Abstract
The concept of endothelial progenitor cells (EPCs) has attracted considerable interest in cardiovascular research, but despite a decade of research there are still no specific markers for EPCs and results from clinical trials remain controversial. Using liquid chromatography–tandem mass spectrometry, we analyzed the protein composition of microparticles (MPs) originating from the cell surface of EPC cultures. Our data revealed that the conventional methods for isolating mononuclear cells lead to a contamination with platelet proteins. Notably, platelets readily disintegrate into platelet MPs. These platelet MPs are taken up by the mononuclear cell population, which acquires “endothelial” characteristics (CD31, von Willebrand factor [VWF], lectin-binding), and angiogenic properties. In a large population-based study (n = 526), platelets emerged as a positive predictor for the number of colony-forming units and early outgrowth EPCs. Our study provides the first evidence that the cell type consistent with current definitions of an EPC phenotype may arise from an uptake of platelet MPs by mononuclear cells resulting in a gross misinterpretation of their cellular progeny. These findings demonstrate the advantage of using an unbiased proteomic approach to assess cellular phenotypes and advise caution in attributing the benefits in clinical trials using unselected bone marrow mononuclear cells (BMCs) to stem cell-mediated repair.
Introduction
Numerous studies have demonstrated that endothelial progenitor cells (EPCs) are present among peripheral blood mononuclear cells (PBMNCs) and represent a subset of circulating bone marrow mononuclear cells (BMCs), which have the capacity to differentiate into endothelial cells in vivo.1 New concepts of stem cell–based therapies for myocardial regeneration resulted in a rapid translation into a clinical context.2-4 Yet, key questions remain unanswered. Importantly, the nomenclature and the phenotype of EPCs are subject to ongoing controversy and there are currently no specific markers that unambiguously identify these cells.5,6 Thus, a more comprehensive approach is needed to analyze their antigenic profiles.
MPs are small membrane vesicles (0.2-1.0 μm) that originate from the plasma membrane and are shed from the cell surface after activation and apoptosis.7 Importantly, MPs retain membrane antigens specific for the parent cell they originate from. Thus, MPs represent an ideal subproteome to clarify the cellular progeny of EPC cultures and mass spectrometry is the instrument of choice for this kind of research.8 In this study, we used a proteomic approach to identify membrane proteins present on MPs in EPC cultures.
Methods
EPC culture
The study was approved by the ethics review board of J. W. Goethe University and King's College London. Peripheral blood was collected from healthy adult volunteers and informed consent was obtained in accordance with the Declaration of Helsinki. EPC cultures were performed as previously described.9,10 In brief, PBMNCs from healthy volunteers were isolated by Lymphoprep (1.077 g/mL; Axis-Shield PoCAS) density barrier centrifugation. The low-density fraction (< 1.077 g/mL) was carefully removed from the interface and washed 3 times with PBS (Dulbecco phosphate-buffered saline; Sigma-Aldrich) containing 2% FBS (fetal bovine serum, filtered and heat inactivated; Gibco, Invitrogen). Immediately after isolation, the cells were counted and 8 × 106 cells were plated on fibronectin-coated (10 μg/mL fibronectin from human plasma; Sigma-Aldrich) 12-well plates containing 1 mL endothelial basal medium (EBM; Cambrex Bio Science) supplemented with 20% FBS, EGM SingleQuots (10 μg/mL epidermal growth factor, 3 μg/mL bovine brain extract, 50 μg/mL gentamicine, 50 μg/mL amphotericin-B, 1 μg/mL hydrocortisone; Cambrex Bio Science) and 10 ng/mL human vascular endothelial growth factor 165 (hVEGF 165; R&D Systems). Before use, the medium was passed through a 0.2-μm filter. After 3 days in culture, the nonadherent cells were removed and fresh EBM medium was added. The medium was changed on day 5 and cells were kept in culture until day 7. The EPC phenotype was confirmed by the presence of endothelial markers and the uptake of 1,19-dioctadecyl-3,39-tetramethylindo-carbocyanine-labeled acetyl low-density lipoprotein (DiI-Ac-LDL) and binding of Ulex europaeus I agglutinin (UEA-1) as described previously.10
Isolation of MPs derived from EPCs
EPC cultures were subject to overnight (O/N) serum deprivation at different time points (eg, at days 3 and 7). Supernatants were collected and centrifuged at 400g for 15 minutes to remove floating cells and cellular nuclei, followed by a second centrifugation step at 12 500g for 5 minutes to remove cell debris and apoptotic bodies. MPs were pelleted from this precleared supernatant in a final centrifugation step at 20 500g for 150 minutes at 4°C (Sorvall RC-6 Plus; Thermo Fisher Scientific).
Electron microscopy
Agarose-enclosed (2% low gelling agarose, 40°C) MP-pellets from the last centrifugation step were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer pH 7.3 to 7.4, postfixed in 1% OsO4, and embedded in Epon epoxy resin. Ultrathin sections (75-nm each) were stained with uranyl acetate and lead citrate. High-resolution transmission electron microscopic (TEM) analysis was done at 80 kV in a JEOL 1200 EX electron microscope (Jeol).
1D gel-LC-MS/MS
For proteomics, MPs from EPC cultures of 4 healthy subjects were pooled to obtain sufficient material. MPs were reconstituted in Laemmli buffer and separated by SDS–polyacrylamide gel electrophoresis (PAGE) gels. Large-format gradient gels (4%-12%) were cast using the a2DE optimizer (NextGen Sciences). After the gels were overlaid with water-saturated butanol (2:1) and left to polymerize overnight, the stacking gel containing 4% to 5% acrylamide weakly buffered at pH 9.0 was cast over the already set resolving gel. Once samples were loaded, a constant 50-mV current was applied as proteins migrated down the stacking gel; at the stacking gel/running gel boundary the current was increased and maintained at 75 mV until the dye front reached the end of the gel. After silver staining, all gel bands were excised, and subject to in-gel tryptic digestion according to published methods modified for use with an Investigator ProGest (Genomic Solutions) robotic digestion system. After enzymatic degradation, peptides were separated by a nanoflow high-performance liquid chromatography (HPLC) system (Ultimate 3000; Dionex) on a reverse-phase column and applied online to a LTQ XL ion-trap tandem mass spectrometer (MS/MS). Spectra were collected from the ion-trap mass analyzer using full ion scan mode over the mass-to-charge (m/z) range 300 to 2000. MS/MS scans were performed on each ion using dynamic exclusion. Database search was performed using the SEQUEST v 28 (rev 13; BioWorks version 3.3.1; ThermoFisher Scientific) against a human/bovine UniProt/SPROT database.11 One missed cleavage per peptide was allowed and carbamidomethylation of cysteine as well as partial oxidation of methionine were assumed. The following filters were applied: Xcorr values of more than 2.0 (singly charged ions), more than 2.5 (doubly charged ions), and more than 3.5 (triply charged ions), deltaCN more than 0.1, a minimum of 2 peptides, and a probability score less than e−003. Scaffold (version 2.0; Proteome Software) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the “PeptideProphet” algorithm.12 Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. A detailed methodology is available on our website http://www.vascular-proteomics.com.
Culture of endothelial cells
Human umbilical venous endothelial cells (HUVECs) were isolated from human umbilical cords and cultured in M199 medium supplemented with 1 ng/mL ECGF, 3 μg/mL ECGS, 10 U/mL heparin, 2.5 μg/mL thymidine, and 5% FBS. The cells were grown in T75 flasks coated with gelatin (Sigma-Aldrich), incubated at 37°C in 5% CO2, and passaged every 2 days.
Culture of THP-1 cell line
The human monocytic THP-1 cell line (ATCC) was cultured in ATCC-formulated RPMI-1640 Medium (ATCC) supplemented with 10% FBS.
Reverse-transcription PCR
Total RNA was extracted from EPCs using the Trizol Plus RNA Purification Kit (Invitrogen) according to the manufacturer's instructions. Total RNA from other cell lines was extracted with the RNeasy Mini Kit and QIAshredder (QIAGEN) according to the manufacturer's protocol. RNA (5 μg) was converted to cDNA using the ImProm-II Promega Reverse Transcription System (Promega). The cDNA products were amplified by polymerase chain reaction (PCR) using human-specific primers for CD31 and integrins (primer sequences are shown in supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The PCR conditions were as follows: 94°C for 4 minutes and then 35 cycles at 94°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute followed by 72°C for 5 minutes. The PCR products were detected by separation on 2% agarose gels (Ultrapure Agarose; Invitrogen) and ethidium bromide staining.
Full blood count
Blood (1 mL) was collected into BD Vacutainer EDTA tubes (4 mL, K2EDTA, BD Vacutainer; Becton Dickinson) and mononuclear cell preparations were obtained after Lymphoprep density barrier centrifugation. The cell preparations were analyzed in duplicate using an automated cell counter (Advia 2120; Siemens Medical Solutions Diagnostic) to determine the number of white cells and platelets present by optical analysis. The different types of white cells present were distinguished by light scatter and myeloperoxidase (MPO) activity after being exposed to a myeloperoxidase enzyme substrate, which was passed through a tungsten light source. The pattern of light scattered (size) and intensity of light absorbed (MPO activity) determined the type of white cells present. Platelets were isovolumetrically sphered before exposure to the laser light source. The platelet count was then determined by the amount of light scattered (size) and their reflective index (activation status).
Fluorescence microscopy
For EPCs, 12-mm glass coverslips were coated with 10 μg/mL fibronectin for 1 hour at room temperature (RT) before PBMNCs were placed on top and cultured for 7 days in EBM complete medium. The slides were removed from the culture at different time points (eg, days 1, 3, 5, and 7). For THP-1 cells, 5 × 105 cells were added to poly-d-lysine–coated coverslips (BD BioCoat Cellware; BD Biosciences). Cells were fixed with 2% paraformaldehyde (PFA) and permeabilized with 0.05% Triton-X (Sigma-Aldrich) for 10 minutes. After blocking with 2% BSA (Sigma-Aldrich) for 15 minutes, cells were stained with primary antibodies anti–PAR-4 (goat; Santa Cruz Biotechnology), anti-CD31 (goat; R&D Systems), anti-VWF (rabbit; Sigma-Aldrich), and anti-integrin αIIb (CD41, mouse; Chemicon International) for 90 minutes in a 1:200 dilution followed by incubation in the dark with anti–goat (for PAR-4: anti–goat IgG TRITC conjugate [Sigma-Aldrich]; for CD31: anti–goat IgG Alexa Fluor 568 [Molecular Probes]), anti–rabbit (for VWF: anti–rabbit IgG TRITC conjugate; Zymax, Invitrogen), or anti–mouse (for CD41: anti–mouse IgG FITC conjugate; Biosource International) conjugated secondary antibodies for 60 minutes. Finally, nuclei were counterstained with DAPI (DAPI Nucleic Acid Stain; Molecular Probes) and slides were covered with fluorescent mounting medium. Images were obtained at room temperature on a Zeiss Axioplan 2 Imaging fluorescence microscope (100×/1.30 numeric aperture [NA] oil objective) equipped with an Axiocam camera (Carl Zeiss) or a Leica TCS SP5 STED (Leica Microsystems) inverted confocal laser scanning microscope with fixed stage microscope DMI4000 B, equipped with helium/neon lasers (633 nm and 543 nm), an argon laser (457-514 nm), a DPSS laser (561 nm), a diode laser (405 nm), and a HCX PL APO 40×, 63×, 100×/1.33 NA oil objective. The acquisition software was Axiovision (Carl Zeiss MicroImaging) and LAS AF software (Leica Microsystems). The images were converted to the RGB mode using Adobe Photoshop version 7.0 (Adobe Systems) and their resolution was set to 300 dpi.
Platelet preparation
Platelets were isolated from healthy subjects as previously described.13 In brief, blood was drawn using acid citrate dextrose as anticoagulant (ACD: 120 mM sodium citrate, 110 mM glucose, 80 mM citric acid, 1:7 vol/vol) and centrifuged for 17 minutes at 200g and 30°C in the presence of indomethacin (10 μM; Sigma-Aldrich). The platelet-rich plasma was then centrifuged for another 10 minutes at 1000g in the presence of prostacyclin (0.1 μg/mL; Sigma-Aldrich). The resulting platelets were resuspended in modified Tyrode-HEPES buffer (145 mM NaCl, 2.9 mM KCl, 10 mM HEPES, 1 mM MgCl2, 5 mM glucose, pH 7.3) at a concentration of 4 × 108/mL.
Preparation of platelet MPs
Platelets were activated by thrombin (0.1 U/mL; Sigma-Aldrich) and their aggregation was monitored with a turbidometric method (Chronolog 490; Chronolog). Platelet MPs were harvested by ultracentrifugation at 100 000g for 90 minutes at 4°C and carefully resuspended in complete medium (RPMI 1640 with 10% FBS). For fluorescence labeling, platelet MPs were incubated with 5 μg/mL CellMask Deep Red plasma membrane stain/lectin (Molecular Probes, Invitrogen) at 37°C for 15 minutes and pelleted again at 100 000g to remove any excess dye.
Platelet MP uptake
THP-1 cells (5 × 105) were cultured as previously described into 12-well culture plates with 1 mL complete medium. Platelet MPs labeled with Deep Red (D/R) lectin were added to the cultures for 2 hours at 37°C. Cell suspensions were washed twice with PBS and placed on poly-d-lysine–coated coverslips (BD BioCoat Cellware; BD Biosciences), and fixed with 2% PFA for 10 minutes at 37°C. In other experiments, isolated platelet MPs were added directly to THP-1 cells and incubated for 2 days at 37°C. The cells were then centrifuged, washed twice in PBS, and applied on poly-d-lysine–coated coverslips and fixed with 2% PFA. The following primary antibodies were used: goat anti-CD31 (R&D Systems), rabbit anti-VWF (Sigma-Aldrich), and mouse anti-integrin αIIb (Chemicon International) for 90 minutes at a dilution of 1:200 followed by secondary antibodies anti–goat IgG Alexa Fluor 568 (Molecular Probes, Invitrogen), anti–rabbit IgG TRITC conjugate (Zymax, Invitrogen), and anti–mouse IgG FITC conjugate (Biosource-International). Nuclei were counterstained with Vectashield mounting medium with DAPI [Vector Laboratories]) for 5 minutes.
Ulex europaeus agglutinin I
THP-1 cells were placed on poly-d-lysine–coated coverslips (BD BioCoat Cellware; BD Biosciences) in the presence or absence of nonactivated platelets and platelet MPs activated by thrombin as described in “Preparation of platelet MPs” above. The adherent cells were fixed with 3.75% PFA and incubated with UEA-1 (Lectin from Ulex europaeus FITC conjugate; Sigma-Aldrich) for 1 hour. Staining of nuclei was performed with DAPI Nucleic Acid Stain (Molecular Probes, Invitrogen) and images were obtained at room temperature on a Leica TCS SP5 STED (Leica Microsystems) inverted confocal laser-scanning microscope.
Flow cytometric analysis
Pelleted MPs from EPC conditioned medium were used for flow cytometry experiments. Labeling for annexin V, CD31, CD41, CD11a, and CD235a was performed to determine the cellular origin of MPs in EPC cultures as reported earlier.14 MPs expressing phosphatidylserine (PS) were labeled using fluoroisothiocyanate-conjugated annexin V (Roche Diagnostics) in the presence or absence (negative control) of CaCl2 (5 mM). The MP pellet was incubated with different fluorochrome-labeled antibodies or their corresponding isotype-matched IgG controls (RT; 30 minutes in the dark). Anti–CD31-phycoerythrin, anti–CD41-phycoerythrin-cyanin5, and anti–CD235a-fluoroisothiocyanate were obtained from Beckman Coulter. Anti–CD11a (LFA-1)–phycoerythrin was purchased from BD Pharmingen. MPs were analyzed on a Coulter EPICS XL flow cytometer (Beckman Coulter). Regions corresponding to MPs were identified in forward light scatter (FCS) and side-angle light scatter (SSC) intensity dot plot representation set at logarithmic gain. The gate for MPs was defined as events with a 0.1- to 1-μm diameter, in comparison with calibrator beads (Megamix fluorescent beads of 0.5, 0.9, and 3 μm in diameter; Biocytex), and plotted on a FL/FSC fluorescence dot plot to determine the MPs labeled by specific antibodies.
In vitro tube formation assay
HUVECs (4 × 104) were placed on Matrigel (10 mg/mL, Matrigel Basement Membrane Matrix, Phenol-Red Free [BD Biosciences], 50 μL/well) in 8-well chamber slides. After attachment, 300 μL EPC conditioned medium was added. The formation of capillary networks was assessed after overnight incubation at 37°C. HUVECs were treated with conditioned medium derived from EPC cultures at different time points (days 3, 5, and 7). For comparison, the particulate fraction was removed from the conditioned medium by filtration (0.1-μm filters). In addition, integrin inhibitors were added to the conditioned medium of EPC cultures: The inhibitory disintegrin echistatin (echistatin α1 isoform; Sigma-Aldrich) is an Arg-Gly-Asp (RGD)–containing snake-venom protein and blocks the function of integrin αVβ3/αIIbβ3.15 A peptide derived from the GP IIIa molecule (GP IIIa-4) was added to inhibit the formation of the GP IIb/IIIa complex.16 Pictures were taken on a Nikon Eclipse TS100 inverted microscope (objective 10×/0.25). The length of the capillaries was measured by the AxioVision 3.0 Software (Carl Zeiss Vision) and expressed as pixels2 from 5 different reference points in duplicate wells. The experiment was repeated twice and the values of the length of the tubes are given as mean plus or minus SD.
Bruneck study
Population recruitment was performed as part of the Bruneck Study.17 The survey area was located in the north of Italy (Bolzano Province). At the 1990 baseline evaluation, the study population was recruited as an age- and sex-stratified random sample of all inhabitants of Bruneck aged 40 to 79 years (125 women and 125 men in the fifth to eighth decades each). Assessments were carried out every 5 years and participation exceeded 90%. In the 2005 evaluation, numbers of early outgrowth EPCs and the formation of colony-forming units (CFUs) were assessed by 2 commonly used methods18 : EPC numbers were identified by double-positive staining for DiI-Ac-LDL and UEA-1 lectin on day 5 of culture. For CFUs, PBMNCs were suspended in Medium 199 (Gibco, Invitrogen) with 20% FBS for 48 hours. Nonadherent cells were replated at 4 × 106 cells/well on human fibronectin–coated plates kept in the same growth medium. After 7 days, CFUs were counted following strict guidelines to ensure consistency. Two trained independent senior investigators blinded to the clinical details of the subject determined the number of EPCs and CFUs. Coefficient of variation was less than 10% in each case.
Statistical analysis
Statistical analysis was performed using the analysis of variance and unpaired Student t test. Results were given as means plus or minus SE. Nonparametric Spearman rank correlation coefficients were obtained using SPSS 12.0 and BMDP software (Statistical Solutions). A P value less than .05 was considered significant.
Results
Proteomic analysis of MPs obtained from EPC cultures
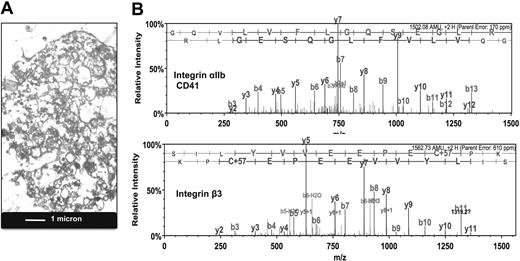
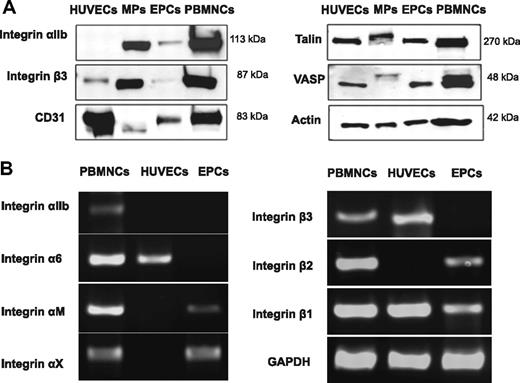
MPs were harvested from the conditioned medium of early outgrowth EPCs and visualized by transmission electron microscopy (Figure 1A). After separation by SDS-PAGE, their protein content was analyzed by liquid chromatography–tandem mass spectrometry. A comprehensive list containing 618 proteins is provided in supplemental Table 2. When the identified proteins were classified based on their Gene Ontology annotation,19 integrin signaling was returned as the top canonical pathway (P < .001). The spectral counts for integrins and other membrane proteins are highlighted in Table 1. Unexpectedly, the most abundant integrins were αIIb and β3, also known as platelet glycoprotein IIb/IIIa (GpIIb/IIIa, CD41/CD61; Figure 1B). Validation of the proteomic data by immunoblotting confirmed that GpIIb/IIIa was enriched in the MP fraction (Figure 2A). Despite its abundance at the protein level, GpIIb/IIIa mRNA was not detectable in EPCs. Similar results were obtained for integrin α6, another integrin found in platelets20 (Figure 2B). Thus, our proteomic analysis of EPC-derived MPs unraveled the presence of platelet proteins among early outgrowth EPCs, a culture method that has been fundamental to many studies published on EPCs to date.9,21,22
Presence of platelet proteins in MPs of EPC cultures. (A) Transmission electron microscopy. Image of MPs harvested from the conditioned medium of EPC cultures. (B) Proteomic analysis. Product ion spectra of doubly charged tryptic peptides identified as the platelet integrin αIIb (GQVLVFLGQSEGLR) and integrin β3 (SILYVVEEPECPK).
Presence of platelet proteins in MPs of EPC cultures. (A) Transmission electron microscopy. Image of MPs harvested from the conditioned medium of EPC cultures. (B) Proteomic analysis. Product ion spectra of doubly charged tryptic peptides identified as the platelet integrin αIIb (GQVLVFLGQSEGLR) and integrin β3 (SILYVVEEPECPK).
Membrane proteins identified in microparticles derived from EPC cultures
| Protein name . | SWISS PROT accession name . | MW, kDa . | Spectra, n . |
|---|---|---|---|
| Integrins, alpha chain | |||
| Integrin alpha-IIb precursor, CD41 antigen | ITA2B_HUMAN | 113 | 156 |
| Integrin alpha-6 precursor, CD49f antigen | ITA6_HUMAN | 127 | 21 |
| Integrin alpha-M precursor, CD11b antigen | ITAM_HUMAN | 127 | 13 |
| Integrin alpha-2 precursor, CD49b antigen | ITA2_HUMAN | 129 | 6 |
| Integrin alpha-X precursor, CD11c antigen | ITAX_HUMAN | 128 | 5 |
| Integrins, beta chain | |||
| Integrin beta-3 precursor, CD61 antigen | ITB3_HUMAN | 87 | 78 |
| Integrin beta-2 precursor, CD18 antigen | ITB2_HUMAN | 85 | 49 |
| Integrin beta-1 precursor, CD29 antigen | ITB1_HUMAN | 88 | 18 |
| Other surface receptors | |||
| Platelet glycoprotein Ib alpha chain CD42b-alpha/CD42b antigen | GP1BA_HUMAN | 69 | 21 |
| Leukocyte antigen MIC3, CD9 antigen | CD9_HUMAN | 25 | 20 |
| Platelet glycoprotein IX, CD42a antigen | GPIX_HUMAN | 19 | 19 |
| 4F2 cell-surface antigen heavy chain, CD98 antigen | 4F2_HUMAN | 58 | 18 |
| Platelet glycoprotein Ib beta chain CD42b-beta/CD42c antigen | GP1BB_HUMAN | 22 | 15 |
| Hyaluronate receptor, CD44 antigen | CD44_HUMAN | 82 | 15 |
| Intercellular adhesion molecule 3 | ICAM3_HUMAN | 59 | 13 |
| Transferrin receptor protein 1, CD71 antigen | TFR1_HUMAN | 85 | 13 |
| Platelet endothelial cell adhesion molecule, CD31 antigen | PECA1_HUMAN | 83 | 9 |
| Leukocyte common antigen, CD45 antigen | CD45_HUMAN | 147 | 7 |
| Leukocyte surface antigen, CD47 antigen | CD47_HUMAN | 35 | 6 |
| Renin receptor | RENR_HUMAN | 39 | 3 |
| Membrane-associated progesterone receptor component 2 | PGRC2_HUMAN | 24 | 4 |
| Fibroblast growth factor receptor 2, CD332 antigen | FGFR2_HUMAN | 92 | 2 |
| Other membrane proteins | |||
| Vesicular integral-membrane protein VIP36 | LMAN2_HUMAN | 40 | 13 |
| Regulating synaptic membrane exocytosis protein 1 | RIMS1_HUMAN | 189 | 2 |
| Nck-associated protein 1-like | NCKPL_HUMAN | 128 | 3 |
| Receptor-associated proteins | |||
| Protein tyrosine phosphatase receptor type C–associated protein | PTCA_HUMAN | 21 | 6 |
| Tyrosine-protein phosphatase nonreceptor type 6 | PTN6_HUMAN | 68 | 5 |
| Growth factor receptor-bound protein 2 | GRB2_HUMAN | 25 | 3 |
| Adipocyte-derived leucine aminopeptidase | ERAP1_HUMAN | 106 | 3 |
| Receptor-type tyrosine-protein phosphatase eta | PTPRJ_HUMAN | 146 | 2 |
| Protein name . | SWISS PROT accession name . | MW, kDa . | Spectra, n . |
|---|---|---|---|
| Integrins, alpha chain | |||
| Integrin alpha-IIb precursor, CD41 antigen | ITA2B_HUMAN | 113 | 156 |
| Integrin alpha-6 precursor, CD49f antigen | ITA6_HUMAN | 127 | 21 |
| Integrin alpha-M precursor, CD11b antigen | ITAM_HUMAN | 127 | 13 |
| Integrin alpha-2 precursor, CD49b antigen | ITA2_HUMAN | 129 | 6 |
| Integrin alpha-X precursor, CD11c antigen | ITAX_HUMAN | 128 | 5 |
| Integrins, beta chain | |||
| Integrin beta-3 precursor, CD61 antigen | ITB3_HUMAN | 87 | 78 |
| Integrin beta-2 precursor, CD18 antigen | ITB2_HUMAN | 85 | 49 |
| Integrin beta-1 precursor, CD29 antigen | ITB1_HUMAN | 88 | 18 |
| Other surface receptors | |||
| Platelet glycoprotein Ib alpha chain CD42b-alpha/CD42b antigen | GP1BA_HUMAN | 69 | 21 |
| Leukocyte antigen MIC3, CD9 antigen | CD9_HUMAN | 25 | 20 |
| Platelet glycoprotein IX, CD42a antigen | GPIX_HUMAN | 19 | 19 |
| 4F2 cell-surface antigen heavy chain, CD98 antigen | 4F2_HUMAN | 58 | 18 |
| Platelet glycoprotein Ib beta chain CD42b-beta/CD42c antigen | GP1BB_HUMAN | 22 | 15 |
| Hyaluronate receptor, CD44 antigen | CD44_HUMAN | 82 | 15 |
| Intercellular adhesion molecule 3 | ICAM3_HUMAN | 59 | 13 |
| Transferrin receptor protein 1, CD71 antigen | TFR1_HUMAN | 85 | 13 |
| Platelet endothelial cell adhesion molecule, CD31 antigen | PECA1_HUMAN | 83 | 9 |
| Leukocyte common antigen, CD45 antigen | CD45_HUMAN | 147 | 7 |
| Leukocyte surface antigen, CD47 antigen | CD47_HUMAN | 35 | 6 |
| Renin receptor | RENR_HUMAN | 39 | 3 |
| Membrane-associated progesterone receptor component 2 | PGRC2_HUMAN | 24 | 4 |
| Fibroblast growth factor receptor 2, CD332 antigen | FGFR2_HUMAN | 92 | 2 |
| Other membrane proteins | |||
| Vesicular integral-membrane protein VIP36 | LMAN2_HUMAN | 40 | 13 |
| Regulating synaptic membrane exocytosis protein 1 | RIMS1_HUMAN | 189 | 2 |
| Nck-associated protein 1-like | NCKPL_HUMAN | 128 | 3 |
| Receptor-associated proteins | |||
| Protein tyrosine phosphatase receptor type C–associated protein | PTCA_HUMAN | 21 | 6 |
| Tyrosine-protein phosphatase nonreceptor type 6 | PTN6_HUMAN | 68 | 5 |
| Growth factor receptor-bound protein 2 | GRB2_HUMAN | 25 | 3 |
| Adipocyte-derived leucine aminopeptidase | ERAP1_HUMAN | 106 | 3 |
| Receptor-type tyrosine-protein phosphatase eta | PTPRJ_HUMAN | 146 | 2 |
EPC indicates endothelial progenitor cell; and MW, molecular weight.
Integrin expression in PBMNCs, HUVECs, and EPCs. (A) Confirmation by immunoblotting. Note that the platelet integrin GpIIb/IIIa was specifically enriched in the MP fraction of EPC cultures. Besides integrins, MPs also contained CD31 (PECAM), although at lower abundance than GpIIb/IIIa, consistent with the spectral counts presented in Table 1. Talin-1 and VASP, 2 downstream mediators of integrin signaling, showed a molecular weight shift indicative of phosphorylation. Actin was used as loading control. (B) No mRNA expression was detectable for GpIIb/IIIa in EPCs. A similar result was obtained for the platelet integrin α6. The less abundant integrins (according to the spectral counts in Table 1) were expressed at the mRNA level. The data are representative of 3 independent experiments.
Integrin expression in PBMNCs, HUVECs, and EPCs. (A) Confirmation by immunoblotting. Note that the platelet integrin GpIIb/IIIa was specifically enriched in the MP fraction of EPC cultures. Besides integrins, MPs also contained CD31 (PECAM), although at lower abundance than GpIIb/IIIa, consistent with the spectral counts presented in Table 1. Talin-1 and VASP, 2 downstream mediators of integrin signaling, showed a molecular weight shift indicative of phosphorylation. Actin was used as loading control. (B) No mRNA expression was detectable for GpIIb/IIIa in EPCs. A similar result was obtained for the platelet integrin α6. The less abundant integrins (according to the spectral counts in Table 1) were expressed at the mRNA level. The data are representative of 3 independent experiments.
The presence of platelets in EPC cultures
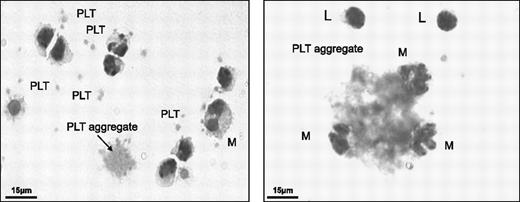
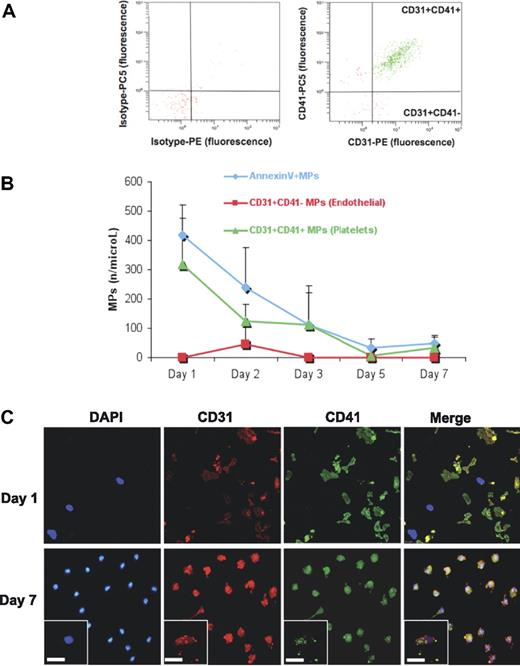
A blood count revealed that despite extensive washing, platelets were not entirely removed from PBMNCs by density barrier centrifugation (Table 2). Platelets as well as platelet aggregates were clearly visible among PBMNCs (Figure 3). Immunostaining for PAR-4 and integrin αIIb (CD41) revealed that platelets disintegrated in culture and platelet proteins were taken up by the mononuclear cell population (Figure 4). Flow cytometric analysis confirmed an abundance of platelet MPs (CD31+CD41+), but a scarcity of endothelial MPs (CD31+CD41−) in EPC cultures (Figure 5A). Consistent with the observed uptake, the number of platelet MPs decreased over time (Figure 5B). On day 1, platelets were in close proximity, but separated from the CD31− mononuclear cell population (Figure 5C). In contrast, by day 7, cells stained positive for CD31 and the platelet-specific integrin αIIb, suggesting an uptake of platelet proteins.
Cellular composition before and after Lymphoprep isolation
| Cell type, cells/mL . | Full blood . | PBMNCs . |
|---|---|---|
| Leukocyte count | 5.85 × 106 (± 0.464) | 2.56 × 106 (± 0.251) |
| Neutrophils | 3.31 × 106 (± 0.358) | 0.04 × 106 (± 0.002) |
| Lymphocytes | 1.84 × 106 (± 0.029) | 1.56 × 106 (± 0.385) |
| Monocytes | 0.36 × 106 (± 0.061) | 0.69 × 106 (± 0.052) |
| Erythrocyte count | 5.43 × 109 (± 0.052) | 0.01 × 109 (± 0.001) |
| Platelet count | 268.33 × 106 (± 15.632) | 15.83 × 106 (± 2.732) |
| Cell type, cells/mL . | Full blood . | PBMNCs . |
|---|---|---|
| Leukocyte count | 5.85 × 106 (± 0.464) | 2.56 × 106 (± 0.251) |
| Neutrophils | 3.31 × 106 (± 0.358) | 0.04 × 106 (± 0.002) |
| Lymphocytes | 1.84 × 106 (± 0.029) | 1.56 × 106 (± 0.385) |
| Monocytes | 0.36 × 106 (± 0.061) | 0.69 × 106 (± 0.052) |
| Erythrocyte count | 5.43 × 109 (± 0.052) | 0.01 × 109 (± 0.001) |
| Platelet count | 268.33 × 106 (± 15.632) | 15.83 × 106 (± 2.732) |
Values are means of 3 independent experiments (± SEM), each performed in duplicate. Bold numbers highlight the abundance of platelets compared to monocytes.
PBMNCs indicates peripheral blood mononuclear cells.
Platelet contamination of PBMNCs. PBMCs were fixed and stained using the Hema Gurr rapid staining set for hematology according to the manufacturer's instructions (VWR International). Platelets and platelet aggregates were present among washed PBMNCs. PLT denotes platelets; M, monocytes; and L, lymphocytes.
Platelet contamination of PBMNCs. PBMCs were fixed and stained using the Hema Gurr rapid staining set for hematology according to the manufacturer's instructions (VWR International). Platelets and platelet aggregates were present among washed PBMNCs. PLT denotes platelets; M, monocytes; and L, lymphocytes.
Cellular uptake of platelet MPs. Intact platelets stained positive for PAR-4 and integrin αIIb (CD41) among the PBMNCs counterstained with DAPI (day 1). Over time, the platelets disintegrated, but platelet proteins remained detectable in EPC cultures (day 3) and were taken up by the adherent cell population (day 5, inset). By day 7, most cells stained positive for platelet markers (insets: scale bar represents 25 μm).
Cellular uptake of platelet MPs. Intact platelets stained positive for PAR-4 and integrin αIIb (CD41) among the PBMNCs counterstained with DAPI (day 1). Over time, the platelets disintegrated, but platelet proteins remained detectable in EPC cultures (day 3) and were taken up by the adherent cell population (day 5, inset). By day 7, most cells stained positive for platelet markers (insets: scale bar represents 25 μm).
Flow cytometric analysis. (A) MPs were harvested from the conditioned medium of EPCs at different time points and analyzed by flow cytometry. Note the scarcity of endothelial MPs (CD31+CD41−), but the abundance of platelet MPs (CD31+CD41+) in EPC cultures (> 90% of all CD31+ MPs). (B) Their decline in the conditioned medium corresponds to the observed uptake by the mononuclear cell population. (C) To evaluate the contribution of platelet MPs to the positivity for “endothelial” markers in EPC cultures, cells were costained for CD31 and integrin αIIb (CD41) on day 1 and day 7. Note that on day 1, CD31 was confined to platelets. By day 7, CD31+ cells were present in EPC cultures. However, these cells were also positive for the platelet-specific integrin αIIb. Scale bar represents 25 μm.
Flow cytometric analysis. (A) MPs were harvested from the conditioned medium of EPCs at different time points and analyzed by flow cytometry. Note the scarcity of endothelial MPs (CD31+CD41−), but the abundance of platelet MPs (CD31+CD41+) in EPC cultures (> 90% of all CD31+ MPs). (B) Their decline in the conditioned medium corresponds to the observed uptake by the mononuclear cell population. (C) To evaluate the contribution of platelet MPs to the positivity for “endothelial” markers in EPC cultures, cells were costained for CD31 and integrin αIIb (CD41) on day 1 and day 7. Note that on day 1, CD31 was confined to platelets. By day 7, CD31+ cells were present in EPC cultures. However, these cells were also positive for the platelet-specific integrin αIIb. Scale bar represents 25 μm.
Uptake of platelet MPs by mononuclear cells
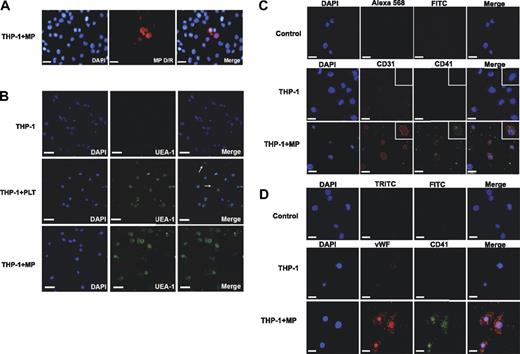
To investigate to what extent marker proteins can be exchanged between cell types, platelet MPs were labeled with a fluorescence-conjugated lectin, which stains cell membranes, and incubated with the THP-1 monocytic cell line. Within 1 to 2 hours, labeled MPs were taken up by 5% to 10% of the THP-1 cells (Figure 6A). Notably, platelets also stained positive for Ulex europaeus I agglutinin (UEA-1), a lectin that has been widely used to demonstrate the endothelial potential of EPCs. After incubation with platelets or platelet MPs, most THP-1 cells bind UEA-1 (Figure 6B). THP-1 cells incubated with platelet MPs for 2 days stained strongly for CD31 (Figure 6C) and VWF (Figure 6D), but the staining colocalized with integrin αIIb (CD41), replicating the observed phenotype in EPC cultures.
Platelet MPs and endothelial characteristics. (A) Platelet MPs were generated by activating platelets with thrombin, labeled with a fluorescence-conjugated lectin (MP D/R), and incubated with the monocytic THP-1 cell line. An uptake of labeled platelet MPs by THP-1 cells was observed within 1 to 2 hours. (B) THP-1 cells bound UEA-1 after coincubation with intact platelets or platelet MPs. PLT denotes platelets; arrows depict platelets among THP-1 cells. After incubation with platelet MPs for 2 days, THP-1 cells stained positive for CD31 (C) and VWF (D), but also for integrin αIIb (CD41) confirming that platelet MPs can be responsible for the markers used to prove the “endothelial” potential of EPCs. Scale bar represents 25 μm.
Platelet MPs and endothelial characteristics. (A) Platelet MPs were generated by activating platelets with thrombin, labeled with a fluorescence-conjugated lectin (MP D/R), and incubated with the monocytic THP-1 cell line. An uptake of labeled platelet MPs by THP-1 cells was observed within 1 to 2 hours. (B) THP-1 cells bound UEA-1 after coincubation with intact platelets or platelet MPs. PLT denotes platelets; arrows depict platelets among THP-1 cells. After incubation with platelet MPs for 2 days, THP-1 cells stained positive for CD31 (C) and VWF (D), but also for integrin αIIb (CD41) confirming that platelet MPs can be responsible for the markers used to prove the “endothelial” potential of EPCs. Scale bar represents 25 μm.
Proangiogenic effect of platelet MPs
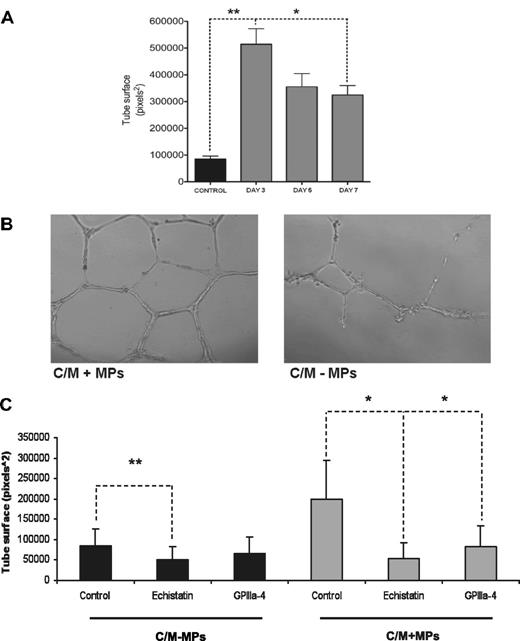
Next, we tested whether platelet MPs influence the angiogenic activity of EPCs.23,24 Conditioned medium of EPC cultures stimulated endothelial tube formation in the Matrigel assay. This proangiogenic effect was more pronounced on day 3 than on days 5 and 7 arguing against an outgrowth of more potent progenitor cells in EPC cultures (Figure 7A). Instead, removing MPs from the conditioned medium by filtration or by ultracentrifugation attenuated capillary network formation (Figure 7B). Moreover, addition of the disintegrin echistatin or the peptide GPIIIa-4, a specific inhibitor against the formation of the platelet integrin complex αIIbβ3,16 reduced the effect of EPC-derived MPs on endothelial tube formation (Figure 7C). Thus, platelet MPs contribute to the proangiogenic effect of the conditioned medium from EPC cultures.
Platelet MPs mediate the proangiogenic effect. (A) The proangiogenic effect of conditioned medium from EPC cultures decreased over time, arguing against an outgrowth of a progenitor cell population. (B) The depletion of MPs from the conditioned medium (C/M-MPs) of EPC cultures reduced endothelial tube formation in the Matrigel assay. (C) The addition of the disintegrin echistatin and the inhibitory peptide GPIIIa-4 against the platelet integrin αIIb attenuated the proangiogenic effect of the conditioned medium in the presence of platelet MPs (C/M + MPs). *P < .05 level; **P < .01. Results were obtained in 3 independent experiments.
Platelet MPs mediate the proangiogenic effect. (A) The proangiogenic effect of conditioned medium from EPC cultures decreased over time, arguing against an outgrowth of a progenitor cell population. (B) The depletion of MPs from the conditioned medium (C/M-MPs) of EPC cultures reduced endothelial tube formation in the Matrigel assay. (C) The addition of the disintegrin echistatin and the inhibitory peptide GPIIIa-4 against the platelet integrin αIIb attenuated the proangiogenic effect of the conditioned medium in the presence of platelet MPs (C/M + MPs). *P < .05 level; **P < .01. Results were obtained in 3 independent experiments.
Correlation between platelet count and EPC numbers in the Bruneck study
Finally, we explored whether platelet counts correlate with numbers of colony-forming units (CFUs)25 or early outgrowth EPCs (double positive for diI-Ac-LDL and lectin) in a large population-based study (n = 526).18 Among leukocytes, the strongest correlation was observed for monocytes (expressed as percentage of total leukocytes, nonparametric Spearman rank correlation coefficient r = 0.215, P < .01). Apart from monocytes, only the platelet count (r = 0.092 and r = 0.089 for CFUs and EPCs, respectively; P < .05) and the product of the platelet count and the mean platelet volume (MPV; r = 0.110 and r = 0.137 for CFUs and EPCs [P < .05 and P < .01], respectively) emerged as a significant predictor for EPC numbers and CFUs in the general population (Table 3).
Correlation of full blood count with numbers of CFUs and early outgrowth EPCs in the Bruneck Study (n = 526)
| . | CFU . | EPC . |
|---|---|---|
| Leukocyte count | 0.321† | 0.258† |
| Neutrophils, % | 0.031 | −0.024 |
| Lymphocytes, % | 0.062 | 0.010 |
| Monocytes, % | 0.215† | 0.108* |
| Erythrocyte count | 0.038 | −0.023 |
| Platelet count | 0.092* | 0.089* |
| Platelet count* MPV | 0.110* | 0.137† |
| . | CFU . | EPC . |
|---|---|---|
| Leukocyte count | 0.321† | 0.258† |
| Neutrophils, % | 0.031 | −0.024 |
| Lymphocytes, % | 0.062 | 0.010 |
| Monocytes, % | 0.215† | 0.108* |
| Erythrocyte count | 0.038 | −0.023 |
| Platelet count | 0.092* | 0.089* |
| Platelet count* MPV | 0.110* | 0.137† |
Values presented are nonparametric Spearman rank correlation coefficients; correlation coefficients in bold are significant at P < .05 (*) or P < .01 (†).
CFU indicates colony forming unit; EPC, endothelial progenitor cell; and MPV, mean platelet volume.
Discussion
Recent publications have cast doubts about the origin of CFUs by demonstrating that they may be clonally derived from the hematopoietic system, possess myeloid progenitor cell activity, and differentiate into phagocytic macrophages.5,6 Thus, there is an urgent need to reassess the cellular progeny of early outgrowth EPCs. Our proteomic data identified membrane proteins of leukocyte origin, but platelet proteins were also present in EPC cultures, suggesting that platelets were not entirely removed by the density barrier centrifugation methods commonly used to isolate PBMNCs. In culture, these platelets disintegrated into smaller MPs resulting in an uptake by the mononuclear cell population. At the time when the outgrowth of EPCs is routinely assessed by fluorescence staining, the platelets have disappeared, but platelet proteins are still detectable. Notably, markers consistent with current definitions of an EPC phenotype, such as CD31 and VWF, are all abundant platelet proteins. In addition, platelets also contain cryptic VEGF receptors, including KDR, which become exposed on the platelet membrane upon stimulation by VEGF.26 Although it can be argued that platelets are readily discerned by their size and the absence of a nucleus, this assumption is fundamentally flawed if platelet proteins can be transferred to other cell types.27 After platelet activation, a myriad of platelet MPs accumulates at sites of tissue injury and leaks into the circulation.7,28 Mononuclear cells readily take up the platelet-derived MPs resulting in a possible exchange of antigens between cell types. Thus, it is important to acknowledge that double-positive cells for “endothelial” and hematopoietic/stem cell markers are not necessarily EPCs and that the intercellular transfer of membrane proteins by extracellular secretory membrane bodies is likely to result in an overestimation of EPCs in areas of tissue injury, if platelet-specific antigens such as CD41 are not included as controls to reliably establish their “endothelial cell” potential.
Importantly, the isolation methods currently used for cell-based therapy in cardiovascular medicine are similar to the one used in this study. Density barrier centrifugation is an equilibrium method that fractionates on the basis of particle density. Platelets will settle on the interface when samples are spun over 1.077 g/mL density barriers (Histopaque, Lymphoprep, Ficoll-Paque, etc) and platelets can be abundant among unselected BMCs (data not shown). Thus, the following observations may be directly relevant to our findings: (1) Clinical trials on cell therapy were inconclusive and the inconsistent therapeutic effect was attributed to the different cell isolation procedures.29-31 (2) BMCs were more potent than PBMNCs in improving clinical outcome and this was considered to be a consequence of their increased “stemness.” (3) The therapeutic potential of purified progenitor cell preparations, however, was not superior to unselected BMCs.32 Although it has been argued that progenitor cells per se may be insufficient and that interactions with other cell types are needed, our findings advise caution in attributing clinical benefits to stem cell–mediated repair. Virtually any manipulation of a bone marrow sample, in particular the shear forces in the needle, will cause platelet activation. Moreover, BMCs also contain megakaryocytes, a heterogeneous cell population with a density ranging from 1.020 to 1.085 g/mL. All megakaryocytes less than 1.077 g/mL will settle on the interface of the buffy coat. The release of MPs from activated megakaryocytes or the budding of protoplatelets may result in a transfer of endothelial and stem cell antigens, including CD34 and CD133,33,34 to the mononuclear cell population used for cell therapy. Notably, a recent study suggested that circulating platelet MPs in plasma are also derived from megakaryocytes.35 Because platelets are rich sources of angiogenic growth factors, variations in platelet contamination are a likely explanation for the divergent results reported in recent clinical trials. At the very least, the presence of platelets and platelet MPs has to be closely monitored and reported in future clinical trials to exclude that stem cell therapy predominantly delivers platelet proteins to areas of myocardial injury.
Besides, platelet MPs could induce an angiogenic monocyte/macrophage phenotype. For example, we have recently demonstrated an up-regulation of thymidine phosphorylase (platelet-derived endothelial growth factor) in CFUs compared with the conventional EPC cultures.10 The replating step in the Hill-colony assay6,25 greatly minimizes platelet contamination. Thymidine phosphorylase, however, is present in platelets and MPs of EPC cultures (supplemental Table 2). Although platelet markers are still detectable by immunostaining, it is currently unclear to what extent other platelet proteins, including thymidine phosphorylase, are transferred and/or induced by platelet MPs. Silencing of thymidine phosphorylase expression using siRNA technology attenuated the angiogenic effect of EPCs arguing against a simple uptake of this intracellular enzyme. Notably, platelet MPs could also activate an angiogenic program by horizontal transfer of mRNA.24
Thus far, studies have shown that the release of factors from activated platelets can enhance the function and the numbers of EPCs, facilitate differentiation of CD34+ cells into endothelial cells, guide their migration to sites of vascular injury, and stimulate colony formation and dil-Ac-LDL uptake in EPC cultures.36-40 Platelets from healthy volunteers even improved the number and function of EPCs from patients.39 Our study provides the first evidence that the characteristics used to demonstrate the “endothelial” potential of EPCs (CD31, VWF, UEA-1 staining) can be explained by an uptake of platelet MPs by mononuclear cells. This finding represents a paradigm shift in our current definition of an EPC phenotype and has important implications for clinical trials using EPCs or unpurified BMCs for stem cell therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr Thomas M. Chiang (Memphis VA Medical Center and Departments of Medicine and Molecular Science, University of Tennessee-Health Science Center, Memphis, TN) for providing the inhibitory peptide for the formation of the GP IIb/IIIa complex. We thank Dr David Bishop-Bailey (Queen Mary, University of London, London, United Kingdom) for providing the THP-1 cell line, and Mr Tom Berry for proofreading the manuscript.
This work emanates from the European Vascular Genomics Network (http://www.evgn.org), a Network of Excellence supported by the European Community's sixth Framework Program for Research Priority 1 “Life sciences, genomics and biotechnology for health” (contract no. LSHM-CT-2003-503254) and the Leducq Foundation LINK project. This work was funded by grants from the King's College Joint Research Committee, the British Heart Foundation, and the Oak Foundation. C.M.B. was supported by a Contrat d'Interface Inserm AP-HP and M.M., by a Senior Research Fellowship of the British Heart Foundation.
Authorship
Contribution: M.P. performed all cell culture experiments and wrote the paper; G.P. performed the Western blots; U.M. performed the proteomic analysis; C.D. performed the FACS experiment; J.G. performed the FBC assay; Q. Xu performed and analyzed the data from the Bruneck Study; C.M.B. performed the flow cytometry experiment; N.W. wrote the paper; C.U. cultivated EPCs; J.W. performed and analyzed the data from the Bruneck Study; M.S. and J.B. performed the electron microscopy; Q. Xiao and S.K. performed and analyzed the data from the Bruneck Study; and M.M. designed the research, performed the proteomic analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manuel Mayr, Cardiovascular Division, King's British Heart Foundation Centre, King's College London, 125 Coldharbour Ln, London SE5 9NU, United Kingdom; e-mail: manuel.mayr@kcl.ac.uk.