After damage to the endothelium, platelets prevent excessive bleeding by adhering to the exposed ECM. In this issue of Blood, Kim and colleagues1 describe a novel function of TGFBIp that potentiates platelet adhesion and activation, a protein with a previously established role in corneal dystrophies.2
When platelets adhere, spread, and become activated, they form a procoagulant surface that stabilizes the wounded area. Platelets adhere to several extracellular matrix (ECM) proteins including collagen, von Willebrand factor (VWF), and fibrinogen.3 However, multiple interactions are necessary to secure platelet adhesion; mice deficient in fibrinogen and VWF still form occlusive thrombi.4 This suggests that other ECM or platelet-derived proteins contribute to adhesive events at the vascular wall. One of these proteins appears to be transforming growth factor beta–induced protein (TGFBIp). Interestingly, TGFBIp has been shown to affect sight. Abnormal deposition of TGFBIp mutants has been linked to blurred vision.2 In the current study, Kim et al go “outside the eye” to discover that platelets store TGFBIp and release it upon thrombin activation. Although future studies are necessary to resolve how TGFBIp accumulates inside platelets, the authors show that TGFBIp facilitates platelet adhesion and spreading and that soluble TGFBIp induces the expression of surface P-selectin and phosphatidylserine. Taken together, these data indicate that activated platelets may release TGFBIp, which, in turn, recruits additional platelets to the site of injury (see figure).
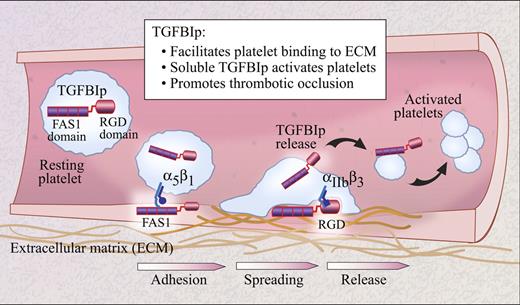
Schematic of the potential roles of TGFBIp in arterial thrombosis. Platelets adhere to the damaged vascular wall by a variety of mechanisms that may include interactions between the FAS1 domain of TGFBIp and integrin α5β1. The RGD-domain of TGFBIp may also contribute to platelet spreading via interactions with integrin αIIbβ3. As platelet agonists are generated at the site of injury, they induce release of TGFBIp from stored pools, which in turn activates other platelets and promotes thrombus formation.
Schematic of the potential roles of TGFBIp in arterial thrombosis. Platelets adhere to the damaged vascular wall by a variety of mechanisms that may include interactions between the FAS1 domain of TGFBIp and integrin α5β1. The RGD-domain of TGFBIp may also contribute to platelet spreading via interactions with integrin αIIbβ3. As platelet agonists are generated at the site of injury, they induce release of TGFBIp from stored pools, which in turn activates other platelets and promotes thrombus formation.
The mechanism behind TGFBIp-induced platelet activation requires further insight. The FAS1 domain of TGFBIp regulates platelet adhesion, in part through interactions with integrin α5β1. However, other partners likely participate since interruption of TGFBIp binding to α5β1 partially interferes with, but does not completely abrogate, platelet adhesion. Once adhesion has occurred, TGFBIp promotes platelet spreading through its RGD domain, most likely through interactions that involve integrin αIIbβ3. In this regard, the authors demonstrate that TGFBIp induces conformational activation of αIIbβ3. These intriguing observations indicate new roles for TGFBIp in platelet activation and provide an impetus for identifying the cognate TGFBIp receptor so that more in-depth mechanistic studies can be pursued.
Interestingly, too much TGFBIp may induce thrombosis. By simulating arterial shear rates, the authors demonstrate that platelets more readily adhere to collagen and form thrombi in the presence of TGFBIp. Furthermore, injections of epinephrine in mice in conjunction with increased levels of TGFBIp (through either coadministration of the recombinant protein or transgenic overexpression of TGFBIp) results in increased pulmonary emboli compared with mice receiving epinephrine alone.
So is TGFBIp an important player in arterial thrombosis? The pulmonary embolism model used by the authors provide evidence in favor of TGFBIp promoting increased platelet aggregation in the vasculature.5 Nevertheless, questions still remain about the in vivo relevance of TGFBIp in thrombus formation. Exploiting different thrombosis models, such as ferric chloride or laser-induced injury, may supply further support that elevated levels of TGFBIp foster thrombosis. The crux of its role, however, will need to be elucidated by other approaches that may include studies in murine platelets lacking TGFBIp or possibly in humans that possess mutations in the TGFBIp gene. These investigative avenues will help us visualize the exact function of TGFBIp in platelet biology and discern whether inhibitors that target TGFBIp may dampen a person's risk for thrombosis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal