Abstract
Human umbilical vein endothelial cell (HUVEC)–released ADAMTS-13 (a disintegrin and metalloprotease with thrombospondin repeats) and HUVEC-secreted von Willebrand factor (VWF) strings were investigated under static conditions that allow the accumulation and analysis of ADAMTS-13. The latter was released constitutively from HUVECs and cleaved the secreted and cell-anchored VWF strings progressively during 15 minutes in Ca2+/Zn2+-containing buffer. HUVEC ADAMTS13 mRNA expression was approximately 1:100 of VWF monomeric subunit expression. In contrast to multimeric VWF stored within Weibel-Palade bodies and secreted rapidly in response to cell stimulation, ADAMTS-13 was released directly from the Golgi to the cell exterior without an organelle storage site. The constitutive release of ADAMTS-13 continued at the same slow rate regardless of the presence or absence of histamine stimulation of HUVECs. Consequently, the percentage of VWF strings cleaved by ADAMTS-13 at VWF Y1605-M1606 decreased as the rate of VWF string secretion was increased by cell stimulation. Blockade of HUVEC ADAMTS-13 activity by antibodies to different ADAMTS-13 domains made it possible to detect the attachment of ADAMTS-13 all along the lengths of HUVEC-secreted VWF strings. Constitutive ADAMTS-13 released from endothelial cells may contribute to the maintenance of cell surfaces free of hyperadhesive VWF multimeric strings.
Introduction
Human endothelial cells synthesize von Willebrand factor (VWF) multimers, package them in Weibel-Palade bodies (WPBs), and secrete them in response to agonist stimulation. The VWF multimers are rapidly propelled outward as long, hyperadhesive VWF strings anchored to the surface of human umbilical vein endothelial cells (HUVECs), as well as arterial and microvascular endothelial cells. Endothelial cells also produce and release functionally active ADAMTS-13 (a disintegrin and metalloprotease with thrombspondin repeats), a zinc/calcium VWF-specific metalloprotease.1 Endothelial cells may be an important source of plasma ADAMTS-13 (along with hepatic stellate cells).2 ADAMTS-13 is absent or severely reduced in many patients with thrombotic thrombocytopenia purpura, either because the enzyme is not produced/released adequately (familial thrombotic thrombocytopenia purpura) or because enzyme activity is inhibited by ADAMTS-13 autoantibodies.3,4
We found recently that interactions between HUVEC ADAMTS-13 and anchored VWF multimeric strings can be evaluated effectively in the absence of flow, platelets, or chemical denaturants.5 Under these conditions, the long VWF strings are rapidly secreted and anchored to the HUVEC surfaces, and ADAMTS-13 progressively accumulates above the HUVECs that produce and release it. When experiments are done under flowing conditions, fluids are passed continuously over endothelial cells in flow chambers without recirculating; consequently, any proteins released slowly from the cells do not reach steady state concentrations. In previous studies when human endothelial cells from umbilical veins or arteries, coronary arteries, the skin microcirculation, or the kidney glomerulus were stimulated by agonists to secrete VWF strings under these flowing conditions, the cleavage of anchored VWF strings by ADAMTS-13 released from the same (and neighboring) cells could not be observed.6,7 For this reason, ADAMTS-13/VWF string cleavage experiments under flow have not provided information about ADAMTS-13 production and release by endothelial cells.
We conducted experiments under static conditions using buffers containing the Ca2+/Zn2+ required for optimal ADAMTS-13 activity to determine whether ADAMTS-13 is (1) stored in any HUVEC granule after synthesis; (2) secreted rapidly from stimulated HUVECs or released slowly and constitutively regardless of cell stimulation; and (3) capable of attaining minimum local concentrations necessary to cleave VWF strings secreted from the same (and neighboring) cultured HUVECs. We also (1) compared the relative rates of transcription and release of ADAMTS-13 and VWF from cultured (but otherwise unaltered) HUVECs; (2) examined the characteristics of ADAMTS-13/VWF string cleavage interactions from stimulated and unstimulated HUVECs; and (3) related our findings to the size of VWF multimers present in normal plasma.
Methods
HUVECs
Endothelial cells were obtained from collagenase-digested human umbilical veins, as previously described.7 Informed consent was obtained in accordance with the Declaration of Helsinki, under protocols approved by the institutional review board of Rice University. Cells were seeded onto 24- and 48-well plates or 1% gelatin-coated glass coverslips placed in culture dishes (9.62 cm2). HUVECs were grown in MCDB 131 (Sigma-Aldrich), including 1.6mM CaCl2 and 1nM ZnSO4, supplemented with microvascular endothelial cell growth factors (Lonza), 3% penicillin-streptomycin-neomycin, and 0.2mM l-glutamine. In most experiments, HUVECs were primary cultures or P1 with cell densities of 5 to 9 × 105 cells/cm2. Cell nuclei were detected and counted using 1nM DAPI (4,6 diamidino-2-phenylindole).
Antibodies
Antibodies to ADAMTS-13.
The BL antibodies (Bethyl Laboratories) are polyclonal goat immunoglobulin (Ig)G generated from distinct peptides in human ADAMTS-13.1,5 BL156 reacts with the Tsp1-4 domain; BL152 and BL153 are against distinct metalloprotease epitopes; BL158 reacts with the CUB-1 domain; and BL159 reacts with the CUB-2 domain.
Antibodies to full-length VWF.
Polyclonal goat anti–human VWF IgG (goat anti–VWF; Bethyl Laboratories) and polyclonal rabbit anti–human VWF IgG (rabbit anti–VWF; Ramco Laboratories) were made against VWF purified from human cryoprecipitate.8
Antibodies to VWF fragments.
Antibodies against intracellular structures.
Endoplasmic reticulum (ER) is a Mab IgG2a against protein disulfide isomerase; mitochondria, rabbit IgG against COX IV; lysosomes, Mab IgG1 antibody against CD63; and Golgi, rabbit IgG antibody against 58K Golgi protein (Abcam).
Fluorescent secondary antibodies.
Antigoat polyclonal antibodies were donkey anti–goat IgG-594 or donkey anti–goat IgG-647. Antirabbit antibodies were F(ab′)2 goat anti–rabbit IgG-488 and goat anti–rabbit IgG-594. Antimouse antibody was goat anti–mouse IgG-488. Each secondary was used at 20 μg/mL (Invitrogen).
Control antibody.
The nonspecific control antibody was goat anti–human IgG (Invitrogen).
Measurement of ADAMTS-13 levels
Ninety-six–well plates (Maxisorb; Nalge Nunc International) were coated overnight with 4 μg/mL goat anti–ADAMTS-13 (BL156) in 0.1M bicarbonate, pH 9.6, at 4°C. Wells were blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS; 9mM CaCl2, 4.9mM MgCl2, 26.7mM KCl, 14.7mM KH2PO4, 1.4M NaCl, 80.6mM Na2HPO4, pH 7.4) for 1 hour at 37°C. Samples were diluted in 1% BSA/PBS and incubated for 1 hour at 37°C. The wells were washed with PBS containing 0.05% Tween-20 before addition of anti–ADAMTS-13 (BL159)–horseradish peroxidase (HRP; 0.5 μg/mL) diluted in 1% BSA/PBS for 1 hour at 37°C. Rewashed wells were color developed with 3,3′,5,5′-tetramethylbenzidine (Invitrogen) and stopped with 2M H2SO4, and the absorbance was recorded at 450 nm. Standard curves were generated by dilutions of normal plasma.
Measurement of total VWF and VWF 140-kD and VWF 176-kD fragments
A similar immunoassay procedure was used to measure levels of total VWF antigen, VWF 140-kD, and VWF 176-kD fragments.9,10 Plates were coated with 1 μg/mL rabbit polyclonal anti–VWF. Levels of total VWF antigen were detected with goat anti–VWF plus rabbit anti–goat IgG-HRP (0.5 μg/mL); VWF 140-kD fragments were detected with Mab LJ140 plus anti–mouse IgG-HRP (1 μg/mL); and VWF 176-kD fragments were detected with Mab LJ176 plus anti–mouse IgG-HRP (1 μg/mL). Detection of VWF 140-kD and 176-kD fragments did not require samples to be immunoprecipitated or concentrated.
Fluorescent microscopy
Images were acquired with a Nikon Diaphot TE300 microscope, SensiCamQE CCD camera (Cooke), and IP Lab software Version 3.9.4r4 (Scanalytics) using Nikon CFI plan-apochromatic fluorescence objectives 60×/1.4 NA oil and 20×/0.45 NA with a Ludl filter wheel and Chroma filter set with single-band excitation filters for fluorescein isothiocyanate/tetramethyl rhodamine iso-thiocyanate/cyanine 5/DAPI.
Conditions of VWF string cleavage by HUVEC ADAMTS-13
Detection of VWF strings.
Histamine-stimulated HUVECs secreted VWF strings that were visualized directly by fluorescent microscopy using VWF antibodies and fluorescently labeled secondary antibodies. Platelets were not present in any of our experiments.
Cleavage of VWF strings by HUVEC ADAMTS-13 occurred in the near absence of shear stress.
The gentle addition and removal of surrounding fluids (requiring < 5 seconds) was the only type of force applied to the secreted VWF. The average shear stress applied was calculated to be 0.048 dyn/cm2 (described below).5 This value is approximately 15-fold less than the lowest mean shear stress estimated to be present continuously in the normal venous circulation.11,12 There was no fluid movement (ie, no shear stress) during the portions of experiments that included measurement of VWF string cleavage.
Shear stress exerted on HUVEC-secreted ultra large VWF strings during fluid addition or removal.
The average shear stress during these brief maneuvers was calculated using the following equation:
where τ equals average shear stress (dyn/cm2 or Pascal); μ equals viscosity (poise or Pascal [× seconds]); Q equals flow rate (mL/s); R equals radius of the culture dish (cm); and h equals height (in cm) of fluid over the cells. The reagents were assumed to be Newtonian fluids with apparent viscosities of 0.76 cpoise. The height (h) of the fluid over the cells was calculated by dividing the volume of fluid added (1 mL) by the area of the culture dish (9.62 cm2). The calculated average shear stress of 0.048 dyn/cm2 was maximal for the experiments because the mathematical model assumed that the application of fluids was directly across the top of the HUVECs, whereas the fluids were actually added to the sides of the culture dishes.
Measurements of VWF string lengths on cell surfaces cleaved by HUVEC-released ADAMTS-13
HUVECs were stimulated with 100μM histamine for 2 minutes and washed with PBS. HUVECs were then incubated for 0 to 2, 5, or 10 to 15 minutes in Ca2+/Zn2+-containing buffer (1mM CaCl2, 0.05mM ZnCl2, 138mM NaCl, 5.5mM glucose, 12mM NaHCO3, 2.9mM KCl, and 0.36mM Na2HPO4, pH 7.4) and washed before fixation. After staining with rabbit anti–VWF (10 μg/mL) plus anti–rabbit IgG-488, VWF string numbers and lengths at 200 × (201 μm × 150 μm), visualized by fluorescence, were measured using calibrated distance units (pixels/μm) with imaging software. In some experiments, HUVECs were incubated with 50 μL of Ca2+/Zn2+-containing buffer or serum-free media plus or minus 20 μg/mL antibody BL156 before fixation.
Detection of antibody-inactivated ADAMTS-13 binding to secreted VWF strings
HUVECs were stimulated with 100μM histamine for 2 minutes, washed with PBS, and then incubated for 20 minutes (without fixative) with antibodies against ADAMTS-13 (BL156, BL152, and BL153; 10 μg/mL) plus anti–goat IgG-594. Cells were then rewashed before addition of rabbit anti–VWF plus anti–rabbit IgG-488 for an additional 20 minutes (also without fixative). Fluorescent images were obtained at ×600.
Analysis of VWF cleavage in HUVEC supernatants
HUVECs grown in 24- or 48-well plates were incubated with serum-free media (MCDB 131 with 1% insulin-transferrin-selenium A and 1% BSA) or with serum-free media containing 100μM histamine at 37°C. Cell supernatant was collected into 10mM EDTA (ethylenediaminetetraacetic acid) after 60 minutes or 48 hours and analyzed by separate immunoassays for total VWF antigen and VWF 176-kD fragments levels.
Endothelial cell locations of ADAMTS-13 and VWF
Primary, first, and second passage HUVECs seeded on glass coverslips for 4 to 12 days were washed with PBS, fixed, and treated with 0.2% Triton X-100. Cells were simultaneously immunostained with goat anti–ADAMTS-13 (BL156; 10 μg/mL) plus donkey anti–goat IgG-647 (20 μg/mL) and antibodies (10 μg/mL) made against intracellular markers for ER (protein disulfide isomerase), the Golgi (58K Golgi protein), lysosomes (CD63), mitochondria (COX IV), or WPBs (VWF, rabbit anti–VWF) plus appropriate secondary fluorescent antibodies (20 μg/mL). HUVECs were also stained with goat anti–VWF plus donkey anti–goat IgG-647 and each of the intracellular makers.
Detection of VWF multimers and VWF fragments
Human VWF was isolated from 10 U of pooled cryoprecipitate by glycine/sodium chloride precipitation and size fractionated (purified VWF [pVWF]).8 Cryoprecipitate-poor plasma was prepared from 1 U of normal plasma by 3 cycles of freeze/thawing and centrifugation and similarly size fractionated (cryosupernatant [CS]).13
Samples of pVWF or CS were either denatured in 8M urea/1% sodium dodecyl sulfate (SDS) and separated by 0.8% or 1% agarose electrophoresis, or reduced with 2-mercaptoethanol in 1% SDS and separated by 5% polyacrylamide gel electrophoresis (PAGE) before transfer to polyvinylidene difluoride membranes. Membranes were overlaid with goat anti–VWF, followed by rabbit anti–goat IgG-HRP, or with Mab LJ140, followed by goat anti–mouse IgG-HRP, and detected with Immuno-Star WesternC reagents (Bio-Rad) for imaging (ChemiDoc XRS; Bio-Rad).
Quantitative polymerase chain reaction
RNA from confluent T-75 flasks of first passage, unstimulated HUVECs was isolated using TRIzol (Invitrogen), chloroform extraction, and isopropanol precipitation. RNA integrity was verified by 260/280 optical density ratios and 1%-agarose-formaldeyde electrophoresis. The RNA was reverse transcribed into cDNAs with Moloney murine leukemia virus reverse transcriptase (Promega). Real-time polymerase chain reaction (PCR) using primers for VWF (aagccaatatagggcctcgt, aagccaatatagggcctcgt), ADAMTS13 (cacaggcctctcttcacaca, ggtgttagggg-agatgctca), and glyceraldehyde-3-phosphate dehydrogenase (gactgagtgtggcagggact, ggcctccaaggagtaagacc) was done using the ABI Prism 7300 system (Applied Biosystems) with general conditions, as follows: 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles (15 seconds at 95°C; 1 minute at 60°C). Samples were amplified in triplicate and detected using quantitative PCR MasterMix Plus for SYBR Green 1 (Eurogentec). Primer efficiencies were determined by reverse transcription of 1 μg to 0.2 ng of RNA and amplification of 20 ng to 0.002 ng of the resulting cDNAs, and calculated by standard protocols.14,15 The primers for human ADAMTS13 were selected from Tsp1 domains 2 to 4 between nucleotides 2041 and 2700 of the mRNA of ADAMTS13. The mRNA sequence selected for amplification did not complement any human gene other than ADAMTS13, according to the BLAST nucleotide program.
Statistics
Data are presented as means with standard deviations (SD). Arrays of data were considered significantly different with P values less than .05, using the 2-tailed paired Student t test.
Results
Comparative effects of HUVEC stimulation on ADAMTS-13 and VWF release
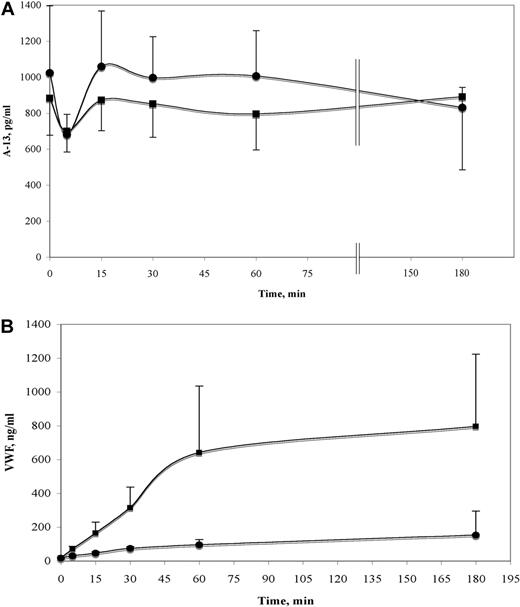
ADAMTS-13 levels (pg/mL) were similar and remained nearly constant in endothelial cell supernatant samples collected from both histamine-stimulated and unstimulated HUVECs over a 3-hour time period. There was a transient initial decrease in ADAMTS-13 levels at 5 minutes after the addition of serum-free media to both histamine-stimulated and unstimulated HUVEC samples (Figure 1A), probably as a consequence of ADAMTS-13 binding to secreted VWF strings. Media addition alone produces evanescent HUVEC perturbation opposed to the strong, sustained stimulation by histamine. In contrast, stimulation of HUVECs resulted in a 6- to 15-fold increase in VWF secretion rate during the first hour compared with unstimulated cells (Figure 1B and Table 1). These results indicate that although VWF is actively secreted from stimulated cells, in contrast, ADAMTS-13 is released via the constitutive pathway that is not coupled to histamine stimulation. Levels of ADAMTS-13 released from HUVECs over longer time periods (24-48 hours) increase 2- to 4-fold over initial levels (data not shown).
Quantification of ADAMTS13 and VWF secreted by unstimulated and histamine-stimulated HUVECs. Culture media was collected from HUVECs grown in 48-well plates at 0, 5, 15, 30, 60, and 180 minutes after the addition of 60 μL of serum-free media with 1% BSA ± 100μM histamine. Samples were collected into 10mM EDTA, and levels of VWF and ADAMTS-13 were measured by immunoassay. (A) There were no significant differences in the ADAMTS-13 levels (pg/mL) measured from the stimulated (•) and unstimulated HUVECs (■) at each time point (n = 8-10). (B) Levels of VWF secreted after histamine stimulation (■) and VWF released in the absence of stimulation (•) were significantly different at each point measured after 0 minutes (n = 5-6).
Quantification of ADAMTS13 and VWF secreted by unstimulated and histamine-stimulated HUVECs. Culture media was collected from HUVECs grown in 48-well plates at 0, 5, 15, 30, 60, and 180 minutes after the addition of 60 μL of serum-free media with 1% BSA ± 100μM histamine. Samples were collected into 10mM EDTA, and levels of VWF and ADAMTS-13 were measured by immunoassay. (A) There were no significant differences in the ADAMTS-13 levels (pg/mL) measured from the stimulated (•) and unstimulated HUVECs (■) at each time point (n = 8-10). (B) Levels of VWF secreted after histamine stimulation (■) and VWF released in the absence of stimulation (•) were significantly different at each point measured after 0 minutes (n = 5-6).
Rates of VWF secretion from stimulated and unstimulated HUVECs
| Time, min . | VWF rate, ng/mL/min, average (range) . | |
|---|---|---|
| Stimulated . | Unstimulated . | |
| 5-15 | 9.5 (4.8-14.3) | 1.65 (1.3-2.0) |
| 15-3 | 9.9 (6.0-13.8) | 1.85 (1.8-1.9) |
| 30-60 | 10.9 (1.9-20.0) | 0.69 (0.01-1.4) |
| 60-180 | 1.29 (1.0-1.6) | 0.49 (0-1.4) |
| Time, min . | VWF rate, ng/mL/min, average (range) . | |
|---|---|---|
| Stimulated . | Unstimulated . | |
| 5-15 | 9.5 (4.8-14.3) | 1.65 (1.3-2.0) |
| 15-3 | 9.9 (6.0-13.8) | 1.85 (1.8-1.9) |
| 30-60 | 10.9 (1.9-20.0) | 0.69 (0.01-1.4) |
| 60-180 | 1.29 (1.0-1.6) | 0.49 (0-1.4) |
Cell supernatants were collected from unstimulated and histamine-stimulated HUVECs after 0, 5, 15, 30, 60, and 180 minutes after addition of serum-free media. Rates of VWF secretion (ng/mL per minute) were calculated from averaged VWF levels (average) and, using the lowest and highest VWF levels (range), measured at each time interval in 5 to 6 determinations.
HUVEC ADAMTS-13 is constitutively released without detectable storage in a cytoplasmic granule
The slow, steady rate of ADAMTS-13 release by HUVECs, unaffected by histamine stimulation over a 3-hour period (Figure 1A), implies that ADAMTS-13 may be constitutively released from the Golgi to the endothelial cell exterior without an intermediate organelle storage site. Our fluorescent staining experiments are supportive and suggest that ADAMTS-13 is not detectably stored in HUVEC mitochondria, WPBs,1 or lysosomes (data not shown).
Endothelial cell production of ADAMTS-13 relative to VWF monomers
Unstimulated HUVECs released 100-fold less ADAMTS-13 (∼1 ng/mL) than VWF (∼100 ng/mL) over 60 minutes (Figure 1). HUVEC transcription of ADAMTS-13 and monomeric VWF in these cells was compared by quantifying HUVEC ADAMTS13 and VWF gene expression by real-time PCR. First passage HUVECs had 107- to 142-fold more copies of VWF mRNA transcripts (encoding VWF monomeric subunits) than ADAMTS13 mRNA (Table 2). The expression ratios between ADAMTS13 and VWF did not differ appreciably with cDNA amounts ranging from 0.2 to 10 ng in the PCR.
Expression of HUVEC ADAMTS13 compared with HUVEC VWF
| cDNA . | Ct . | ΔCt . | Ratio of ADAMTS13 to VWF . |
|---|---|---|---|
| 10 ng | 107-fold lower | ||
| ADAMTS13 | 29.83 ± 1.33 | 7.4 ± 1.93 | |
| VWF | 22.43 ± 2.29 | ||
| 2 ng | 142-fold lower | ||
| ADAMTS13 | 29.2 ± 1.36 | 7.85 ± 1.56 | |
| VWF | 21.34 ± 1.45 |
| cDNA . | Ct . | ΔCt . | Ratio of ADAMTS13 to VWF . |
|---|---|---|---|
| 10 ng | 107-fold lower | ||
| ADAMTS13 | 29.83 ± 1.33 | 7.4 ± 1.93 | |
| VWF | 22.43 ± 2.29 | ||
| 2 ng | 142-fold lower | ||
| ADAMTS13 | 29.2 ± 1.36 | 7.85 ± 1.56 | |
| VWF | 21.34 ± 1.45 |
Expression of HUVEC ADAMTS13 and VWF mRNA was quantified by real-time PCR. The data are generated from the difference in threshold cycle (Ct) for each gene amplified from either 2 ng or 10 ng of cDNA. The fold difference in expression was calculated (after normalizing to glyceraldehyde-3-phosphate dehydrogenase) by the equation: fold difference = efficiencyΔCt. RNA was extracted and reverse-transcribed, and the resulting cDNA was amplified in triplicate in 5 separate experiments.
Although the mRNA level for the VWF (monomers) is more than 100-fold higher than the level of ADAMTS13 mRNA, each VWF multimeric string secreted by HUVECs is composed of 20 to 50 (or more) VWF monomer subunits.16 Consequently, the synthetic ratio of ADAMTS-13 molecules to VWF multimeric strings is likely to be less disproportionate.
Cleavage of cell-anchored long VWF strings by ADAMTS-13 released by HUVECs
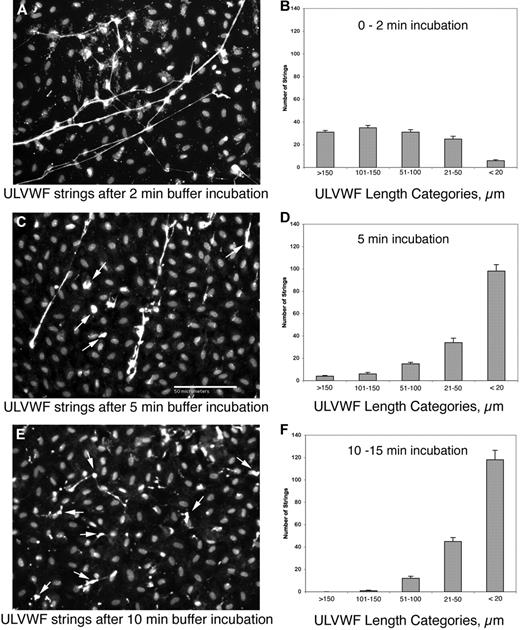
The ADAMTS-13 released by HUVECs is capable of cleaving long VWF strings secreted from the same HUVECs under the nonflowing in vitro conditions that allow released ADAMTS-13 to accumulate. HUVECs were stimulated with histamine to secrete HUVEC-anchored VWF strings, washed, and incubated with Ca2+/Zn2+-containing buffer for 0 to 15 minutes. The cell-anchored VWF strings were then fixed at different time intervals, stained with polyclonal anti-VWF and fluorescent secondary antibody, and then counted and measured. After the shortest incubation time (0-2 minutes), 75% of the VWF strings were longer than 100 μm and only 5% of the strings were shorter than 20 μm (Figure 2A-B). After 5 minutes incubation, only 16% of the VWF strings were more than 100 μm in length (Figure 2C-D), and after 10 to 15 minutes fewer than 10% of VWF strings exceeded 100 μm (Figure 2E-F). These results demonstrate that increasing incubation times in Ca2+/Zn2+ buffer shifted the VWF string length distribution toward the smaller size categories (Figure 2 and Table 3). The only ADAMTS-13 present under these conditions was released from the HUVECs.1 After ADAMTS-13–mediated cleavage, the residual short VWF strings that remained attached to HUVECs often retracted into a globular form (Figure 2C,E).
Cleavage of HUVEC-anchored VWF strings by HUVEC-released ADAMTS13. HUVECs were stimulated for 2 minutes with 100μM histamine, washed, and incubated statically for 0 to 2 minutes (A-B), 5 minutes (C-D), or 10 to 15 minutes (E-F) in Ca2+/Zn2+-containing buffer. The cells were fixed and stained with rabbit anti–VWF plus goat anti–rabbit IgG-488 and DAPI. The lengths of VWF strings were measured from microscope fields (201 μm × 150 μm; ×200) after each incubation time (n = 10), and were categorized as follows: lengths > 150 μm; 101 to 150 μm; 51 to 100 μm; 21 to 50 μm; and <20 μm. Representative images and graphs (means ± SD) are shown for each time interval. The arrows on panels C and E indicate cleaved VWF strings. The panels demonstrate that endogenous ADAMTS-13 released from HUVECs progressively cleaved VWF strings anchored to the same and/or nearby cells into smaller forms.
Cleavage of HUVEC-anchored VWF strings by HUVEC-released ADAMTS13. HUVECs were stimulated for 2 minutes with 100μM histamine, washed, and incubated statically for 0 to 2 minutes (A-B), 5 minutes (C-D), or 10 to 15 minutes (E-F) in Ca2+/Zn2+-containing buffer. The cells were fixed and stained with rabbit anti–VWF plus goat anti–rabbit IgG-488 and DAPI. The lengths of VWF strings were measured from microscope fields (201 μm × 150 μm; ×200) after each incubation time (n = 10), and were categorized as follows: lengths > 150 μm; 101 to 150 μm; 51 to 100 μm; 21 to 50 μm; and <20 μm. Representative images and graphs (means ± SD) are shown for each time interval. The arrows on panels C and E indicate cleaved VWF strings. The panels demonstrate that endogenous ADAMTS-13 released from HUVECs progressively cleaved VWF strings anchored to the same and/or nearby cells into smaller forms.
Length of VWF strings after cleavage by HUVEC ADAMTS-13
| . | 0-2 minutes . | 5 minutes . | 10-15 minutes . |
|---|---|---|---|
| Average length, μm | 107 ± 57 | 28 ± 36 | 19 ± 19 |
| Size range, μm | 11-248 | 2-205 | 2-107 |
| . | 0-2 minutes . | 5 minutes . | 10-15 minutes . |
|---|---|---|---|
| Average length, μm | 107 ± 57 | 28 ± 36 | 19 ± 19 |
| Size range, μm | 11-248 | 2-205 | 2-107 |
Histamine-stimulated HUVECs were incubated in Ca2+/Zn2+-containing buffer for 0 to 2, 5, and 10 to 15 minutes, fixed, and stained with rabbit anti–VWF plus goat anti–rabbit IgG-488. VWF string lengths (± SD) were measured from microscope views (201 μm × 150 μm) at ×200 at each incubation time during 10 experiments.
In control experiments, neither nonspecific anti–human IgG plus fluorescent reporting antibody nor secondary fluorescent antibody alone produced fluorescent VWF string staining patterns (data not shown).
Specificity of HUVEC-released ADAMTS-13 cleavage of VWF strings
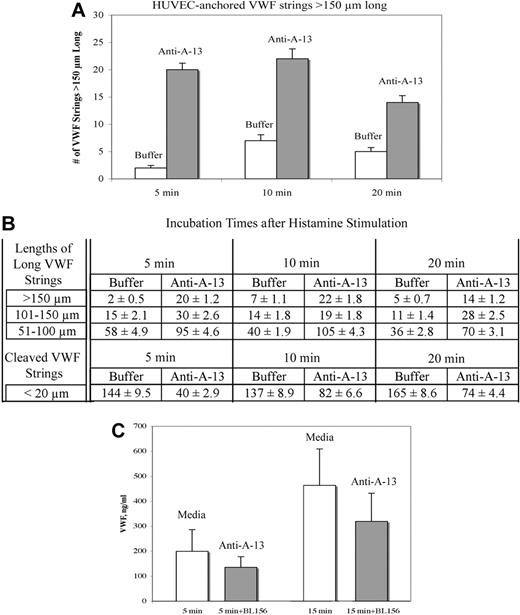
The presence of antibody against ADAMTS-13 (BL156; 20 μg/mL) during histamine stimulation of HUVECs, and during the subsequent 5-, 10-, and 20-minute HUVEC incubation periods in Ca2+/Zn2+ buffer, resulted in 3- to 10-fold more cell-anchored long VWF strings more than 150 μm in length than in the absence of ADAMTS-13 antibody (Figure 3A-B). Concurrently, in the presence of anti–ADAMTS-13, the number of short VWF strings (< 20 μm) decreased 2- to 3-fold (Figure 3B). These observations indicate that the antibody against ADAMTS-13 specifically inhibited the ADAMTS-13 activity that was released (only) from the HUVECs under these conditions.
Specific blockade of VWF string cleavage by antibody to ADAMTS13. HUVECs were stimulated with 100μM histamine in buffer ± 20 μg/mL anti–ADAMTS-13 (BL156) for 2 minutes. After washing, the cells were incubated for 5, 10, and 20 minutes in Ca2+/Zn2+-containing buffer alone or buffer containing 20 μg/mL anti–ADAMTS-13. After incubation, the cells were fixed and stained with rabbit anti–VWF plus goat anti–rabbit IgG-488. The number and lengths of cell-anchored VWF strings were measured from microscope fields (201 μm × 150 μm; ×200) from 3 to 4 experiments at each time point and shown as means ± SD. (A) VWF strings with lengths exceeding 150 μm after 5-, 10-, and 20-minute incubations with Ca2+/Zn2+-containing buffer alone (□) or buffer containing 20 μg/mL anti–ADAMTS-13 (▩). (B) Lengths of long VWF strings (> 150 μm, 101-150 μm, and 51-100 μm) and lengths of cleaved VWF strings (< 20 μm) in the presence and absence of anti–ADAMTS-13 after each incubation time. A greater number of long VWF strings was quantified in the presence of anti–ADAMTS-13. (C) HUVECs were stimulated with 100μM histamine for 2 minutes, washed, and incubated in serum-free media ± 20 μg/mL anti–ADAMTS-13. Levels of VWF released into cell supernatants and collected into 10mM EDTA after 5 minutes (P = .05; n = 10) and 15 minutes (P = .01; n = 8) were significantly lower in supernatants from cells incubated with antibodies to ADAMTS-13.
Specific blockade of VWF string cleavage by antibody to ADAMTS13. HUVECs were stimulated with 100μM histamine in buffer ± 20 μg/mL anti–ADAMTS-13 (BL156) for 2 minutes. After washing, the cells were incubated for 5, 10, and 20 minutes in Ca2+/Zn2+-containing buffer alone or buffer containing 20 μg/mL anti–ADAMTS-13. After incubation, the cells were fixed and stained with rabbit anti–VWF plus goat anti–rabbit IgG-488. The number and lengths of cell-anchored VWF strings were measured from microscope fields (201 μm × 150 μm; ×200) from 3 to 4 experiments at each time point and shown as means ± SD. (A) VWF strings with lengths exceeding 150 μm after 5-, 10-, and 20-minute incubations with Ca2+/Zn2+-containing buffer alone (□) or buffer containing 20 μg/mL anti–ADAMTS-13 (▩). (B) Lengths of long VWF strings (> 150 μm, 101-150 μm, and 51-100 μm) and lengths of cleaved VWF strings (< 20 μm) in the presence and absence of anti–ADAMTS-13 after each incubation time. A greater number of long VWF strings was quantified in the presence of anti–ADAMTS-13. (C) HUVECs were stimulated with 100μM histamine for 2 minutes, washed, and incubated in serum-free media ± 20 μg/mL anti–ADAMTS-13. Levels of VWF released into cell supernatants and collected into 10mM EDTA after 5 minutes (P = .05; n = 10) and 15 minutes (P = .01; n = 8) were significantly lower in supernatants from cells incubated with antibodies to ADAMTS-13.
Reduced amounts of soluble VWF are released from histamine-stimulated HUVECs into cell supernatants in the presence of antibodies to ADAMTS-13 (BL156; 20 μg/mL) compared with stimulated cells incubated in serum-free media alone (Figure 3C).
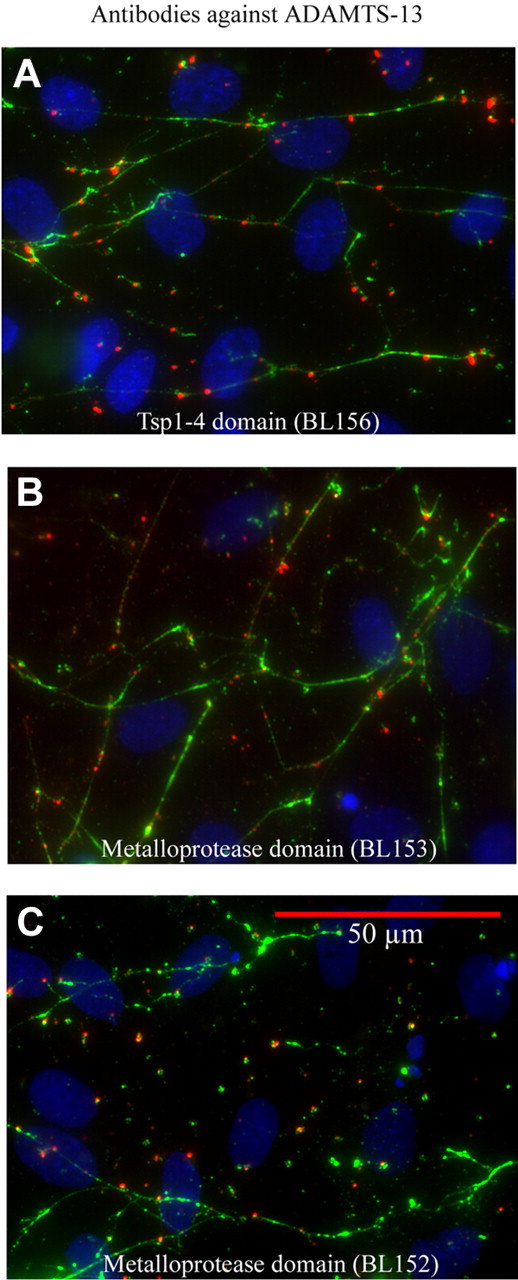
Inactivation of ADAMTS-13 enables its detection attached to uncleaved long VWF strings
Endogenous ADAMTS-13 released from HUVECs over a 20-minute experimental period was observed attached to secreted, anchored long VWF strings in the presence of any of 3 distinct ADAMTS-13 antibodies individually capable of blocking ADAMTS-13 activity. These antibodies included the following: BL156 (against the Tsp1-4 domain; Figure 4A); BL152 (against the metalloprotease domain; Figure 4B); and BL153 (second distinct antibody against the metalloprotease domain; Figure 4C). (The binding of any of these 3 IgG antibodies to ADAMTS-13 blocks ADAMTS-13 activity, regardless of the domain epitope target, because the molecular size of antibody and enzyme is similar in size.) After histamine stimulation to induce VWF secretion, HUVECs were separately incubated (without fixation) with the different antibodies to ADAMTS-13 and fluorescent secondary antibodies for 20 minutes. This was followed by the addition of anti-VWF and fluorescent secondary antibody to visualize the VWF strings. Under these conditions, the activity of endogenous ADAMTS-13 released from the HUVECs during the 20-minute incubation period was blocked and the inactivated enzyme was observed attached to intact, uncleaved, long VWF strings.
HUVEC-released ADAMTS13 attached to HUVEC-secreted VWF strings. HUVECs were stimulated with histamine for 2 minutes, washed, and incubated for 20 minutes (without fixative) with polyclonal goat antibodies made against different domains of human ADAMTS-13: (A) BL156 (Tsp1-4 domain); (B) BL152 (metalloprotease domain); and (C) BL153 (distinct peptide within metalloprotease domain); and secondary anti-goat IgG-594 (red). The cells were rewashed before staining with rabbit anti–VWF plus anti-rabbit IgG-488 (green) to visualize VWF strings. Cell nuclei were stained blue with DAPI. The images magnified ×600, represent 6 to 12 experiments, and demonstrate that blocking the activity of HUVEC-released ADAMTS-13 enables the ADAMTS-13 bound only to uncleaved VWF strings to be detected.
HUVEC-released ADAMTS13 attached to HUVEC-secreted VWF strings. HUVECs were stimulated with histamine for 2 minutes, washed, and incubated for 20 minutes (without fixative) with polyclonal goat antibodies made against different domains of human ADAMTS-13: (A) BL156 (Tsp1-4 domain); (B) BL152 (metalloprotease domain); and (C) BL153 (distinct peptide within metalloprotease domain); and secondary anti-goat IgG-594 (red). The cells were rewashed before staining with rabbit anti–VWF plus anti-rabbit IgG-488 (green) to visualize VWF strings. Cell nuclei were stained blue with DAPI. The images magnified ×600, represent 6 to 12 experiments, and demonstrate that blocking the activity of HUVEC-released ADAMTS-13 enables the ADAMTS-13 bound only to uncleaved VWF strings to be detected.
Without the addition of antibodies to ADAMTS-13 (buffer alone in Figure 2E), or in experiments when control antibodies (goat anti–human IgG plus secondary goat IgG-594, or secondary antibodies alone) were added to stimulated HUVECs during the 20-minute incubation period, only a few HUVEC-anchored VWF strings remained. The addition of antibodies to ADAMTS-13 (or antibody to VWF, followed by anti–ADAMTS-13) prevented VWF string cleavage, and allowed the protease to be observed attached to HUVEC-anchored VWF strings.
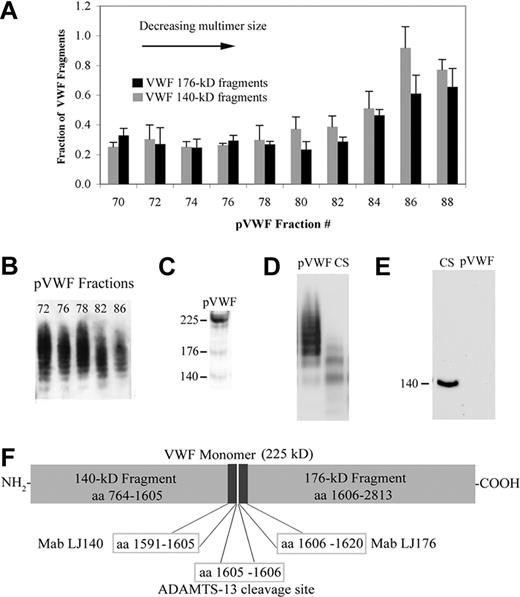
Plasma VWF multimeric size is determined by the extent of ADAMTS-13 cleavage of HUVEC-secreted VWF strings at Y1605-6M sites of the VWF A2 domain
Figure 5 demonstrates that (1) the decreasing size of soluble VWF multimers is the result of increasing ADAMTS-13 cleavage of A2 Y1605-M1606 sites in VWF monomeric subunits; and (2) this cleavage results in an increased proportion of 176-kD and 140-kD fragments produced by Y1605-M1606 cleavage. Fractions of pVWF containing the largest plasma VWF multimers (fractions 70-80) contained relatively low percentages of 176-kD and 140-kD fragments (Figure 5A-B) per total quantity of VWF antigen.9,10 This indicates that the soluble VWF multimers in these aliquots were produced by ADAMTS-13 cleavage of relatively few A2 1605-6 sites on VWF strings as these were secreted from HUVECs. The percentage of both 176-kD and 140-kD fragments per total quantity of VWF antigen was higher in pVWF aliquots without the largest plasma-type VWF multimers (fractions 82-88; Figure 5A-B), indicating that multimers in these fractions resulted from additional cleavage of VWF A2 1605-6 sites. Each VWF multimer cleaved by ADAMTS-13 at 1605-6 sites contains one fragmented subunit at opposite ends of the polymer, separated by intact VWF subunits. The cleaved fragments on each end can be either one of 176-kD and one of 140-kD, or 2 of 176-kD, or 2 of 140-kD fragments. The proportion of 176-kD and 140-kD fragments to intact VWF subunits increases with each successive 1605-6 cleavage as the multimers become smaller. For example, a VWF multimer can contain 10 intact subunits with one 176-kD and one 140-kD fragment on each end, and a smaller VWF multimer can contain 2 intact subunits also with one 176-kD and one 140-kD fragment on each end. In this example, the ratio of either 176-kD or 140-kD fragment to total intact VWF subunit would be 1:10 for the larger multimer and 1:2 for the smaller form.
Quantification of VWF 176-kD and 140-kD fragments in plasma-derived pVWF. (A) VWF 140-kD fragments (gray) and 176-kD fragments (black) in size-fractionated pVWF were detected with Mab LJ140 and Mab LJ176 by immunoassay, and expressed as percentages of VWF fragments per total quantity of VWF antigen. Results are means ± SD. (B) VWF multimer immunoblot (1% agarose) showing multimer size ranges in pVWF fractions. (C) pVWF (multimer sizes similar to 82) was reduced and separated by SDS-5% PAGE. The immunoblot using polyclonal goat anti–VWF displays intact VWF monomer (225 kD) and faint bands for the 140-kD and 176-kD fragments. (D) Nonreduced samples separated by SDS-0.8% agarose demonstrate the larger plasma VWF multimers present in pVWF and the smaller multimers in CS. (E) Reduced samples of CS and pVWF were separated by SDS-5% PAGE and immunoblotted with Mab LJ140. The VWF 140-kD fragment was undetectable in pVWF samples that contained 1000-fold more VWF antigen (8 μg) than CS samples (8.4 ng). Immunoblots represent similar results obtained in 4 to 5 experiments. (F) Target epitopes of Mab LJ140 and Mab LJ176 that react specifically with either fragment 140-kD or 176-kD flanking the ADAMTS-13 cleavage site within the A2 domain of the VWF monomeric subunit. Cleavage of VWF at peptide bond M1605-Y1606 by ADAMTS-13 results in an increased proportion of 140-kD and 176-kD fragments relative to total VWF protein.
Quantification of VWF 176-kD and 140-kD fragments in plasma-derived pVWF. (A) VWF 140-kD fragments (gray) and 176-kD fragments (black) in size-fractionated pVWF were detected with Mab LJ140 and Mab LJ176 by immunoassay, and expressed as percentages of VWF fragments per total quantity of VWF antigen. Results are means ± SD. (B) VWF multimer immunoblot (1% agarose) showing multimer size ranges in pVWF fractions. (C) pVWF (multimer sizes similar to 82) was reduced and separated by SDS-5% PAGE. The immunoblot using polyclonal goat anti–VWF displays intact VWF monomer (225 kD) and faint bands for the 140-kD and 176-kD fragments. (D) Nonreduced samples separated by SDS-0.8% agarose demonstrate the larger plasma VWF multimers present in pVWF and the smaller multimers in CS. (E) Reduced samples of CS and pVWF were separated by SDS-5% PAGE and immunoblotted with Mab LJ140. The VWF 140-kD fragment was undetectable in pVWF samples that contained 1000-fold more VWF antigen (8 μg) than CS samples (8.4 ng). Immunoblots represent similar results obtained in 4 to 5 experiments. (F) Target epitopes of Mab LJ140 and Mab LJ176 that react specifically with either fragment 140-kD or 176-kD flanking the ADAMTS-13 cleavage site within the A2 domain of the VWF monomeric subunit. Cleavage of VWF at peptide bond M1605-Y1606 by ADAMTS-13 results in an increased proportion of 140-kD and 176-kD fragments relative to total VWF protein.
Quantification of VWF fragments in the fractionated pVWF aliquots was similar using either Mab LJ176 or LJ140 directed against peptides flanking either side of the 1605-6 ADAMTS-13 cleavage sites in the A2 domain of VWF monomers (Figure 5A,F). Samples of fractionated CS, consisting of only the smallest VWF multimers,13 contained many-fold more VWF 140-kD fragments (indicating increased 1605-6 VWF multimer cleavage events) than the largest pVWF forms fractionated according to size. This is demonstrated in Figure 5C through E.
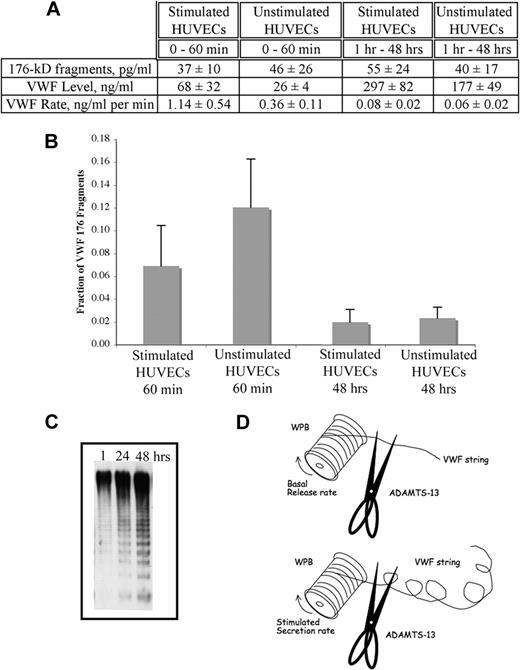
ADAMTS-13 cleavage of VWF strings secreted from stimulated and unstimulated HUVECs occurs at the position 1605-6 of the VWF A2 domain
The VWF strings secreted by HUVECs were cleaved by ADAMTS-13, and then released in soluble form into the supernatants above stimulated and unstimulated cells. These soluble VWF multimeric forms were analyzed for the extent of VWF 176-kD fragments relative to the total soluble VWF antigen in the supernatant, as a measurement of the extent of ADAMTS-13–mediated 1605-6 cleavage of A2 domains in the monomeric subunits of VWF strings that occurred as the strings were secreted from the HUVECs. After VWF string cleavage, total soluble VWF antigen accumulated at a 3-fold slower rate over the initial 60 minutes in the supernatants of unstimulated HUVECs (0.36 ng/mL VWF per minute) compared with the supernatants of histamine-stimulated HUVECs (1.14 ng/mL VWF per minute; Figure 6A). During this initial 60-minute period, the more slowly accumulating soluble VWF in the supernatants of unstimulated HUVECs contained an approximately 2-fold higher ratio of VWF 176-kD fragments per total VWF antigen than the supernatants of histamine-stimulated cells (Figure 6B; P = .014). These data indicate that there is more ADAMTS-13–mediated cleavage of the relatively few VWF strings secreted at a slow rate from unstimulated HUVECs, and less ADAMTS-13 cleavage of the many VWF strings secreted rapidly in response to cell stimulation. During the course of 48 hours, the rate of total soluble VWF antigen levels that accumulated in samples collected from the supernatants of unstimulated and initially histamine-stimulated HUVECs almost equalized (0.06 vs 0.08 ng/mL VWF per minute, respectively; Figure 6A). This is because the rate of histamine-stimulated VWF string secretion from HUVECs progressively slowed, whereas the slow secretion of VWF strings from unstimulated cells remained approximately unchanged. In consequence, the ratios of VWF 176-kD fragments to total soluble VWF antigen also nearly equalized in the supernatants of stimulated and unstimulated HUVECs (Figure 6B).
Quantification of ADAMTS13 cleavage sites 1605-6 in VWF A2 domains in VWF strings secreted by stimulated and unstimulated HUVECs. HUVECs grown in 24-well plates were incubated with 200 μL of serum-free media or stimulated with 100μM histamine in the same media at 37°C. Culture media was collected after 60 minutes or 48 hours into 10mM EDTA and analyzed for total VWF antigen and VWF 176-kD fragments by immunoassay. (A) VWF 176-kD fragment levels (pg/mL) were quantified using Mab LJ176 against amino acids M1606-P1620 of VWF monomers, and total VWF antigen levels (ng/mL) were measured using a polyclonal antibody against purified VWF. (B) Ratio of VWF 176-kD fragments per total VWF antigen after 60 minutes and 48 hours from histamine-stimulated and unstimulated HUVECs. Results are means ± SD (n = 12) and demonstrate that there is more ADAMTS-13–mediated cleavage of the few VWF strings secreted at a slow rate from unstimulated HUVECs than the many VWF strings secreted rapidly in response to cell stimulation at 60 minutes. (C) VWF multimer immunoblot representative of soluble VWF collected 1, 24, and 48 hours after HUVEC histamine stimulation in serum-free media. (D) Cartoon illustrating the relationship between rate of VWF string secretion and VWF cleavage multimer length. WPBs, represented by a spool of thread, release longer VWF strings before ADAMTS-13 cleavage under stimulation (rapid release rates) than under unstimulated conditions (basal rate). HUVEC-released ADAMTS-13, represented by the pair of scissors, is slow and continuous and remains constant regardless of the rate of VWF string secretion.
Quantification of ADAMTS13 cleavage sites 1605-6 in VWF A2 domains in VWF strings secreted by stimulated and unstimulated HUVECs. HUVECs grown in 24-well plates were incubated with 200 μL of serum-free media or stimulated with 100μM histamine in the same media at 37°C. Culture media was collected after 60 minutes or 48 hours into 10mM EDTA and analyzed for total VWF antigen and VWF 176-kD fragments by immunoassay. (A) VWF 176-kD fragment levels (pg/mL) were quantified using Mab LJ176 against amino acids M1606-P1620 of VWF monomers, and total VWF antigen levels (ng/mL) were measured using a polyclonal antibody against purified VWF. (B) Ratio of VWF 176-kD fragments per total VWF antigen after 60 minutes and 48 hours from histamine-stimulated and unstimulated HUVECs. Results are means ± SD (n = 12) and demonstrate that there is more ADAMTS-13–mediated cleavage of the few VWF strings secreted at a slow rate from unstimulated HUVECs than the many VWF strings secreted rapidly in response to cell stimulation at 60 minutes. (C) VWF multimer immunoblot representative of soluble VWF collected 1, 24, and 48 hours after HUVEC histamine stimulation in serum-free media. (D) Cartoon illustrating the relationship between rate of VWF string secretion and VWF cleavage multimer length. WPBs, represented by a spool of thread, release longer VWF strings before ADAMTS-13 cleavage under stimulation (rapid release rates) than under unstimulated conditions (basal rate). HUVEC-released ADAMTS-13, represented by the pair of scissors, is slow and continuous and remains constant regardless of the rate of VWF string secretion.
Discussion
ADAMTS-13 is synthesized by HUVECs, and then slowly and continuously (constitutively) released. Under static conditions, the absence of movement allows the ADAMTS-13 that is released from the HUVECs to accumulate in the fluid bathing the cells. We were able to capture in real time, and quantify, the proteolytic cleavage by HUVEC-released ADAMTS-13 of hyperadhesive VWF multimeric strings anchored to the HUVECs.
VWF cleavage by ADAMTS-13 requires one or more accessible A2 domains in the monomeric subunits that comprise each VWF multimeric string. This specific cleavage occurs at susceptible VWF A2 Y1605-M1606 peptide bond targets. Even in the absence of flow, the conformational change in A2 domains as the result of VWF propulsive secretion17 and anchorage to endothelial cells probably involves A2 unfolding and increased ADAMTS-13 access.18
ADAMTS-13 antigen was found in HUVEC endoplasmic reticulum and periodically in the Golgi apparatus. ADAMTS-13 antigen was not detected in mitochondria, WPBs,1 or lysosomes. HUVECs release ADAMTS-13 at similar slow rates from both histamine-stimulated and unstimulated cells. These results suggest that HUVEC ADAMTS-13 is synthesized, exported to the ER lumen, processed through the Golgi, and then released slowly and continuously (constitutively) to the cell exterior without an intermediate storage site.19
In contrast to ADAMTS-13, VWF multimeric strings are secreted rapidly from HUVECs in response to histamine stimulation. VWF strings are secreted at a slower, basal rate from unstimulated cells. Using immunofluorescence, we detected internal VWF in the Golgi, but only concentrated and stored in WPBs. No other pool of VWF potentially designated for basal secretion was detected.20 These latter findings are compatible with those of Tsai et al, and the recent work of Giblin et al, who reported that periodic WPB exocytosis accounts for approximately 80% of VWF release from unstimulated HUVECs.21,22
Antibodies made against 3 distinct regions of human ADAMTS-131,5,23 prevented HUVEC-released ADAMTS-13 from cleaving long VWF strings secreted from, and anchored to, the same and adjacent histamine-stimulated HUVECs. The presence of anti–ADAMTS-13 during HUVEC stimulation, and in the subsequent static buffer incubation periods, increased the number of long (>150 μm) HUVEC-anchored VWF strings. With ADAMTS-13 activity blocked in these experiments, the binding of inactivated ADAMTS-13 could be visualized directly at several points along the long, uncleaved, cell-anchored VWF strings.
When HUVECs were stimulated under static conditions by histamine to secrete many cell-anchored VWF strings rapidly, the local concentration of slowly and continuously released HUVEC ADAMTS-13 was not initially sufficient to cleave many of the strings. The uncleaved VWF strings could be observed, measured, and quantified by fluorescence microscopy. Over the course of 15 minutes, as the slow HUVEC release of ADAMTS-13 continued unchanged, the rate of HUVEC secretion of VWF strings progressively slowed from the initial rapid rate observed immediately after the addition of histamine. The cell-anchored VWF strings were then cleaved by the accumulating ADAMTS-13 that had been released slowly from the HUVECs.
The slow and continuous HUVEC release of ADAMTS-13 antigen was independent of HUVEC histamine stimulation. The ratio of HUVEC-released ADAMTS-13 to anchored VWF strings was initially low after the rapid secretion of many VWF strings from stimulated HUVECs. The low concentration of ADAMTS-13 resulted in only a few potential ADAMTS-13 cleavage events per VWF string. For this reason, much longer VWF multimers were released into solution by ADAMTS-13 cleavage of many VWF strings secreted rapidly by histamine-stimulated HUVECs at only a few VWF A2 1605-6 sites. This produced a low percentage of 176-kD VWF fragments per total VWF antigen.
In contrast, the ratio of HUVEC-released ADAMTS-13 to anchored VWF strings was relatively high during the periodic, basal secretion of only a few VWF strings from unstimulated cells, providing many potential ADAMTS-13 cleavage events per VWF string. The soluble VWF multimers released by ADAMTS-13 cleavage of these few VWF strings were cleaved at many VWF A2 1605-6 sites, as demonstrated by the high percentage of 176-kD VWF fragments per total VWF antigen.
Our results suggest that the number of ADAMTS-13 cleavage events on each VWF multimeric string and, consequently, the range of VWF multimeric sizes produced are directly related to the rate of VWF string secretion by stimulated and unstimulated endothelial cells. Analysis of VWF forms from normal human plasma indicated that the largest VWF multimers had been cleaved at only a few 1605-6 sites, as demonstrated by the extremely low ratio of the 140-kD fragments to total VWF antigen. This suggests that large plasma VWF multimers may have originated from VWF strings that were secreted rapidly from stimulated endothelial cells (Figure 6D). In contrast, the small VWF multimers in the CS fraction of normal plasma were cleaved at many 1605-6 sites, as demonstrated by the extremely high ratio of the 140-kD fragments to total VWF antigen (Figure 5D-E). The later result suggests that small plasma VWF multimers may have originated from VWF strings that were secreted slowly from unstimulated endothelial cells (Figure 6D).
VWF multimeric strings, once freed into solution, may have few (or no) additional A2 domains accessible to ADAMTS-13 cleavage. There is no evidence that endothelial cell VWF strings that have been cleaved by ADAMTS-13 as they are secreted (either a few or many times) into soluble VWF forms can then be cleaved again in plasma. Although it has been demonstrated previously that purified human VWF multimers can be cleaved by ADAMTS-13 into smaller forms in vitro in the presence of VWF denaturants (guanidine)24 or during VWF unfolding induced by extremely high shear forces (3351 and 4761 seconds−1),25 these are nonphysiologic processes. In normal blood vessels, wall shear rates range from 20 to 200 seconds−1 in veins and 500 to 1600 seconds−1 in arterioles.11,12
In conclusion, we have described the relationship between the secretion and cleavage rate of HUVEC VWF strings by HUVEC-released ADAMTS-13 under static conditions. The findings provide insight into the generation of the range of VWF multimers in plasma. HUVEC ADAMTS-13, released slowly and continuously (constitutively), is capable by itself of cleaving long, anchored VWF strings secreted in small numbers from the same and neighboring unstimulated cultured cells. This could be an important endothelial cell mechanism to counteract platelet adhesion/aggregation onto anchored VWF strings if microvascular blood flow is occluded, restricting the access of plasma ADAMTS-13. The slow and continuous released HUVEC ADAMTS-13 alone is inadequate to cleave the large number of VWF strings secreted rapidly from the same and nearby endothelial cells stimulated by an agonist that induces intense VWF string secretion.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We appreciate the mathematical contributions of Enrique Munoz of the Rice University Bioengineering Department.
This work was supported by grants from the Mary R. Gibson Foundation and by grants HL-78784 and HL-31950 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: N.A.T. designed and performed the experiments, did the analysis, prepared the figures, and drafted/edited the manuscript; L.N. was responsible for endothelial cell culture and multimer analysis; Z.M.R. first identified the ADAMTS-13 cleavage site in VWF and generated the Mabs against the amino acids flanking this site that recognize the VWF 176-kD and 140-kD fragments; and J.L.M. designed some experiments and assisted with writing and editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nancy A. Turner, Department of Bioengineering, Rice University, 6500 Main St, Houston, TX 77030; e-mail: nturner@rice.edu.

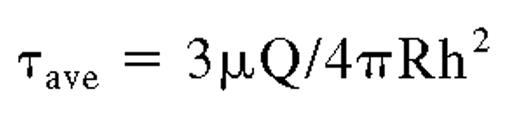
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal