While plasma-derived and recombinant coagulation FVIII may largely share the same amino acid sequence and restore coagulation equally well, Qadura and colleagues demonstrate in this issue of Blood that these molecules appear quite different to the immune system.
A major complication of treatment of hemophilia A is the formation of inhibitory antibodies against the therapeutic factor VIII (FVIII) protein, which occurs with an overall incidence of 20% to 30% of patients. Using hemophilia A mice and in vitro assays, Qadura et al find that a plasma-derived FVIII product containing von Willebrand factor (VWF) activates dendritic cells (a critical antigen-presenting cell for the initiation of a specific immune response) quite differently than recombinant FVIII.1 Similarly, when injected into the hemophilia mice, the cytokine profiles of responding T cells differed substantially after administration of the 2 FVIII formulations, suggesting that different subsets of T-helper cells were being stimulated. The response to recombinant FVIII had a bias toward Th1 response but also involved production of the regulatory cytokine IL-10, while plasma-derived protein caused mostly a Th2 response and up-regulation of TGF-β, a cytokine with immune-suppressive properties. In addition, plasma-derived FVIII promoted FoxP3+ Treg responses and caused lower-titer inhibitor and total antibody (IgG) formation compared with recombinant FVIII.1
A controversial debate over potential differences in the immunogenicity of FVIII products has been ongoing for years. Plasma-derived products may vary in purity and VWF content. Recombinant products produced in mammalian tissue culture are of high purity, do not contain VWF, and may be full-length or B domain–deleted FVIII. Some studies had claimed a higher propensity of recombinant products to cause inhibitor formation, which, however, may have been influenced by the retrospective nature of such analyses and has not been substantiated by prospective studies.2 Questions that fuel the discussion of immunogenicity of factor products include the safety of switching treatment of a patient from one product to another, matching of products to specific patient populations, and the role of VWF in immune responses.3,4 Similar discussions are ongoing about which product and dose to use in immune tolerance induction (ITI) protocols. Different hemophilia treatment centers report diverse experiences. For example, a recent report documents a higher success rate using a plasma-derived, VWF-containing product compared with recombinant FVIII in elimination of inhibitors by ITI.3 It has been hypothesized that VWF could prevent binding of inhibitors by masking epitopes on the C2 domain or otherwise interfere with antibody binding. Qadura et al propose an alternative mechanism, namely antigenic competition for processing by antigen-presenting cells. Interpretation of these animal data are somewhat complicated by murine immune responses to human VWF that may not occur in patients. Nonetheless, this new study provides the hemophilia research community with the exciting perspective of having an animal model available that allows studies that address immunogenic differences between FVIII products, doses, and treatment protocols. In addition, such studies can determine involvement of T regulatory cells in controlling immune responses, as evidence is mounting that active suppression of antibody formation by this subset of T cells is a critical component of maintaining tolerance to coagulation factors.5,6
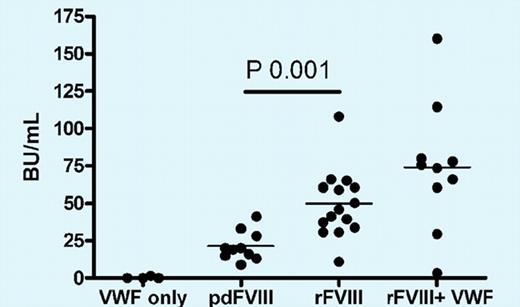
Administration of plasma-derived human FVIII (a product that contains VWF) to hemophilia A mice induces a lower inhibitor response than recombinant FVIII. Interestingly, the inhibitor titers cannot be reduced by administration of a mixture of recombinant FVIII with VWF, suggesting that antigenic properties of FVIII complexed to VWF in plasma are not identical to those obtained from in vitro mixing of these molecules. See the complete figure in the article beginning on page 871.
Administration of plasma-derived human FVIII (a product that contains VWF) to hemophilia A mice induces a lower inhibitor response than recombinant FVIII. Interestingly, the inhibitor titers cannot be reduced by administration of a mixture of recombinant FVIII with VWF, suggesting that antigenic properties of FVIII complexed to VWF in plasma are not identical to those obtained from in vitro mixing of these molecules. See the complete figure in the article beginning on page 871.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■