Abstract
Lenalidomide plus dexamethasone is effective for the treatment of relapsed and refractory multiple myeloma (MM); however, toxicities from dexamethasone can be dose limiting. We evaluated the efficacy and safety of lenalidomide monotherapy in patients with relapsed and refractory MM. Patients (N = 222) received lenalidomide 30 mg/day once daily (days 1-21 every 28 days) until disease progression or intolerance. Response, progression-free survival (PFS), overall survival (OS), time to progression (TTP), and safety were assessed. Overall, 67% of patients had received 3 or more prior treatment regimens. Partial response or better was reported in 26% of patients, with minimal response 18%. There was no difference between patients who had received 2 or fewer versus 3 or more prior treatment regimens (45% vs 44%, respectively). Median values for TTP, PFS, and OS were 5.2, 4.9, and 23.2 months, respectively. The most common grade 3 or 4 adverse events were neutropenia (60%), thrombocytopenia (39%), and anemia (20%), which proved manageable with dose reduction. Grade 3 or 4 febrile neutropenia occurred in 4% of patients. Lenalidomide monotherapy is active in relapsed and refractory MM with acceptable toxicities. These data support treatment with single-agent lenalidomide, as well as its use in steroid-sparing combination approaches. The study is registered at http://www.clinicaltrials.gov as NCT00065351.
Introduction
Multiple myeloma (MM) is an incurable plasma cell malignancy, previously associated with a median survival time of less than 3 years.1 In the United States, it is estimated that there are 19920 new cases of MM per year and 10690 deaths annually.2 Autologous stem cell transplantation (SCT) is a treatment of choice for younger patients with MM, but almost all patients relapse and become refractory to treatment.1,3,4 In relapsed patients, responses to treatment are characteristically short, with a median survival of less than 1 year in patients who have received 2 or more prior treatment regimens.5 The introduction of novel agents has transformed the management of MM, with the impact of thalidomide, bortezomib, and, most recently, lenalidomide improving treatment outcomes for patients with MM.6
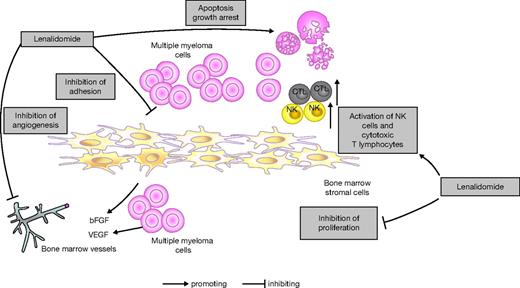
Lenalidomide (Revlimid; Celgene Corporation) is an oral IMiD immunomodulatory drug with broad pleiotropic activities including direct induction of apoptosis, inhibition of angiogenesis, immunomodulatory effects such as activation of T cells and natural killer cells, and cadherin modulation of cytokinesis (Figure 1).7-9 It has shown clinical benefit as monotherapy in patients with relapsed or refractory MM in both phase 1 and phase 2 studies, with a large randomized phase 2 trial concluding that a 30-mg dose given once daily for 3 weeks, with 1 week off therapy, was both well tolerated and active compared with 15 mg given twice daily.10-12 The addition of low-dose dexamethasone in this study was also shown to be feasible and effective in patients progressing on lenalidomide alone, with responses observed in 29% of patients.11
Lenalidomide mechanism of action. Based on Davies et al7 and Hideshima et al.8,9 bFGF indicates basic fibroblast growth factor; CTL, cytotoxic T lymphocytes; NK, natural killer; and VEGF, vascular endothelial growth factor.
After the publication of 2 important phase 3 trials, lenalidomide in combination with dexamethasone was confirmed to have important efficacy in the treatment of patients with relapsed or refractory MM who have received at least 1 prior treatment regimen.13,14 This led to its approval by the US Food and Drug Administration, European Medicines Agency, and other regulatory boards. However, the addition of dexamethasone to lenalidomide has been associated with significant adverse events, especially when given at high doses, including deep-vein thrombosis (DVT), infections, and hyperglycemia.13-15 It is, therefore, important to investigate steroid-sparing approaches for lenalidomide as part of MM therapy, and in particular for steroid-intolerant patients.
In this phase 2 study, we evaluated the efficacy and safety of single-agent lenalidomide (30 mg once daily) in the treatment of patients with relapsed and refractory MM.
Methods
This was a multicenter, single-arm, open-label study of lenalidomide monotherapy in patients with relapsed and refractory MM. Patients were enrolled between July 28, 2003, and May 13, 2004, at 30 centers in the United States. The study was approved by the Institutional Review Board of each participating institution in accordance with federal regulations and the Declaration of Helsinki, and all patients gave written informed consent before entering the study.
Patient eligibility criteria
Patients aged 18 years or older were eligible to participate if they had relapsed after achieving at least stable disease with prior therapy, and then had disease progression during salvage anti-MM therapy. They must have received 2 or more prior treatment regimens (defined as therapy with a single agent or combination of agents, not including SCT) and have documented evidence of disease progression during, or within 60 days after completion of, treatment with a salvage regimen used just before study entry. Measurable disease was defined as 0.5 g/dL or more M-protein in serum or 0.2 g or more/24-hour urinary M-protein excretion. Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, and a negative pregnancy test within 7 days before commencing treatment in women of childbearing potential. Patients with a known hypersensitivity to thalidomide or with prior exposure to lenalidomide were excluded. Laboratory parameters required for patient eligibility included: an absolute neutrophil count of 1 × 109/L (1000 cells/mm3) or higher; an adequate platelet count defined as 75 × 109/L (75000/mm3) or higher for patients in whom less than 50% of bone marrow nucleated cells were plasma cells and 30000/mm3 or higher for patients in whom 50% or more of bone marrow nucleated cells were plasma cells; serum aspartate aminotransferase/alanine aminotransferase levels less than 3.0 × the upper limit of normal; a serum creatinine level less than 221 μM (2.5 mg/dL); and a serum total bilirubin level less than 34.2 μM (2.0 mg/dL).
Treatment
Lenalidomide was administered at a dose of 30 mg (a 25-mg lenalidomide capsule and a 5-mg lenalidomide capsule) given once daily on days 1 to 21 of every 28-day cycle until disease progression or unacceptable toxicity. To monitor treatment compliance, reconciliation of lenalidomide capsules was completed at each scheduled study visit.
Assessment of study outcomes
The primary efficacy end point was achievement of at least a partial response (defined as best response including complete response [CR] or partial response [PR] at any time during the first 6 cycles). Secondary end points included assessment of overall response rate (ORR; defined as CR + PR + minimal response [MR]), CR, PR, MR, stable disease, progressive disease, duration of response, progression-free survival (PFS), time to progression (TTP), overall survival (OS) duration, 1-year survival rate, and safety.
Investigator-evaluated response was assessed according to the European Group for Blood and Marrow Transplant (EBMT) criteria.16 ORR in this relapsed and refractory MM population was calculated as CR plus PR plus MR as recommended by the American Society of Hematology/US Food and Drug Administration Workshop on Clinical Endpoints in Multiple Myeloma.17 Duration of response was measured from the time of best response (≥ PR or ≥ MR) to progression of disease, including death due to MM. TTP was estimated as the time from the start of lenalidomide treatment to the first occurrence of any of the following events: disease progression with TTP measured at the date of the first assessment of tests required to determine progression; and discontinuation from treatment due to disease progression as determined by the investigator, whether or not confirmed by EBMT criteria. PFS was assessed in the same way as TTP, except that patient deaths occurring during the treatment period were counted as progression in the PFS calculation. Median OS was defined as the time from the start of lenalidomide treatment to death due to any cause. A subgroup analysis was also performed based on the number of investigator-reported prior treatment regimens for MM (≤ 2 vs ≥ 3). Adverse events were graded according to National Cancer Institute Common Toxicity Criteria (Version 2.0), and were assessed at each patient visit.
Statistical analysis
The probability of a response in each category was estimated using the proportion of patients meeting the criteria of each response category (ie, the number of patients with response in each category divided by the number of patients evaluated). Exact 2-sided 95% confidence intervals for the probability of a response of at least PR were calculated. For TTP, duration of response, and OS, the Kaplan-Meier procedure was used to characterize the survival function. Median time to events and the respective 2-sided 95% confidence intervals were calculated for each of these variables.
To evaluate the impact of prognostic factors on response to lenalidomide, univariate and multivariate analyses were conducted using age (≤ 65 years, > 65 years), sex, ECOG performance status (0, 1, ≥ 2), prior SCT (univariate: 0, 1, 2; multivariate: yes, no), prior anti-MM regimens (univariate: 1, 2, 3, > 3; multivariate: ≤ 3, > 3), prior radiotherapy (yes, no), and prior thalidomide treatment (multivariate only: yes, no).
The study was designed as a collaborative effort by the primary investigator (P.R.), coinvestigators, and the sponsor, Celgene Corporation. Statistical analysis was performed by the sponsor. All authors had full access to the primary data and the final analysis. An independent data and safety monitoring committee reviewed ongoing safety and efficacy data throughout the study. The study is registered at http://www.clinicaltrials.gov as NCT00065351.
Results
Baseline characteristics
All 222 patients enrolled received at least 1 dose of lenalidomide and were included in both the intent-to-treat (ITT) and safety populations. Baseline demographic and disease-related characteristics of patients are shown in Table 1. Most patients had an ECOG performance status of 0 or 1 (82%) and the median time from diagnosis was 3.7 years (range, 0.4-19.8 years). Overall, 67% of patients received 3 or more prior anti-MM treatment regimens and 45% of patients had received 1 or more prior autologous stem cell transplants. A total of 80% of patients had received prior treatment with thalidomide and 43% had received prior treatment with bortezomib.
Baseline demographic and disease-related characteristics (intent-to-treat population)
| Characteristic . | . |
|---|---|
| Median age (range), y | 64 (38-88) |
| Sex, no. (%) | |
| Male | 123 (55) |
| Female | 99 (45) |
| Median time from first pathologic diagnosis (range), y | 3.7 (0.4-19.8) |
| ECOG performance status, no. (%) | |
| 0 | 71 (32) |
| 1 | 112 (50) |
| 2 | 26 (12) |
| Missing | 13 (6) |
| Median no. of prior treatment regimens including SCT (range) | 5 (3-21) |
| No. of prior autologous SCTs, no. (%) | |
| 0 | 122 (55) |
| 1 | 9 (4) |
| More than 1 | 91 (41) |
| No. of prior anti–multiple myeloma treatment regimens, not including SCT, no. (%) | |
| 1 | 18 (8) |
| 2 | 55 (25) |
| 3 | 47 (21) |
| More than 3 | 102 (46) |
| Prior therapies, no. (%) | |
| Radiotherapy | 96 (43) |
| Thalidomide | 177 (80) |
| Dexamethasone | 152 (68) |
| Bortezomib | 96 (43) |
| Melphalan | 94 (42) |
| Doxorubicin | 19 (9) |
| Characteristic . | . |
|---|---|
| Median age (range), y | 64 (38-88) |
| Sex, no. (%) | |
| Male | 123 (55) |
| Female | 99 (45) |
| Median time from first pathologic diagnosis (range), y | 3.7 (0.4-19.8) |
| ECOG performance status, no. (%) | |
| 0 | 71 (32) |
| 1 | 112 (50) |
| 2 | 26 (12) |
| Missing | 13 (6) |
| Median no. of prior treatment regimens including SCT (range) | 5 (3-21) |
| No. of prior autologous SCTs, no. (%) | |
| 0 | 122 (55) |
| 1 | 9 (4) |
| More than 1 | 91 (41) |
| No. of prior anti–multiple myeloma treatment regimens, not including SCT, no. (%) | |
| 1 | 18 (8) |
| 2 | 55 (25) |
| 3 | 47 (21) |
| More than 3 | 102 (46) |
| Prior therapies, no. (%) | |
| Radiotherapy | 96 (43) |
| Thalidomide | 177 (80) |
| Dexamethasone | 152 (68) |
| Bortezomib | 96 (43) |
| Melphalan | 94 (42) |
| Doxorubicin | 19 (9) |
All patients were treated with lenalidomide, N = 222.
ECOG indicates Eastern Cooperative Oncology Group; and SCT, stem cell transplantation.
Response evaluation
In the ITT population, the primary end point of CR plus PR was achieved in 26% (95% confidence interval [CI], 25.7-26.5), including 5 patients with CR (2%) and 53 with PR (24%; Table 2). Forty patients had an MR (18%), giving an ORR (CR + PR + MR) of 44% (95% CI, 43.7-44.6). During treatment with lenalidomide, 48% of patients achieved stable disease. Of the 222 patients enrolled, 183 patients had a postbaseline M-protein measurement at cycle 2 or beyond; the ORR in this population was 51% (CR, 3%; PR, 29%; and MR, 20%), with 32% of patients achieving a PR or better. The reasons for treatment discontinuation in the 39 patients without a postbaseline M-protein measurement at cycle 2 or beyond were as follows: adverse events (n = 16); death (n = 9); lack of therapeutic effect as determined by the investigator (n = 9); patient withdrew consent (n = 4); and major protocol violation (n = 1).
Summary of clinical responses for the intent-to-treat population (N = 222)
| Response, no. (%) . | Patients treated with lenalidomide, N = 222 . | Patients with 2 or fewer prior treatment regimens, n = 73 . | Patients with 3 or more prior treatment regimens, n = 149 . | Patients with prior thalidomide treatment, n = 177 . | Patients with prior bortezomib treatment, n = 96 . |
|---|---|---|---|---|---|
| Complete response (CR) | 5 (2) | 1 (1) | 4 (3) | 3 (2) | 3 (3) |
| Partial response (PR) | 53 (24) | 18 (25) | 35 (23) | 38 (22) | 27 (28) |
| Minimal response (MR) | 40 (18) | 14 (19) | 26 (17) | 31 (18) | 14 (15) |
| Stable disease | 107 (48) | 35 (48) | 72 (48) | 90 (51) | 41 (43) |
| Progressive disease | 8 (4) | 1 (1) | 7 (5) | 8 (5) | 7 (7) |
| Not evaluable/known | 9 (4) | 4 (5) | 5 (3) | 7 (4) | 4 (4) |
| CR + PR | 58 (26) | 19 (26) | 39 (26) | 41 (23) | 30 (31) |
| CR + PR + MR | 98 (44) | 33 (45) | 65 (44) | 72 (41) | 44 (46) |
| Response, no. (%) . | Patients treated with lenalidomide, N = 222 . | Patients with 2 or fewer prior treatment regimens, n = 73 . | Patients with 3 or more prior treatment regimens, n = 149 . | Patients with prior thalidomide treatment, n = 177 . | Patients with prior bortezomib treatment, n = 96 . |
|---|---|---|---|---|---|
| Complete response (CR) | 5 (2) | 1 (1) | 4 (3) | 3 (2) | 3 (3) |
| Partial response (PR) | 53 (24) | 18 (25) | 35 (23) | 38 (22) | 27 (28) |
| Minimal response (MR) | 40 (18) | 14 (19) | 26 (17) | 31 (18) | 14 (15) |
| Stable disease | 107 (48) | 35 (48) | 72 (48) | 90 (51) | 41 (43) |
| Progressive disease | 8 (4) | 1 (1) | 7 (5) | 8 (5) | 7 (7) |
| Not evaluable/known | 9 (4) | 4 (5) | 5 (3) | 7 (4) | 4 (4) |
| CR + PR | 58 (26) | 19 (26) | 39 (26) | 41 (23) | 30 (31) |
| CR + PR + MR | 98 (44) | 33 (45) | 65 (44) | 72 (41) | 44 (46) |
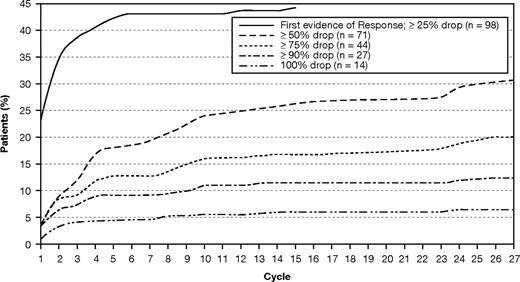
For the ITT population, the median time to first response after treatment with lenalidomide monotherapy was 2.8 months. The median duration of response for patients who experienced PR or better while on treatment was 12.6 months, and the median duration of response for patients who experienced a CR, PR, or MR was 8.4 months. Figure 2 displays the cumulative response distribution in patients who achieved CR, PR, or MR.
Maximum serum M-protein drop over time in patients who achieved at least a minimal response.
Maximum serum M-protein drop over time in patients who achieved at least a minimal response.
Response results for the ITT population were further analyzed by stratification of patients who had received 2 or fewer versus 3 or more prior MM treatment regimens. ORR (CR + PR + MR) was 45% of patients with 2 or fewer prior treatment regimens compared with 44% in those patients with 3 or more prior treatment regimens (P = .82; Table 2). In those who had received prior treatment with bortezomib, the ORR was 46% (44 of 96 patients), with 3 patients (3%) achieving a CR; 27 (28%), a PR; and 14 (15%), an MR. Among patients who had undergone prior SCT, the ORR was 39% (39 of 99 patients), including 3 patients with a CR, 21 with a PR, and 15 with an MR. In patients who had received prior treatment with thalidomide, the ORR was 41% (72 of 177 patients), including 3 patients (2%) who achieved a CR, 38 (22%) who achieved a PR, and 31 (18%) who achieved an MR. The ORR was 35% in patients who were refractory to prior thalidomide therapy (n = 20; defined as progression while receiving any thalidomide-containing regimen with no initial response), 25% in patients who relapsed after prior response to any thalidomide-containing regimen, and 43% in patients who were sensitive to treatment with thalidomide (Table 3).18 It should be noted that some patients in the above analyses were counted separately for each of these categories as they received more than 1 prior treatment including thalidomide, bortezomib, or stem cell transplant before receiving lenalidomide.
Summary of clinical response for patients with prior thalidomide therapy (n = 177)
| Prior treatment with thalidomide . | Response, no. (%) . | |
|---|---|---|
| CR + PR . | CR + PR + MR . | |
| All patients, n = 177 | 41 (23) | 72 (41) |
| Thalidomide | ||
| Refractory,* n = 20 | 6 (30) | 7 (35) |
| Relapsed,† n = 12 | 2 (17) | 3 (25) |
| Sensitive, n = 144 | 33 (23) | 62 (43) |
| Unknown, n = 1 | 0 | 0 |
| Prior treatment with thalidomide . | Response, no. (%) . | |
|---|---|---|
| CR + PR . | CR + PR + MR . | |
| All patients, n = 177 | 41 (23) | 72 (41) |
| Thalidomide | ||
| Refractory,* n = 20 | 6 (30) | 7 (35) |
| Relapsed,† n = 12 | 2 (17) | 3 (25) |
| Sensitive, n = 144 | 33 (23) | 62 (43) |
| Unknown, n = 1 | 0 | 0 |
CR indicates complete response; MR, minimal response; and PR, partial response.
Refractory defined as immediate disease progression (no initial response) in any of the regimen sequences within which they received thalidomide. The definition is consistent with Wang et al.18
Relapsed defined as progression following initial response to any thalidomide-containing regimen.
Several factors were evaluated to determine whether patients were associated with response to lenalidomide (Table 4). In univariate and multivariate analyses, no independent prognostic factors were identified as predictors of an inferior response to lenalidomide, including prior SCT or the number of prior anti-MM treatment regimens.
Clinical response within subgroups for the intent-to-treat population
| . | Response, no. (%) . | |
|---|---|---|
| CR + PR . | CR + PR + MR . | |
| Total group, N = 222 | 58 (26) | 98 (44) |
| Age | ||
| 65 y or younger, n = 125 | 30 (24) | 52 (42) |
| Older than 65 y, n = 97 | 28 (29) | 46 (47) |
| Sex | ||
| Male, n = 123 | 36 (29) | 55 (45) |
| Female, n = 99 | 22 (22) | 43 (43) |
| ECOG performance status* | ||
| 0, n = 71 | 18 (25) | 26 (37) |
| 1, n = 112 | 30 (27) | 52 (46) |
| 2, n = 26 | 7 (27) | 14 (54) |
| No. of prior SCTs | ||
| 0, n = 122 | 32 (26) | 57 (47) |
| 1, n = 9 | 2 (22) | 3 (33) |
| 2 or more, n = 91 | 24 (26) | 38 (42) |
| No. of prior anti–multiple myeloma treatment regimens | ||
| 1, n = 18 | 3 (17) | 6 (33) |
| 2, n = 55 | 16 (29) | 27 (49) |
| 3, n = 47 | 11 (23) | 18 (38) |
| More than 3, n = 102 | 28 (27) | 47 (46) |
| Prior radiotherapy | ||
| Yes, n = 96 | 28 (29) | 42 (44) |
| No, n = 126 | 30 (24) | 56 (44) |
| Median duration of treatment, d | 400.5 | 266.0 |
| . | Response, no. (%) . | |
|---|---|---|
| CR + PR . | CR + PR + MR . | |
| Total group, N = 222 | 58 (26) | 98 (44) |
| Age | ||
| 65 y or younger, n = 125 | 30 (24) | 52 (42) |
| Older than 65 y, n = 97 | 28 (29) | 46 (47) |
| Sex | ||
| Male, n = 123 | 36 (29) | 55 (45) |
| Female, n = 99 | 22 (22) | 43 (43) |
| ECOG performance status* | ||
| 0, n = 71 | 18 (25) | 26 (37) |
| 1, n = 112 | 30 (27) | 52 (46) |
| 2, n = 26 | 7 (27) | 14 (54) |
| No. of prior SCTs | ||
| 0, n = 122 | 32 (26) | 57 (47) |
| 1, n = 9 | 2 (22) | 3 (33) |
| 2 or more, n = 91 | 24 (26) | 38 (42) |
| No. of prior anti–multiple myeloma treatment regimens | ||
| 1, n = 18 | 3 (17) | 6 (33) |
| 2, n = 55 | 16 (29) | 27 (49) |
| 3, n = 47 | 11 (23) | 18 (38) |
| More than 3, n = 102 | 28 (27) | 47 (46) |
| Prior radiotherapy | ||
| Yes, n = 96 | 28 (29) | 42 (44) |
| No, n = 126 | 30 (24) | 56 (44) |
| Median duration of treatment, d | 400.5 | 266.0 |
CR indicates complete response; ECOG, Eastern Cooperative Oncology Group; MR, minimal response; PR, partial response; and SCT, stem cell transplantation.
Total number of patients does not equal 222 due to 13 patients having missing ECOG data.
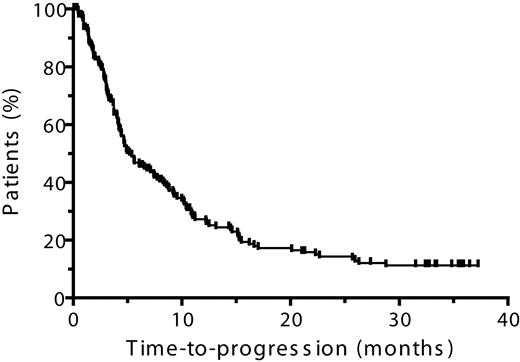
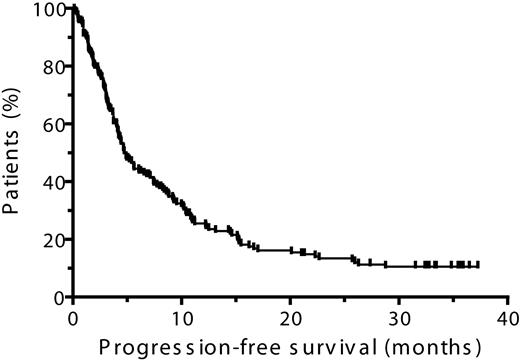
Time to progression, progression-free survival, and overall survival
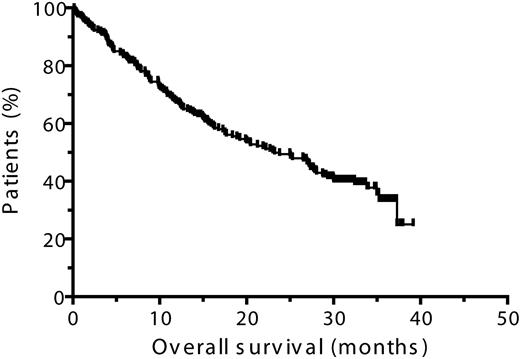
In the ITT population, the median TTP was 5.2 months and the median PFS was 4.9 months (Table 5, Figures 3–4). The median duration of OS was 23.2 months (Table 5, Figure 5) and the 1-year survival rate was 67%. In patients who achieved CR, PR, or MR, both median TTP and median PFS were 10.4 months each, with a median OS of 28.0 months (Table 5). The 1-year survival rate in patients who achieved at least an MR was 79% and in those who achieved at least a PR was 73%.
Summary of efficacy outcomes in patients treated with lenalidomide monotherapy
| Median values, mo . | Overall, N = 222 . | CR + PR, n = 58 . | CR + PR + MR, n = 98 . |
|---|---|---|---|
| Progression-free survival | 4.9* | 14.5 | 10.4 |
| Time to progression | 5.2† | 14.5 | 10.4 |
| Overall survival | 23.2‡ | 33.9 | 28.0 |
| Median values, mo . | Overall, N = 222 . | CR + PR, n = 58 . | CR + PR + MR, n = 98 . |
|---|---|---|---|
| Progression-free survival | 4.9* | 14.5 | 10.4 |
| Time to progression | 5.2† | 14.5 | 10.4 |
| Overall survival | 23.2‡ | 33.9 | 28.0 |
CR indicates complete response; MR, minimal response; and PR, partial response.
Patients who had disease progression or died: 73%.
Patients who had disease progression: 69%.
Patients who died: 60%.
Safety and lenalidomide dose management
The median daily dose of lenalidomide in the study was 25 mg (range, 5-30 mg) and the median duration of treatment was 4.2 months (range, 0.06-38.0 months), with 29% of patients receiving lenalidomide for 9 months or longer. Median duration of treatment was longer for patients who responded to lenalidomide therapy (8.9 months for those who achieved at least an MR, and 13.4 months for those who achieved at least a PR), but there was no significant difference in median daily dose (22.9 mg for patients who achieved at least an MR, and 21.4 mg for those who achieved at least a PR). Reasons for treatment discontinuation were as follows: lack of therapeutic effect as determined by the investigator (51%, n = 113); adverse event (16%, n = 35); patient withdrew consent (5%, n = 12); death (5%, n = 10); major protocol violation (1%, n = 2); lost to follow-up (1%, n = 2); and other (2%, n = 5). Of note for patients who withdrew consent, the median duration of treatment was 3.0 months (range, 0.2-21.3 months) and ORR was 38%, with 1% achieving at least a PR. This suggests that 6% of patients discontinued treatment early in the course of the study, before the best response could be achieved.
Safety evaluation in all patients who received at least 1 dose of lenalidomide revealed that the most frequently reported grade 3 or 4 adverse events were neutropenia (60%), thrombocytopenia (39%), and anemia (20%; Table 6); only 4% of patients had febrile neutropenia. Grade 3 or 4 thrombotic events were reported in 10 (5%) patients during the study. Thrombotic events included investigator-reported DVT (4%) and pulmonary embolism (1%). Five (5%) of 99 patients who received thromboprophylaxis (including 2 patients who discontinued prophylaxis ≥ 6 months before the thrombotic event) and 5 (4%) of 123 patients with no prophylaxis experienced a thrombotic event. Thrombotic events were experienced by 7 (4%) of 170 patients who received erythropoietin at any time during the study, and 3 (6%) of 52 patients with no erythropoietin administration. No cases of lenalidomide-related grade 3 or 4 peripheral neuropathy were reported, with grade 2 peripheral neuropathy reported in 6 (3%) patients.
Grade 3 or 4 adverse events reported in more than 10% of all patients treated (safety population; N = 222)
| Adverse event, no. (%) . | Grade 3 . | Grade 4 . | Grade 3 or 4 . |
|---|---|---|---|
| Neutropenia | 91 (41) | 42 (19) | 133 (60) |
| Thrombocytopenia | 63 (28) | 23 (10) | 86 (39) |
| Anemia NOS | 37 (17) | 7 (3) | 44 (20) |
| Pneumonia NOS | 22 (10) | 5 (2) | 27 (12) |
| Fatigue | 26 (12) | 1 (1) | 27 (12) |
| Adverse event, no. (%) . | Grade 3 . | Grade 4 . | Grade 3 or 4 . |
|---|---|---|---|
| Neutropenia | 91 (41) | 42 (19) | 133 (60) |
| Thrombocytopenia | 63 (28) | 23 (10) | 86 (39) |
| Anemia NOS | 37 (17) | 7 (3) | 44 (20) |
| Pneumonia NOS | 22 (10) | 5 (2) | 27 (12) |
| Fatigue | 26 (12) | 1 (1) | 27 (12) |
NOS indicates not otherwise specified.
Dose reduction or interruption of study treatment due to an adverse event was required in 176 (79%) patients, with a median time to the first dose reduction or interruption of 43 days (range, 2.0-385 days). The most common reasons for lenalidomide dose modification were neutropenia (43%) and thrombocytopenia (28%). Growth factor support was required before dose reduction in 59% of patients (21%, 23%, and 23% during the first 3, 6, and 12 months, respectively). Adverse events led to discontinuation of lenalidomide therapy in 39 (18%) patients, most frequently because of neutropenia (5%), thrombocytopenia (4%), and renal failure (3%). Renal failure leading to discontinuation of therapy was considered to be related to lenalidomide in 4 (2%) patients.
By the end of the study, 132 deaths were reported among the 222 subjects who were treated with lenalidomide. Of 22 patients who died within 30 days after the last dose of lenalidomide, 5 cases were assessed as possibly related to lenalidomide therapy, including myopericarditis, syncope, septic shock, pneumonia, cardiorespiratory arrest, sudden death, pulmonary embolism, neutropenia, thrombocytopenia, and anemia.
Patients proceeding to transplantation
A total of 25 (11.3%) patients proceeded to high-dose therapy including bone marrow transplantation and/or SCT. Of the 23 patients who underwent SCT, transplantation was performed 6 months after the last dose of lenalidomide in 15 patients, at 12 months in 5 patients, at 18 months in 2 patients, and at 30 months in 1 patient. No significant difficulties with stem cell mobilization were proactively reported from any participating center.
Discussion
The results of this phase 2 study demonstrate that lenalidomide monotherapy at a dose of 30 mg given once daily is an active therapy producing meaningful long-term benefit in patients with relapsed and refractory MM. Importantly, patients who had received prior thalidomide or prior bortezomib responded, and the toxicities profile of this steroid-sparing approach, in an otherwise relatively sick population, was favorable.
Overall response rates with lenalidomide monotherapy were similar for patients who had received 2 or fewer prior treatment regimens versus 3 or more prior treatment regimens (45% vs 44%, respectively). Specifically, patients responded after prior treatment with either thalidomide (ORR, 41%) or bortezomib (ORR, 46%), or after undergoing prior SCT (39%), suggesting that lenalidomide is effective regardless of the type of prior therapy. Responses were also similar in patients who were relapsed, refractory, or sensitive to prior thalidomide treatment (ORR, 35%, 25%, and 43%, respectively).
Patients treated with lenalidomide monotherapy in this study achieved a response rate (PR or better) of 26%, with overall median TTP and OS values of 5.2 months and 23.2 months, respectively. These results are comparable with those seen in the SUMMIT trial where patients refractory to prior chemotherapy were treated with bortezomib monotherapy for 7 months (ORR, 28%; median TTP, 7 months; and median OS, 17 months).19,20 Although the response rate with bortezomib in the APEX trial was higher (43%), the median TTP (6.2 months) and OS (29.8 months) advantage was more marginal.21 Moreover, this difference could be attributed to the selection criteria of the APEX study, which enrolled less heavily pretreated patients (a median of 2 prior therapies) and excluded patients who were refractory to dexamethasone.22 Combination therapy with lenalidomide and dexamethasone produced response rates (PR or better) of 60% to 61%, median TTP of 11.1 to 11.3 months, and OS of 29.6 months. These results suggest that the combination of lenalidomide plus dexamethasone is more effective than lenalidomide alone in patients with relapsed or refractory MM.13,14 However, these studies also enrolled less heavily pretreated patients (> 30% had only 1 prior therapy) and also excluded patients who were resistant to dexamethasone.
The median OS in the present study is consistent with the OS reported in patients receiving single-agent lenalidomide at a dose of 30 mg once daily and twice daily (28 and 27 months, respectively) in the antecedent phase 2 randomized study,11 and is among the longest reported in this heavily pretreated patient population. In addition, the median PFS was 4.9 months compared with 7.7 months and 3.9 months seen in patients treated with lenalidomide once daily and twice daily, respectively.11 Again, this likely reflects a more resistant population in the current study, as well as confirming that single daily dosing is active.11
Lenalidomide monotherapy had manageable tolerability in this patient population. Neutropenia, thrombocytopenia, and anemia were the most frequently reported grade 3 or 4 adverse events, with the incidence of grade 4 adverse events being relatively low. Although grade 3 or 4 neutropenia was reported in 60% of patients, febrile neutropenia was reported in only 4% of patients.
MM is associated with an increased incidence of venous thromboembolism, in the order of 5% to 10%,23,24 and treatment with lenalidomide in combination with high-dose dexamethasone has been shown to further increase the risk of venous thrombotic events in patients with relapsed or refractory MM.13,14 Therefore, the rate of grade 3 or 4 DVT observed (4%) is within the range expected in this patient population. Furthermore, the rate of DVT in this study is consistent with that observed with lenalidomide monotherapy in MM.11,25 As well as the treatment being steroid-free, anticoagulant use in almost half of the patients enrolled may have contributed to the relatively low rate of confirmed DVT in this study.
Myelosuppression was managed by dose interruption or reduction, and growth factor support. Importantly, no grade 3 or 4 treatment-related peripheral neuropathy was observed and grade 2 events occurred in only 3% of treated patients. Overall, the safety data demonstrate that lenalidomide monotherapy was generally well tolerated when administered to patients with relapsed and refractory MM.
The encouraging side effect profile and activity shown by lenalidomide monotherapy provide a powerful platform for rationally informed combinations with other agents, and preliminary results are very promising.26-28 Ongoing studies are investigating various lenalidomide-based combination options including bortezomib with or without dexamethasone, bevacizumab plus dexamethasone, and CCI-779.26,28
In conclusion, the encouraging ORR, duration of response, TTP, and OS results of this study demonstrate that oral lenalidomide monotherapy at a dose of 30 mg once daily is an effective and well tolerated therapy for patients with relapsed and refractory MM. These data provide a strong rationale for the use of lenalidomide as a single agent in selected patients, in steroid-sparing combination approaches, an important consideration in this patient population, and as maintenance therapy. Further studies in these settings are currently ongoing.3
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the work performed by individual research teams at all participating study sites; Caitlin Conant and Nicole Carreau for their administrative support with the paper; and a special thank you to all of our patients and their families for their participation.
This study was supported by research funding from Celgene Corporation. The authors received editorial support in the preparation of this paper funded by Celgene; the authors were fully responsible for all content and editorial decisions, and received no financial support or other form of compensation related to the development of the paper.
Authorship
Contribution: P.R. designed the study, recruited subjects, collected data, and wrote the paper; S.J., M.H., J.B., S.S., D.I., S.F.W., W.B., A.Z.B., and R.V. recruited subjects for the study; K.C.A., J.Z., M.O., and R.K. designed the study; Z.Y. and L.K. performed the statistical analysis; and all authors were involved in analyzing and interpreting the data and critical review of paper content. The authors were fully responsible for content and editorial decisions for this paper.
Conflict-of-interest disclosure: P.R. has participated in Advisory Boards and has been a Speakers Bureau member for Millennium Pharmaceuticals and Celgene Corporation. S.J. has consulted for Celgene Corporation and Millennium Pharmaceuticals and received honoraria from Celgene Corporation, Millennium Pharmaceuticals, and Ortho Biotech Canada. M.H. is an employee at Celgene Corporation. J.B. has been a Speakers Bureau member for, received honoraria from, consulted for, and received research support from Celgene Corporation. S.S. has participated in Advisory Boards and has been a Speakers Bureau member for Millennium Pharmaceuticals and Celgene Corporation. D.I., S.F.W., and A.Z.B. declare no competing financial interests. W.B. has participated in Advisory Boards for Millennium Pharmaceuticals and has been a Speakers Bureau member for Celgene Corporation. R.V. has received honoraria from Celgene Corporation. L.K. and J.Z. are employees and have equity ownership at Celgene Corporation. Z.Y. and M.O. and are employees at Celgene Corporation. R.K. is an employee at Celgene Corporation. K.C.A. has participated in Advisory Boards for Celgene Corporation, Millennium Pharmaceuticals, and Novartis; has consulted for and received research support from Celgene Corporation and Millennium Pharmaceuticals; and has consulted for Novartis.
Correspondence: Paul Richardson, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Dana 1B02, Boston, MA 02115; e-mail: paul_richardson@dfci.harvard.edu.