γ/IL-4–primed PCMCs were pulsed or not with 10 μg/mL OVAp. After washing, APCs were cocultured with effector OT-II T cells for 10 minutes. Cells were settled onto polylysine-coated slides; fixed; and stained for tubulin, PKCθ, and IFN-γ; and analyzed by confocal laser scanning microscopy. (A) Representative staining for IFN-γ (red), tubulin (green), and PKCθ (blue) of a PCMCIFN-γ/IL-4/OT-II T-cell conjugate, each staining is represented alone or merged with DIC images; PKCθ staining is also shown as pseudocolor intensity. Arrow 1 points to a synapse where IFN-γ is polarized toward the PCMCs and PKCθ is enriched at the OT-II T cell/PCMC contact site; arrow 2 points to a synapse where PKCθ only is enriched. (B) OT-II T-cell/PCMC conjugates were randomly selected and scored for the recruitment of PKCθ to the cell–cell contact site either alone or in combination with IFN-γ polarization.  indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
CD4+ T cells polarize their secretory machinery toward IFN-γ/IL-4–primed PCMCs. IFN-γ/IL-4–primed PCMCs were pulsed or not with 10 μg/mL OVAp. After washing, APCs were cocultured with effector OT-II T cells for 10 minutes. Cells were settled onto polylysine-coated slides; fixed; and stained for tubulin, PKCθ, and IFN-γ; and analyzed by confocal laser scanning microscopy. (A) Representative staining for IFN-γ (red), tubulin (green), and PKCθ (blue) of a PCMCIFN-γ/IL-4/OT-II T-cell conjugate, each staining is represented alone or merged with DIC images; PKCθ staining is also shown as pseudocolor intensity. Arrow 1 points to a synapse where IFN-γ is polarized toward the PCMCs and PKCθ is enriched at the OT-II T cell/PCMC contact site; arrow 2 points to a synapse where PKCθ only is enriched. (B) OT-II T-cell/PCMC conjugates were randomly selected and scored for the recruitment of PKCθ to the cell–cell contact site either alone or in combination with IFN-γ polarization.  indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
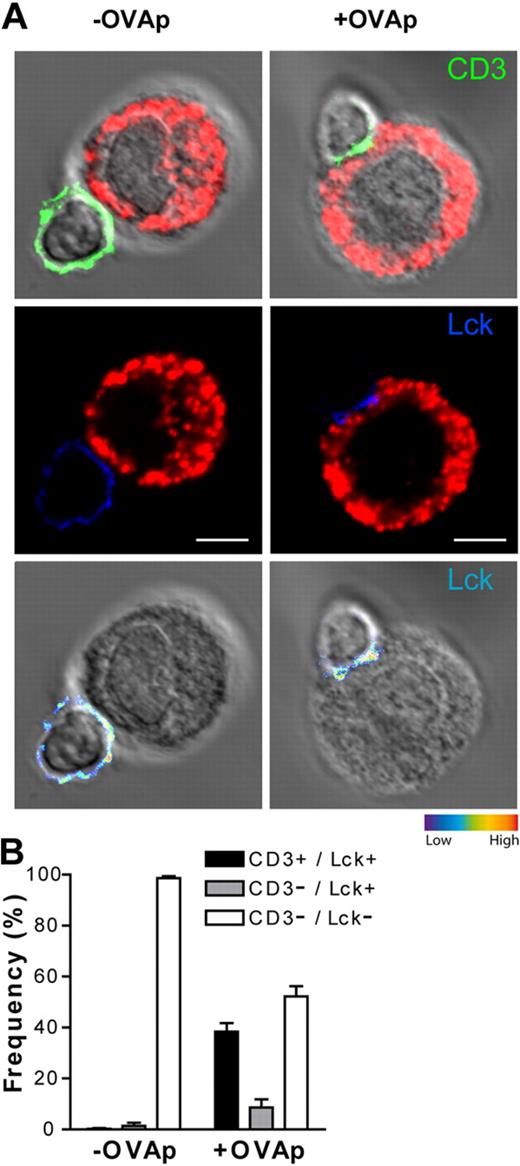
To define whether the observed directionality of the T cell/PCMC interactions was due to specific TCR engagement at the IS, we stained cell conjugates with antibodies directed against the TCR/CD3 complex (anti-CD3ϵ antibodies) and with antibodies against p56lck (a key signaling component of the TCR-mediated signaling cascade). As shown in Figure 5, in a significant fraction of conjugated T cells TCR/CD3 and p56lck were enriched at the contact site with OVAp-pulsed PCMCs (∼ 40% enriched both CD3ϵ and p56lck of ∼ 300 total scored T cells from 3 independent experiments).
Enrichment of TCR/CD3 complex and of p56lck at the T cell/PCMCIFN-γ/IL-4 contact site. IFN-γ/IL-4–primed PCMCs were pulsed or not with 10 μg/mL OVAp. After washing, APCs were cocultured with effector OT-II T cells for 10 minutes. Cells were settled onto polylysine-coated slides, fixed, and stained for mast cell granules with avidin-sulforhodamine 101 (red), CD3ϵ (green), and p56lck (blue). (A) Representative staining for CD3ϵ (green), and p56lck (blue) of a PCMCIFN-γ/IL-4/OT-II T-cell conjugate. Staining is represented merged with DIC images; p56lck staining is also shown as pseudocolor intensity. (B) OT-II T cell/PCMC conjugates were randomly selected and scored for the recruitment of p56lck to the cell-cell contact site either alone or in combination with CD3ϵ.  indicates conjugates exhibiting recruitment of p56lck alone; ■, conjugates exhibiting polarization of both p56lck and CD3ϵ; □, conjugates exhibiting neither p56lck nor CD3 polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
indicates conjugates exhibiting recruitment of p56lck alone; ■, conjugates exhibiting polarization of both p56lck and CD3ϵ; □, conjugates exhibiting neither p56lck nor CD3 polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
Enrichment of TCR/CD3 complex and of p56lck at the T cell/PCMCIFN-γ/IL-4 contact site. IFN-γ/IL-4–primed PCMCs were pulsed or not with 10 μg/mL OVAp. After washing, APCs were cocultured with effector OT-II T cells for 10 minutes. Cells were settled onto polylysine-coated slides, fixed, and stained for mast cell granules with avidin-sulforhodamine 101 (red), CD3ϵ (green), and p56lck (blue). (A) Representative staining for CD3ϵ (green), and p56lck (blue) of a PCMCIFN-γ/IL-4/OT-II T-cell conjugate. Staining is represented merged with DIC images; p56lck staining is also shown as pseudocolor intensity. (B) OT-II T cell/PCMC conjugates were randomly selected and scored for the recruitment of p56lck to the cell-cell contact site either alone or in combination with CD3ϵ.  indicates conjugates exhibiting recruitment of p56lck alone; ■, conjugates exhibiting polarization of both p56lck and CD3ϵ; □, conjugates exhibiting neither p56lck nor CD3 polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
indicates conjugates exhibiting recruitment of p56lck alone; ■, conjugates exhibiting polarization of both p56lck and CD3ϵ; □, conjugates exhibiting neither p56lck nor CD3 polarization. Approximately 100 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
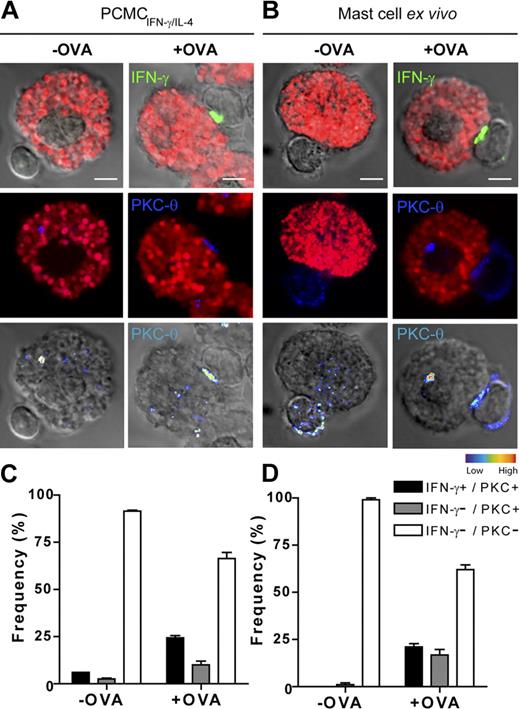
To further document the formation of cognate interactions between OT-II T cells and PCMCs, we investigated the enrichment of PKCθ at the IS and the polarization of the T-cell secretory machinery in conditions in which PCMCsIFN-γ/IL-4 had captured and processed ovalbumin. As shown in Figure 6, OT-II T cells interacting with PCMCs previously incubated for 16 hours with 400 μg/mL ovalbumin (and identified by avidin-sulforhodamine 101 staining) exhibited an enrichment of PKCθ (∼ 35%) and IFN-γ–polarized secretion (∼ 25%) toward the antigen-presenting PCMCs.
IFN-γ/IL-4–primed PCMCs and freshly isolated mast cells process whole ovalbumin and form functional ISs. IFN-γ/IL-4–primed PCMCs (A) or IFN-γ/IL-4–primed peritoneal mast cells (B) were pulsed or not for 16 hours with 400 μg/mL ovalbumin. After washing, APCs were cocultured with effector OT-II T cells for 2.5 hours. Cells were fixed, permeabilized, and stained for mast cell granules with avidin-sulforhodamine 101 (red), PKCθ (blue), and IFN-γ (green) and analyzed by confocal laser scanning microscopy. (Top) IFN-γ (green) and avidin-sulforhodamine 101 (red) are merged with DIC images; (middle) avidin-sulforhodamine 101 (red) and PKCθ staining (blue) are shown; (bottom) PKCθ staining is shown as pseudocolor intensity merged with DIC images. (C-D) For each condition, OT-II T-cell/APC conjugates were randomly selected and scored for the recruitment of PKCθ to the cell–cell contact site either alone or in combination with IFN-γ polarization.  indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 50 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 50 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
IFN-γ/IL-4–primed PCMCs and freshly isolated mast cells process whole ovalbumin and form functional ISs. IFN-γ/IL-4–primed PCMCs (A) or IFN-γ/IL-4–primed peritoneal mast cells (B) were pulsed or not for 16 hours with 400 μg/mL ovalbumin. After washing, APCs were cocultured with effector OT-II T cells for 2.5 hours. Cells were fixed, permeabilized, and stained for mast cell granules with avidin-sulforhodamine 101 (red), PKCθ (blue), and IFN-γ (green) and analyzed by confocal laser scanning microscopy. (Top) IFN-γ (green) and avidin-sulforhodamine 101 (red) are merged with DIC images; (middle) avidin-sulforhodamine 101 (red) and PKCθ staining (blue) are shown; (bottom) PKCθ staining is shown as pseudocolor intensity merged with DIC images. (C-D) For each condition, OT-II T-cell/APC conjugates were randomly selected and scored for the recruitment of PKCθ to the cell–cell contact site either alone or in combination with IFN-γ polarization.  indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 50 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
indicates conjugates exhibiting recruitment of PKCθ alone; ■, conjugates exhibiting polarization of both PKCθ and IFN-γ; □, conjugates exhibiting neither PKCθ nor IFN-γ polarization. Approximately 50 conjugates were analyzed per experiment. Histograms represent means ± SEM of 3 independent experiments. Bar = 5μm.
Finally, we used an additional ex vivo model to investigate T cell/mast cell cognate interactions. In a first set of experiments, freshly isolated peritoneal mast cells were tested for their capacity to activate OT-II T cells. As shown in supplemental Figure 7,interaction of OT-II T cells with freshly isolated mast cells, primed with IFN-γ and IL-4 (under the same conditions as PCMCs) and pulsed with OVAp, resulted in T-cell activation to IFN-γ production and proliferation. We next investigated whether freshly isolated peritoneal mast cells could form ISs with OT-II T cells. Freshly isolated mast cells were IFN-γ/IL-4 primed, cocultured 16 hours with 400 μg/mL ovalbumin, and conjugated for 2.5 hours with OT-II T cells. In these conditions we detected an enrichment of PKCθ (∼ 36%) and polarization of IFN-γ secretion (∼ 20%) toward mast cells (Figure 6), comparable with that observed in OT-II T cells interacting with PCMCs, thus validating the use of PCMCs as a model of antigen-presenting mast cells.
Together these results show that CD4+ T cells form ISs and polarize their secretory machinery toward both peptide-loaded and native antigen-loaded mast cells, providing the first morphologic evidence in support of a role of mast cells as APCs for T lymphocytes.
PCMCs are functionally activated during cognate interaction with OT-II T cells
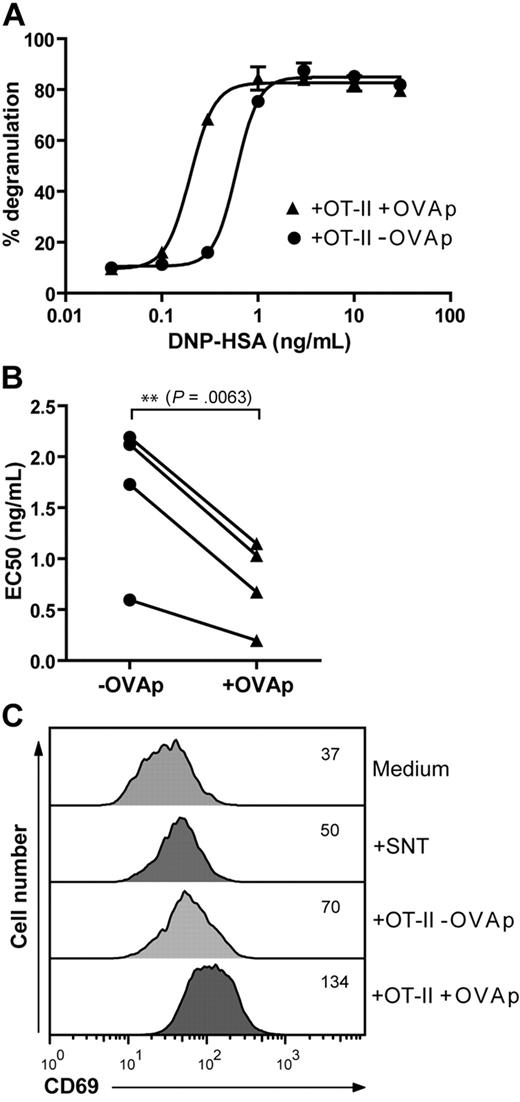
To investigate whether PCMCs are functionally activated during cognate interaction with OT-II T cells, we evaluated the effect of antigen-specific T cell/PCMC interactions on DNP-HSA–induced degranulation of IgE-sensitized mast cells. As shown in Figure 7A and B, PCMCsIFN-γ/IL-4 pulsed with OVAp and cocultured with effector OT-II exhibited lower threshold responses to DNP-HSA stimulation than did unpulsed PCMCsIFN-γ/IL-4. Moreover, PCMCsIFN-γ/IL-4 increased their surface expression of the activation marker CD69 after cognate interaction with OT-II T cells (Figure 7C). CD69 up-regulation could not be mimicked by supernatant from activated OT-II cells, indicating that PCMCIFN-γ/IL-4 activation is not mediated by soluble factors but results from cell–cell contact.
PCMCIFN-γ/IL-4 activation after antigen-specific interaction with OT-II T cells. (A-B) PCMCIFN-γ/IL-4s either unpulsed or pulsed with 10 μg/mL OVAp were cocultured with OT-II T cells in the presence of anti-DNP IgE (1 μg/mL) for 16 hours. (A) Ability to release β-hexosaminidase in response to increasing concentrations of DNP-HSA. Data are from 1 representative experiment of 4 performed in triplicates. (B) EC50 (half maximal effective concentration) of DNP-HSA from 4 independent experiments. Difference between groups was evaluated by a paired Student t test by using GraphPad Prism software. (C) PCMCsIFN-γ/IL-4 either unpulsed or pulsed with 10 μg/mL OVAp were cocultured with OT-II T cells for 16 hours. In parallel samples PCMCs were treated for 16 hours with supernatant from activated OT-II (SNT; 50%, from OT-II cultured for 6 days with anti-CD3/anti-CD28–coated beads) or medium alone. Expression of CD69 on PCMCs was analyzed by flow cytometry; numbers indicate MFI. Data are from 1 representative experiment of 3.
PCMCIFN-γ/IL-4 activation after antigen-specific interaction with OT-II T cells. (A-B) PCMCIFN-γ/IL-4s either unpulsed or pulsed with 10 μg/mL OVAp were cocultured with OT-II T cells in the presence of anti-DNP IgE (1 μg/mL) for 16 hours. (A) Ability to release β-hexosaminidase in response to increasing concentrations of DNP-HSA. Data are from 1 representative experiment of 4 performed in triplicates. (B) EC50 (half maximal effective concentration) of DNP-HSA from 4 independent experiments. Difference between groups was evaluated by a paired Student t test by using GraphPad Prism software. (C) PCMCsIFN-γ/IL-4 either unpulsed or pulsed with 10 μg/mL OVAp were cocultured with OT-II T cells for 16 hours. In parallel samples PCMCs were treated for 16 hours with supernatant from activated OT-II (SNT; 50%, from OT-II cultured for 6 days with anti-CD3/anti-CD28–coated beads) or medium alone. Expression of CD69 on PCMCs was analyzed by flow cytometry; numbers indicate MFI. Data are from 1 representative experiment of 3.
Taken together the above-mentioned results indicate that, on cognate interaction with helper T cells, PCMCs receive stimulatory signals that enhance their biologic functions.
Discussion
In the present study we used PCMCs and freshly isolated peritoneal mast cells, primed with IFN-γ and IL-4, as potential APCs for CD4+ T cells. We report that cognate interactions are formed between mast cells and effector CD4+ T cells, resulting in their activation.
It was previously reported that mouse bone marrow–derived mast cells (BMMCs) could synthesize functional class II molecules36 and could present antigenic peptides to CD4+ T-cell hybridoma.37 However, a recent study by Kambayashi et al35 casted doubts on the possibility that BMMCs might present antigens to CD4+ T cells, by showing that, although BMMCs could uptake antigens, they did not express functional class II molecules on their surface. That study also showed that activation of CD4+ T cells by antigen-loaded BMMCs was indirect and depended on cross-presentation of Ag determinants by adjacent DCs after phagocytosis.35 More recently, the same investigators reported that BMMCs can, under certain circumstances, activate CD4+ T cells via direct antigen presentation, thus reproposing the issue of direct antigen presentation by mast cells.38 In addition, recent studies, by showing that basophils, which are closely related to mast cells, can serve as APCs are able to initiate T helper type 2 responses, support the idea that “nonconventional” APCs can play a central role in the development of adaptive immune-responses.39-41
Our results extend these previous studies and provide experimental evidence of direct antigen presentation by mast cells at the single-cell level by (1) showing antigen presentation by PCMCs, a cellular system that more closely mimics tissue mast cells28 ; (2) presenting the first morphologic evidence of IS formation and T-cell polarization at the T-cell/PCMC contact site; and (3) showing cognate interactions between T cells and freshly isolated peritoneal mast cells.
We present strong morphologic evidence of the antigen-presenting role of individual mast cells. Our results show that T cells exhibit the enrichment of PTyr and F-actin at the contact site with cognate PCMCs, indicating that these early events of IS formation are triggered at the cell-cell interface. We also show the sustained enrichment of PKCθ (a key signaling component of TCR-mediated signal transduction, that is known to translocate for prolonged periods of time at the IS,42-44 at the T cell/mast cell contact site. Finally, we provide evidence for the polarization of tubulin cytoskeleton and of IFN-γ–filled Golgi apparatus toward mast cells and of CD3 and p56lck enrichment at the T cell/mast cell interface, showing that individual T cells in conjugation with mast cells are specifically activated via their TCR.
In the present study only effector (that have low activation threshold45,46 ) and not naive OT-II T cells exhibited functional responses when cocultured with peptide-pulsed PCMCsIFN-γ/IL-4. This selective stimulation of effector cells should be sufficient for an antigen-presenting role of mast cells in vivo, considering their preferential location in tissues and, therefore, their susceptibility to encounter effector lymphocytes.
Our results show that the capacity of mast cells to activate CD4+ T cells is quantitatively relevant, as shown by the amount of IFN-γ released after antigen triggering. However, IFN-γ production remained weaker than that obtained using mature DCs. This can be partially explained by the lower level of expression of MHC class II and costimulation molecules on the PCMC surface and by the fact that only a fraction of PCMCs (30%-45%) expressed MHC class II, probably because of the heterogeneity of the PCMC population (Figure 1).
In our cellular model, the best condition to prime PCMCs for MHC class II expression was 3 days of culture in the presence of IFN-γ and IL-4. The role of IFN-γ in positively regulating MHC class I and II expression on different cell types is well documented.47 Moreover, it is well known that IL-4 and IFN-γ represent the major inducers of class II in the B-lymphoid and monocytic/macrophage lineages, respectively.48,49 Here, we show an intriguing and unexpected cooperation between IL-4 and IFN-γ on the same cellular subset: although IL-4 cannot by itself induce class II expression on PCMCs, it enhances the IFN-γ–induced expression of these molecules. This indicates that IFN-γ is necessary and sufficient to induce MHC class II expression on a fraction of PCMCs, yet the presence of IL-4 optimizes the APC phenotype of these cells. These observations suggest that by responding to both IFN-γ and IL-4 (possibly produced by local Th1 T cells or natural killer T cells, undergoing simultaneous activation in an inflamed tissue), mast cells could provide platforms for antigen presentation to both Th1 and Th2 cells.
What could be the functional role of the observed cognate interactions between mast cells and CD4+ T lymphocytes? We propose that antigen presentation by mast cells to effector T lymphocytes is instrumental to optimize bidirectional crosstalks between these cells in the course of immune responses (supplemental Figure 8).
From the “T helper cell point of view,” the possibility that tissue mast cells might detect, internalize, and present antigens make them important “refueling” cells able to restimulate in situ effector CD4+ T lymphocytes. In other words, mast cells, although less efficient than professional APCs, might offer strategically located platforms for antigen presentation that would locally boost the activation state of effector T lymphocytes, thus favoring their biologic responses. The fact that mast cells produce IL-16 and several chemokines such as CCL3, CCL4, CCL5, CCL203,19 that have specific receptors on effector CD4+ lymphocytes, makes T cell–mast cell encounters in tissues likely.
From the “mast cell point of view,” the observation that cognate interactions can be established with T cells suggests that activated T cells might boost mast cell functions. Evidence in support of this hypothesis comes from our observation that on cognate interaction with OT-II T cells, PCMCs increase their sensitivity to anti-DNP IgE/DNP-HSA stimulation. Thus, after cognate interaction with Th cells, mast cells might lower their threshold for IgE-mediated stimulation and might become more efficient in antigen presentation to T cells. These observations extend previous studies showing that mast cells exhibit enhanced responses after close contact with activated T cells.16,17
It is tempting to speculate that, on one hand, the APC role of tissue mast cells might have a positive effect on the development of adaptive immune responses, but, on the other hand, it might become detrimental in autoimmune diseases (supplemental Figure 8). By responding to Th-derived signals via class II up-regulation and antigen presentation, mast cells can contribute to create an activation loop favorable to the establishment of inflammation. Activated mast cells would then strongly contribute to the inflammatory milieu and would also participate to tissue damage via release of their granule content, thus favoring epitope spreading in the course of the autoimmune process. A recent report showing that regulatory T cells suppress mast cells during inflammatory processes is in agreement with the idea that mast cells might play a role in the establishment of autoimmunity.50
In conclusion, our results shed new light on mast cell function. We show that among the heterogeneous mast cell population only a fraction is able to successfully present antigens to effector CD4+ T lymphocytes, yet this cognate interaction results in cell-cell cooperation at the IS. Further research, looking at mast cell biology from this new angle, will be instrumental to broaden our understanding of their contribution to immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sabina Mueller, Sylvie Guerder, and Jean-Charles Guéry for critical reading of the manuscript; Denis Hudrisier for generously sharing reagents; Fatima L'Faqihi (IFR30, plateau technique cytométrie, Toulouse) and Sophie Allart (IFR30, Plateau technique Imagerie cellulaire, Toulouse) for assistance.
This work was supported by grants from la Ligue contre le Cancer “Equipe labellisée 2009” and Fondation BNP-PARIBAS (S.V.) and ANR-MRAR-015-01 (R.L.)
Authorship
Contribution: N.G., N.E., L.T.M., and E.E. performed experiments; N.G. and E.E. analyzed results and made the figures; R.L. provided reagents and discussed results; and S.V. and E.E. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Espinosa, Inserm U563, Centre de Physiopathologie Toulouse-Purpan, Université Toulouse III, 31024 Toulouse, France; e-mail: eric.espinosa@inserm.fr.
References
Author notes
S.V. and E.E. contributed equally to this study.