The cell surface phenotype used to define an EPC, in one commonly used in vitro assay, may arise from an uptake of contaminating platelet MPs by cultured mononuclear cells, resulting in a gross misinterpretation of the assay results.
Since the first description of a circulating endothelial progenitor cell (EPC) in human blood possessing the ability to form blood vessels de novo (postnatal vasculogenesis), investigations of the role of EPCs in cardiovascular disease and repair have exploded.1 The rapid translation of approaches showing favorable EPC-mediated amelioration of ischemic disease in animal models to clinical trials of EPC or EPC-related cell infusions into human patients suffering from peripheral arterial disease or myocardial infarction has also been remarkable, though the benefits reported thus far have been far less than anticipated from the preclinical data. Some of the potential reasons for the failure of EPCs to deliver more robust results in human clinical trials have already been reviewed.2,3
One of the greatest challenges in studying human EPC biology is the lack of a specific marker to unequivocally identify this circulating cell subset.4 At present, human EPCs are identified and quantitated using 3 general approaches. One may use flow cytometry and monoclonal antibodies to delineate certain cell surface antigens such as CD34, CD133, and vascular endothelial growth factor 2 receptor (KDR) that may enrich for cells that display some ability to participate in new blood vessel formation. However, the peripheral blood cells displaying these antigens do not possess actual in vivo vasculogenic activity, though ample data from clinical studies suggest some role for these cells in maintaining cardiovascular health.4
In another set of assays, one may quantify colony-forming cells using a colony forming unit-Hill (CFU-Hill) or an endothelial colony-forming cell (ECFC) assay. Recent studies indicate that the CFU-Hill assay measures a heterogenous mixture of hematopoietic cells that ultimately permit outgrowth of alternatively activated macrophages having significant stimulatory effects on angiogenesis but fail to contribute to postnatal vasculogenic acitivity directly.5 In contrast, the ECFC assay permits identification of clonal colonies of endothelium with a hierarchy of levels of proliferative potential that form human blood vessels when seeded within 3-dimensional collagen/fibronectin gels and implanted into immunodeficient mice.6
Finally, some investigators simply plate peripheral blood mononuclear cells on fibronectin-coated tissue culture plates and, after 3 to 4 days, remove and discard the nonadherent cells to permit recovery of the adherent cell fraction.7 The adherent cells are then generally assessed for the ability to ingest acetylated low-density lipoprotein (acLDL) and to bind Ulex europaeus agglutinin 1 (UEA1) plant lectin and, if positive, are defined as EPCs. The function of these cultured cells in promoting new vessel formation in preclinical animal models has led to use of a similar culture protocol to generate human progenitor cells for transplantation in some human clinical trials.8
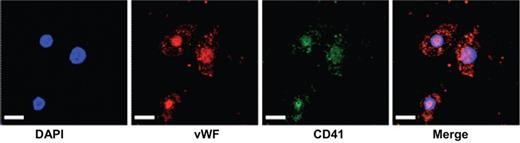
In this issue, Prokopi et al9 report that proteomic analysis reveals the presence of platelet microparticles (MPs) in a standard assay for putative EPCs. Peripheral blood mononuclear cells (PBMNCs) were isolated and plated on fibronectin for EPC culture. MPs present in the EPC-conditioned medium were isolated and analyzed by proteomic approaches and numerous platelet enriched proteins were detected. Using a variety of tests, the authors demonstrate that platelets are a normal contaminant in the PBMNC population and these cells rapidly disintegrate to form MPs under the EPC culture conditions. Platelet MP-derived proteins were found to be taken up and displayed by the adherent PBMNC (despite lack of mRNA for the same proteins in the PBMNC; see figure). While the EPC-conditioned medium stimulated endothelial tube formation in the Matrigel assay, removal of the platelet MPs from the EPC-conditioned medium attenuated this angiogenic effect. Additional data from a large clinical trial indicated that, apart from circulating monocytes, only the platelet count emerged as a significant predictor for patient circulating EPC concentrations in the general population.
Cultured monocytic cell line stained with DAPI for nuclear detection, von Willebrand factor (VWF), CD41 (glycoprotein IIb), and merged image, demonstrating the uptake of platelet-derived MPs by the adherant cells. Scale bar 25 μm. See the complete figure in the article beginning on page 723.
Cultured monocytic cell line stained with DAPI for nuclear detection, von Willebrand factor (VWF), CD41 (glycoprotein IIb), and merged image, demonstrating the uptake of platelet-derived MPs by the adherant cells. Scale bar 25 μm. See the complete figure in the article beginning on page 723.
While the results of Prokopi et al implicate platelet MP transfer of proteins to PBMNC as a major variable that could result in overestimation of EPC numbers in this particular assay, the study failed to examine which PBMNCs take up the MPs and whether all of the angiogenic effects of the adherent PBMNCs are platelet MP-derived. These results suggest that this particular assay for EPCs is not reliable due to the inability to discriminate between MP transfer of platelet cell surface proteins and host cell display of similar transcribed/translated proteins. These data also point out the ongoing need for development of specific functional assays to define EPCs and to continue to strive for discovery of novel unique EPC-restricted cell surface markers.
Conflict-of-interest disclosure: M.C.Y. is a cofounder and consultant for EndGenitor Technologies Inc in Indianapolis, IN. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal