Abstract
Asparaginase (ASP) therapy is associated with depletion of antithrombin (AT) and fibrinogen (FG). Potential toxicities include central nervous system thrombosis (CNST) and hemorrhage. Historical practice at the Izaak Walton Killam Health Centre (IWK) involves measuring AT and FG levels after ASP administration and transfusing fresh-frozen plasma (FFP) or cryoprecipitate (CRY) to prevent thrombotic and hemorrhagic complications. To determine whether this reduced these complications in children with acute lymphoblastic leukemia (ALL), incidence, outcome, and clinical characteristics of ASP-related CNST in ALL patients at IWK were compared with a similar cohort from BC Children's Hospital (BCCH), where prophylaxis was not performed. Costs associated with preventative versus expectant management were estimated. From 1990 to 2005, 240 patients were treated at IWK and 479 at BCCH. Seven BCCH patients developed venous CNST (1.5%), compared with none at IWK. CNST occurred exclusively during induction. Six patients received anticoagulation and continued ASP. All 7 patients remain in remission. National Cancer Institute high-risk ALL predicted CNST risk (P = .02), whereas sex, age, race, and body mass index did not. Neither FFP nor CRY protected against CNST, suggesting prophylaxis is unwarranted for unselected ALL patients. However, prophylactic replacement for HR patients in induction may be cost-effective.
Introduction
The cure rate for childhood acute lymphoblastic leukemia (ALL) has markedly improved in the last few decades through the use of multiagent chemotherapy.1 Asparaginase (ASP), a proteolytic enzyme, is a highly effective chemotherapeutic agent for ALL.2-5 Human lymphoblasts require exogenous asparagine for survival. Treatment with ASP induces a relative asparagine deficiency, ultimately resulting in leukemia cell death.6 ASP is associated with several severe complications, including an increased risk of developing thromboses, particularly in the central nervous system (CNS).7-13 Several studies have identified a direct association between the administration of ASP and development of thromboses.8,14-16 In particular, CNS thromboses (CNSTs) after ASP are reported to occur in approximately 1% to 3% of patients.17
Patients with ALL generally have comparatively higher circulating plasma levels of prothrombotic factors, such as thrombin, compared with age-matched healthy controls.18 Treatment with ASP results in decreased plasma levels of endogenous anticoagulants, such as antithrombin (AT).17,18 Given this presumptive additive mechanism, preventative therapy with fresh-frozen plasma (FFP), which contains AT, has the potential to normalize the plasma anticoagulant/procoagulant balance and decrease the incidence of thrombosis. However, other factors may also influence the risk of thrombosis including insertion of central venous catheters, hereditary thrombophilia, type of ASP used (Escherichia coli has been associated with higher incidence of thrombosis than Erwinia-derived ASP), and exposure to steroids (particularly prednisone).17,19 Paradoxically, ASP may also lead to a reduction in fibrinogen (FG) levels, resulting in delayed coagulation and risk of hemorrhage. Cryoprecipitate (CRY) contains high levels of FG and may contain some asparagine, making it potentially beneficial as prophylaxis for ASP-induced bleeding and thrombosis.
The long-time practice at the Izaak Walton Killam Health Centre in Halifax, Nova Scotia (IWK) has been to treat low AT or FG levels secondary to ASP (“Definitions”) with FFP or CRY, respectively. This practice is not widely performed across North America. However, some support for replacement therapy comes from the PARKAA (Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase) study.10,20 This study demonstrated a nonsignificant trend toward efficacy with a decreased incidence of thrombotic events in the AT-treated group despite an absence of correlative difference in plasma levels of AT and thrombin.10,20 The generalizability of the PARKAA study was limited by premature trial closure due to inadequate patient recruitment. Thus, although there is some theoretic support for prophylaxis, conclusive evidence for primary prevention is lacking.17
In this study, we examined whether prophylactic correction of decreased AT and FG levels by FFP or CRY, respectively, reduced the incidence of clinically evident CNST or hemorrhage in a population of children with ALL receiving ASP. To undertake this study, we compared the IWK cohort with a similar ALL cohort that did not receive FFP and CRY prophylaxis. Both the IWK and BC Children's Hospital in Vancouver, BC (BCCH) are legacy Children's Cancer Group institutions using identical treatment protocols with matched frequency and dosing for ASP. Supportive care is comparable, save for prophylactic product replacement, which is not practiced at BCCH. We assessed whether clinical factors including patient age, sex, body mass index (BMI), race, or ALL risk group predisposed patients with ALL to developing CNST after ASP. In addition, we estimated costs of a prophylactic strategy to avoid CNST versus expectant management of CNST events.
Methods
A retrospective chart review of all patients with ALL treated between 1990 and 2005 through the institutional databases at the IWK and BCCH was conducted. Data were abstracted from both electronic and paper versions of the medical records by L.S.A. and J.N.B. at the IWK and M.D. and D.D. at BCCH. Data analysis for all patients occurred at the IWK using unique identifiers. Demographic data included treatment protocol, weight, and height at the start of therapy, National Cancer Institute (NCI) leukemia risk group, and incidence of CNST. Details of clinical presentation, investigations, and management were also recorded for patients with CNST. For patients at the IWK, AT and FG levels, as well as frequency of replacement therapy with CRY and FFP, were recorded. As many of the IWK patients received continuing care in their local communities, every effort was made to contact these institutions to provide the most comprehensive set of data.
The study was approved by IWK and BCCH ethics review boards.
Definitions
A clinically significant CNST or hemorrhage was defined as one that presented with CNS-related symptoms and was documented by computed tomography or magnetic resonance imaging (MRI). Patient age was defined as the age at diagnosis. Obesity was defined as a body mass index (BMI) greater than or equal to the 95th percentile for patients older than 2 years.21 ALL risk group refers to risk as defined by NCI criteria.22 High-risk (HR) patients were identified by white blood cell (WBC) count higher than 50 ×109/L (50 000/μL) and/or age younger than 1 year or older than 10 years at diagnosis.
Criteria for prophylaxis at the IWK has changed over time. For FFP, before October 2001, prophylaxis was recommended for AT levels less than 50% of the lower limit of normal range. Subsequently, an absolute AT value of less than 50 U/mL was deemed appropriate for replacement. For the purpose of this study, any replacement provided at an AT level of 40 to 60 U/mL was deemed in accordance with these criteria by consensus opinion. For CRY, before October 2001, prophylaxis was recommended for fibrinogen levels less than 50% of the lower limit of normal. Subsequently, an absolute FG level less than 1 g/L was used for replacement. For the purpose of this study, CRY replacement at levels of 0.8 to 1.2 g/L was deemed in keeping with these criteria. If FFP or CRY was provided outside of these criteria by individual physician decision, these administrations were designated as “outside criteria.”
Cost analysis
Cost analysis used current estimates obtained at the IWK. All values are in Canadian dollars. Cost for length of stay on the oncology unit is $1200/day and for an intensive care unit bed, $2592/day. Cost for an MRI of the head is $662. AT and FG levels cost $16 and $12 per test, respectively. Cost per transfusion is $99 for either FFP or CRY (excluding nursing administration costs).
Statistical analysis
The comparative rates of thromboses between the 2 centers were analyzed using Fisher exact test, because CNST is a rare event. Fisher exact test was also used in a univariate analysis to examine the following as predictors of thrombosis: obesity, race (white vs other), NCI leukemia risk group (high vs standard risk), sex, and whether FFP or CRY prevented CNST. Logistic regression analysis was used to examine age as a continuous variable as a risk factor for CNST. Multivariate analysis by logistic regression modeling was used to assess these same parameters in both the entire cohort and in subsets defined by race and leukemia risk group.
Results
A total of 719 patients with a new diagnosis of ALL were included from 1990 to 2005, with 240 patients seen at the IWK and 479 at BCCH. There were no patients with CNS thrombosis at the IWK. At BCCH, 7 children (1.5%) had a symptomatic CNST and all occurred during the induction phase of therapy. All of these patients received prednisone as the steroid. All CNSTs were venous in origin, with 6 involving the sagittal sinus and 1 involving the transverse sinus. There were no episodes of CNS hemorrhage. Patients who had a CNST presented with either generalized (5/7) or focal seizures (2/7), and required additional hospital stays (mean, 13 days; range, 4-27 days), including intensive care unit admissions (3/7), and treatment with one or more anticonvulsants (duration of anticonvulsants: mean, 262 days; range, 14-877 days). Six patients received anticoagulation and had ASP therapy continued. One patient received no anticoagulation, but ASP was discontinued. All 7 remain in remission from ALL at a median follow-up of 11.7 years (Table 1).
Outcomes for the 7 patients with CNS thrombosis (none treated with prophylactic AT or FG replacement)
| . | Patient no. . | ||||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | |
| Leukemia risk | High | High | High | Standard | High | High | High |
| Location of thrombosis | Sagittal sinus | Sagittal sinus | Sagittal sinus | Sagittal sinus | Left transverse sinus | Sagittal sinus | Sagittal sinus |
| Phase of therapy | Induction | Induction | Induction | Induction | Induction | Induction | Induction |
| Symptoms | Focal and generalized seizures, headaches, vomiting | Focal seizures, headaches, vomiting | Generalized seizures | Generalized seizures | Generalized seizures | Generalized seizures, unilateral weakness | Focal seizures, unilateral weakness, motor dysphagia |
| Primary treatment | LMWH | LMWH | Heparin | None | LMWH | Heparin | LMWH |
| Secondary treatment | Warfarin | Warfarin | Warfarin | None | None | LMWH | None |
| Thrombosis outcome | Persistence of clot | Partial resolution of clot | Resolution of clot | Resolution of clot | Resolution of clot | Resolution of clot | Partial resolution of clot |
| Symptoms outcome | Resolved | Resolved | Resolved | Resolved | Resolved | Resolved | Resolved |
| ASP | Continued | Continued | Continued | Discontinued | Continued | Continued | Continued |
| Leukemia outcome | CR1 | CR1 | CR1 | CR1 | CR1 | CR1 | CR2 |
| . | Patient no. . | ||||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | |
| Leukemia risk | High | High | High | Standard | High | High | High |
| Location of thrombosis | Sagittal sinus | Sagittal sinus | Sagittal sinus | Sagittal sinus | Left transverse sinus | Sagittal sinus | Sagittal sinus |
| Phase of therapy | Induction | Induction | Induction | Induction | Induction | Induction | Induction |
| Symptoms | Focal and generalized seizures, headaches, vomiting | Focal seizures, headaches, vomiting | Generalized seizures | Generalized seizures | Generalized seizures | Generalized seizures, unilateral weakness | Focal seizures, unilateral weakness, motor dysphagia |
| Primary treatment | LMWH | LMWH | Heparin | None | LMWH | Heparin | LMWH |
| Secondary treatment | Warfarin | Warfarin | Warfarin | None | None | LMWH | None |
| Thrombosis outcome | Persistence of clot | Partial resolution of clot | Resolution of clot | Resolution of clot | Resolution of clot | Resolution of clot | Partial resolution of clot |
| Symptoms outcome | Resolved | Resolved | Resolved | Resolved | Resolved | Resolved | Resolved |
| ASP | Continued | Continued | Continued | Discontinued | Continued | Continued | Continued |
| Leukemia outcome | CR1 | CR1 | CR1 | CR1 | CR1 | CR1 | CR2 |
ASP indicates asparaginase; LMWH, low-molecular-weight heparin; CR1, first complete remission; and CR2, second complete remission.
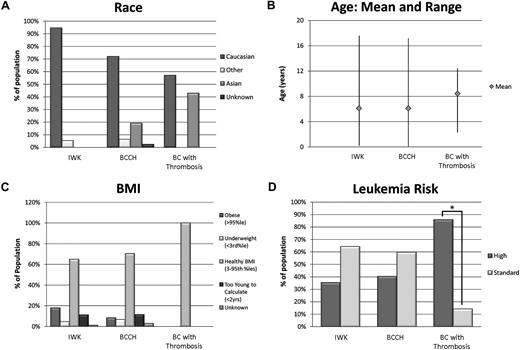
Demographics are shown in Table 2. Males were more commonly affected by CNST (5/7). Distribution of race appeared consistent with that of the geographic area. Interestingly, 3 of 7 patients affected by CNST were of Asian descent, which comprised only 19% of BCCH patients. Importantly, 6 of 7 patients (86%) who developed a CNST were in the NCI HR category, with 4 of these HR patients older than 10 years. One patient younger than 1 year, and 1 patient with WBC count higher than 50 ×109/L (50 000/μL). Sex (P = .48), race (P = .13), age (P = .11), and BMI (P = .60) were not found to be statistically significant risk factors for CNST (Table 2, Figure 1). The only significant risk factor identified for CNST was NCI HR ALL at diagnosis both by univariate (P = .02) and multivariate (P = .04) analyses. Those children with HR ALL had 9.6 greater odds of developing CNST compared with those with standard-risk disease (95% CI, 1.2-80).
Patient demographics and risk factors for CNST
| Demographic . | IWK (%) . | All BCCH patients (%) . | CNST, all BCCH (%) . |
|---|---|---|---|
| No. of patients | 240 | 479 | 7 |
| Sex | |||
| Female | 110 (46) | 204 (43) | 2 (29) |
| Male | 130 (54) | 275 (57) | 5 (71) |
| Risk | |||
| High | 85 (35) | 192 (40) | 6 (86) |
| By age | 59 | 104 | 5 |
| By WBC count | 26 | 88 | 1 |
| Standard | 154 (64) | 283 (59) | 1 (14) |
| Unknown | 1 | 4 | 0 |
| Race | |||
| White | 227 (95) | 345 (72) | 4 (57) |
| Asian | 0 | 92 (19) | 3 (43) |
| Other | 13 (5) | 30 (6) | 0 |
| Unknown | 0 | 12 | 0 |
| Age, y | |||
| Range | 0.2-17.6 | 0-17.2 | 2.3-12.1 |
| Mean | 6.1 | 6.1 | 8.4 |
| Unknown | 1 | 0 | 0 |
| BMI | |||
| Obese (higher than 95th percentile) | 43 (18) | 40 (8) | 0 |
| Healthy BMI | 156 (65) | 33 (70) | 7 (100) |
| Underweight (lower than 3rd percentile) | 11 (4) | 32 (7) | 0 |
| Too young (< 2 y) | 27 | 55 | 0 |
| Unknown | 3 | 10 | 0 |
| FFP prophylaxis | 86 (37) | 0 | 0 |
| CRY prophylaxis | 163 (68) | 0 | 0 |
| Demographic . | IWK (%) . | All BCCH patients (%) . | CNST, all BCCH (%) . |
|---|---|---|---|
| No. of patients | 240 | 479 | 7 |
| Sex | |||
| Female | 110 (46) | 204 (43) | 2 (29) |
| Male | 130 (54) | 275 (57) | 5 (71) |
| Risk | |||
| High | 85 (35) | 192 (40) | 6 (86) |
| By age | 59 | 104 | 5 |
| By WBC count | 26 | 88 | 1 |
| Standard | 154 (64) | 283 (59) | 1 (14) |
| Unknown | 1 | 4 | 0 |
| Race | |||
| White | 227 (95) | 345 (72) | 4 (57) |
| Asian | 0 | 92 (19) | 3 (43) |
| Other | 13 (5) | 30 (6) | 0 |
| Unknown | 0 | 12 | 0 |
| Age, y | |||
| Range | 0.2-17.6 | 0-17.2 | 2.3-12.1 |
| Mean | 6.1 | 6.1 | 8.4 |
| Unknown | 1 | 0 | 0 |
| BMI | |||
| Obese (higher than 95th percentile) | 43 (18) | 40 (8) | 0 |
| Healthy BMI | 156 (65) | 33 (70) | 7 (100) |
| Underweight (lower than 3rd percentile) | 11 (4) | 32 (7) | 0 |
| Too young (< 2 y) | 27 | 55 | 0 |
| Unknown | 3 | 10 | 0 |
| FFP prophylaxis | 86 (37) | 0 | 0 |
| CRY prophylaxis | 163 (68) | 0 | 0 |
CNST indicates central nervous system thrombosis; BCCH, BC Children's Hospital; BMI, body mass index; FFP, fresh-frozen plasma; and CRY, cryoprecipitate.
Risk factors for CNS thrombosis. Leukemia risk group was the only risk factor for CNST by both univariate (P = .02) and multivariate (P = .04) analyses. Race (P = .13), age (P = .11), and BMI (P = .60) were not found to be statistically significant risk factors for CNST. CNST indicates central nervous system thrombosis; and BMI, body mass index.
Risk factors for CNS thrombosis. Leukemia risk group was the only risk factor for CNST by both univariate (P = .02) and multivariate (P = .04) analyses. Race (P = .13), age (P = .11), and BMI (P = .60) were not found to be statistically significant risk factors for CNST. CNST indicates central nervous system thrombosis; and BMI, body mass index.
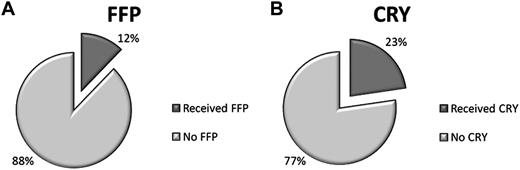
Overall, 86 patients (12%) of the total study population (n = 719) received FFP, and 163 (23%) received CRY (Figure 2). No patient had a significant transfusion reaction reported. FFP administration did not significantly reduce the risk of CNST, even when the HR cohort was examined separately. At the IWK, 37% of children received FFP (mean, 3 transfusions; range, 1-20) with 66% of these cases meeting criteria. Similarly, CRY administration did not significantly effect outcome for either HR (P = .34) or standard-risk (P > .99) patients. Of the 68% who received CRY (mean, 3 transfusions; range, 1-21), 83% of these patients were managed according to criteria (Table 2).
Prophylaxis for CNS thrombosis. The percentage of all 719 patients who received (A) FFP or (B) CRY prophylaxis during the study period. FFP indicates fresh-frozen plasma; and CRY, cryoprecipitate.
Prophylaxis for CNS thrombosis. The percentage of all 719 patients who received (A) FFP or (B) CRY prophylaxis during the study period. FFP indicates fresh-frozen plasma; and CRY, cryoprecipitate.
Financial analysis was performed to estimate the cost of managing a CNST compared with prophylaxis. The mean cost for managing a CNST considering only length of hospital stay and MRI imaging was $19 171 per patient. This results in a minimum total cost of $134 197 to care for the 7 patients who had a CNST. To evaluate the cost of prophylaxis, we performed an intention-to-treat analysis, incorporating measuring AT and FG levels and administering FFP and CRY transfusions to the entire IWK cohort. Patients who underwent prophylaxis received a mean of 12 AT and 13 FG levels and a mean of 3 FFP or CRY transfusions. The median cost to provide prophylaxis to all patients at the IWK was $497 per patient (mean, $622; range, $0-$5435; standard deviation, $580; 95% CI, $548-$695). If this median cost is applied to calculate the price of providing prophylaxis to all 479 patients treated at BCCH, the total cost is $238 063. If only the HR subgroup is considered at the IWK, the median cost of prophylaxis was $339 (mean, $514; range, $0-$2343; standard deviation, $480; 95% CI, $411-$617). If this median cost is applied to calculate the price of providing prophylaxis to only the 192 HR patients at BCCH, the cost is $65 088.
Discussion
There are several studies in the literature demonstrating increased thrombosis risk in children with ALL.7,8,10,13,16-19,23 A recent meta-analysis from Caruso et al19 estimated the occurrence of symptomatic thrombosis during treatment, from diagnosis to the end of the maintenance, to be 5.2% overall and 2.9% for CNST. The majority of non-CNST thromboses involved the upper limbs.19 Ascertainment of upper-limb thrombosis is clinically more subjective and often in association with a central venous device; thus, we chose to focus on CNST. In our study, the rate of CNST was somewhat lower, at 1.5% in the BCCH population and 1% overall.
Children with ALL have increased thrombin generation at the time of diagnosis, but thromboembolic events typically occur once therapy has begun, implicating an interaction of the disease process with therapy.24 ASP and steroids induce a hypercoaguable state by suppression of natural anticoagulation, especially AT and plasminogen, and by elevations in the factor VIII/von Willebrand factor complex, but how and why this occurs is not entirely clear. It may be related to an inflammatory response and/or the capability of tumor cells to generate procoagulant molecules and activate endothelial cells, platelets, and macrophages.24
Several approaches have been considered for correcting ASP-induced AT deficiency. Low-dose warfarin may effectively prevent CNST and has the advantage of being neither a blood product nor AT independent, but it is difficult to maintain an effective and safe dose during chemotherapy.25 FFP prophylaxis, as used in our study, is another option, although both procoagulant and anticoagulant proteins are contained within FFP. AT concentrates are available and may be effective as suggested by the PARKAA study.20 A more recent option may be direct thrombin inhibitors (DTIs). Kuhle et al26 showed in vitro that DTIs provide consistent anticoagulant responses independent of AT levels in plasma from children with ALL. In contrast, low-molecular-weight heparin effects were profoundly influenced by endogenous AT levels.26 Although DTIs appear a promising strategy, to date only one agent, argatroban, is Food and Drug Administration–approved for children with heparin-induced thrombocytopenia.27 Clinical studies in children with ALL have not yet been undertaken.
Seven children at BCCH who did not receive prophylaxis had a CNST, compared with none at the IWK, where 37% and 68% of patients received FFP and CRY prophylaxis, respectively. However, we did not have enough power to demonstrate a protective effect of either FFP or CRY on CNST or hemorrhage. Interestingly, we observed that all CNSTs were venous, occurred during induction and predominantly in NCI HR patients due to age. These observations are consistent with those of Caruso et al19 who found 29% of thrombotic events occurred in the CNS venous system, predominantly during induction therapy in children with ALL, and with Athale et al's prospective analysis, identifying ALL patients older than 10 years and those with HR disease as having a significantly higher incidence of symptomatic thromboses.8 Interestingly, all patients with CNST had received prednisone as the steroid during induction. Prednisone may be associated with a higher risk of CNST compared with dexamethasone.19,28,29
Although the numbers of CNST were small and all of these patients remain in remission from ALL, the clinical impact of a CNST on both the patient and their families is significant and prevention remains highly desirable. Although not proven by our study, our data suggest that targeting prophylaxis to this HR cohort during induction may be a more reasonable strategy compared with universal prophylaxis throughout ALL therapy. This proposition is supported by the recent CAPELAL study in which adult patients with ALL or lymphoblastic lymphoma who received prophylactic AT concentrates during induction had a substantially lower rate of thrombosis (5%) compared with those who did not (13%, P = .04).30 Similarly, in the PARKAA study, a trend toward fewer thromboses was seen in the arm that received prophylactic AT concentrates compared with the untreated arm (28% vs 37%, P = .43).10,20 In addition, in the PARKAA study, the prevalence of asymptomatic thromboses on routine imaging was 37% overall (far higher than the 5% clinically identified), indicating that a considerable number of thromboses may be missed.10 The clinical significance of asymptomatic lesions remains uncertain, but may reflect a larger burden of illness than previously anticipated.
Race was not statistically significantly correlated with CNST (P = .13), although the trend toward a disproportionate number of Asian children who developed a CNST was intriguing. Historically, black and Hispanic patients with ALL have not fared as well as their white counterparts.31,32 Our study suggests that it might be informative to examine whether race, with an expected divergence in inherited polymorphisms, predisposes to thromboses associated with ASP. Children with an underlying inherited thrombophilia may also have an increased risk for developing thromboses during ALL therapy.19,33 A meta-analysis of 5 studies showed that the presence of at least one genetic prothrombotic factor was associated with an 8-fold increase in thrombosis risk in children with ALL. By contrast, others have suggested thrombophilia does not play a major role.10,23,34 Five of the 7 patients with CNST had thrombophilia workups completed, but no evidence of an inherited thrombophilia. Further investigation into the contribution of inherited thrombophilia to CNST during ALL therapy in children is warranted.
Using intention-to-treat analysis, we found that a prophylactic strategy for the entire BCCH cohort would have been more costly than treatment for the 7 patients who suffered a CNST. HR ALL patients appear to be particularly at risk, although we have insufficient evidence to definitively determine whether age or elevated white blood cell count is the critical risk factor. Thus, we propose a prophylactic strategy targeted to all BCCH HR patients. The comparative cost of prophylaxis was half that required to treat the 7 affected patients ($65 088 vs $134 197). Moreover, the cost estimate for CNST treatment is likely an underestimate, not taking into account costs of anticoagulant and anticonvulsant therapies, specialist consultations, electroencephalographies, and other medical investigations. In addition, the cost for prophylaxis is more likely an overestimate, as the IWK strategy intervened at each ASP exposure throughout therapy by measuring both FG and AT levels and replacing with CRY or FFP, respectively. Our study suggests that CNS hemorrhage is exceedingly rare, with no documented cases in either treated or untreated patients, thus evidence is lacking to support CRY replacement for low FG levels. By comparison, our data indicate that CNST prophylaxis could be restricted to HR patients during induction and may be both medically and financially justified.
Our data suggest that CNST is a rare event in patients with ALL receiving ASP and occurs preferentially during the induction phase of chemotherapy. This study is the first to focus on CNST as a consequence of ASP and directly evaluate the impact of prophylaxis with FFP or CRY on a large cohort of patients treated at 2 institutions with otherwise comparable care. However, being a retrospective examination of a rare event, our study lacks the statistical power to make definitive recommendations regarding prophylaxis for CNST. We believe a large multicenter prospective trial comparing targeted prophylactic strategies using modern therapies, such as DTIs, to conclusively establish the efficacy and cost-effectiveness of thrombosis prophylaxis in ALL is warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jocelyn Jaques for database support, Emily Murray for assistance with data collection, and Leota Dickey for assistance with data collection and review of laboratory standards.
Authorship
Contribution: L.S.A. collected and analyzed data and assisted with the writing of the manuscript; M.D. collected and analyzed data; C.V.F. helped design the study, assisted with data analysis, and edited the manuscript; D.D. participated in study design, and data collection and analysis, and edited the manuscript; V.E.P. analyzed data and edited the manuscript; H.W. performed statistical analysis; L.P. analyzed data and supervised statistical analysis; M.Y. generated the patient database and participated in data analysis; C.F. participated in data collection and organization; D.R.B. originated the prophylactic regimens and participated in study design; and J.N.B. designed the study, collected and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason N. Berman, Division of Pediatric Hematology/Oncology, Departments of Pediatrics and Microbiology/Immunology, Dalhousie University, IWK Health Centre, PO Box 9700, 5850/5980 University Ave, Halifax, NS B3K 6R8 Canada; e-mail: jason.berman@iwk.nshealth.ca.