Abstract
Epstein-Barr virus (EBV)–specific T-cell preparations, generated by stimulating immune donor lymphocytes with the autologous virus-transformed B-lymphoblastoid cell line (LCL) in vitro, can be used to target EBV-positive malignancies. Although these preparations are enriched for EBV antigen–specific CD8+ T cells, most also contain a CD4+ T-cell population whose specificity is unknown. Here, we show that, although CD4+ T-cell clones derived from such cultures recognize HLA class II–matched LCLs but not mitogen-activated B lymphoblasts, many (1) do not map to any known EBV antigen, (2) can be raised from EBV-naive as well as EBV-immune persons, and (3) can recognize a broad range of human B lymphoma–derived cell lines irrespective of EBV genome status, providing those lines to express the relevant HLA class II–restricting allele. Importantly, such CD4+ clones not only produce IFNγ but are also cytotoxic and can control the outgrowth of HLA-matched lymphoma cells in cocultivation assays. We infer that such CD4+ T cells recognize cellular antigens that are preferentially up-regulated in EBV-transformed but not mitogen-activated B lymphoblasts and that are also expressed in a range of B-cell malignancies. Such antigens are therefore of potential value as targets for CD4+ T cell–based immunotherapy.
Introduction
Many immunologic approaches to cancer treatment seek to target tumors through their expression of “tumor-associated antigens.” Often these are cellular proteins that become recognizable to the T-cell repertoire through mutation or expression at supraphysiologic levels or expression outside their usual immunologically protected niche.1,2 For virus-associated malignancies, the range of potential target antigens increases to include virus-coded proteins as well as cellular proteins abnormally expressed as a result of virus-induced transformation.
One example of successful T cell–based immunotherapy involves an Epstein-Barr virus (EBV)–associated malignancy, posttransplant B-lymphoproliferative disease (PTLD). EBV is a growth-transforming herpesvirus whose infection of the B-cell system is normally controlled by host T-cell surveillance.3 In immunosuppressed patients lacking such control, EBV-infected B cells grow out as PTLD lesions expressing the same panel of EBV latent proteins (the nuclear antigens EBNA-1, -2, -3A, -3B, -3C, and -LP and the latent membrane proteins LMP-1 and -2) as seen in EBV-transformed B-lymphoblastoid cell lines (LCLs) in vitro.4,5 Importantly, PTLD can be cured by adoptively transferring effector T-cell preparations made in vitro by stimulating peripheral blood mononuclear cells (PBMCs) from the patient6 or an HLA-matched EBV-immune donor7-9 with their autologous LCL. The clinical effectiveness of these preparations is thought to reflect their enrichment in CD8+ T cells specific for EBV latent antigens, in particular the immunodominant EBNA-3A, -3B, and -3C proteins.10,11 Such polyclonal effectors are cytotoxic and kill HLA class I–matched LCL targets efficiently in vitro.6-9
Interestingly these same LCL-stimulated preparations also contain varying proportions of CD4+ T cells.12,13 Although poorly characterized, these CD4+ T cells also appear to recognize and kill LCLs,8,14 and, in a recent trial, their presence in adoptively transferred preparations has been linked with better clinical responses.15 Furthermore, in that trial, LCL-stimulated T cells also appeared to be effective against rare cases of EBV-positive Burkitt (BL) and Hodgkin (HL) lymphomas arising in patients after receiving a transplant.15 Importantly, such tumors do not express the EBNA-3 antigens targeted by CD8+ T cells5 and, in the case of BL, have a global processing defect that renders the tumor impervious to all CD8+ T-cell recognition.16,17 Hence, the CD4+ T-cell component may have been more important in targeting BL and HL, particularly because both tumors are HLA class II positive and, at least in the case of BL, are known to present endogenously expressed antigens to CD4+ T cells.18,19
We therefore set out to examine the antigenic specificity and target cell range of CD4+ T cells within LCL-stimulated populations. Two earlier findings cautioned against the assumption that such effectors are necessarily EBV specific. First, although EBV-immune persons possess CD4+ T-cell memory to several defined EBV latent protein epitopes, LCL stimulation is a relatively inefficient means of reactivating these responses in vitro20,21 because many such epitopes are poorly displayed on the LCL surface.22,23 Second, clonal analysis of T cells harvested from EBV-infected PBMC cultures has identified CD4+ T cells that recognize EBV-transformed but not mitogen-activated B cells yet appear not to map to any of the known EBV-encoded target epitopes.20 However, because such reactivities were detected in cultures from 2 EBV-immune donors and not from the 1 EBV-naive donor tested, their target specificity remained an open question.
Here, we show that HLA class II–restricted cytotoxic CD4+ T-cell clones reactive to the LCL can in fact be generated by LCL stimulation from EBV-naive as well as EBV-immune donors. These effectors do not map to EBV target proteins but appear to recognize cellular antigens whose expression is up-regulated not just in EBV-infected B cells but also in several human B-lymphoma lines irrespective of their EBV status.
Methods
Cell preparations and depletions
Blood was taken (with informed consent in accordance with the Declaration of Helsinki and with approval of Dudley Local Ethical Research Committee, Dudley, United Kingdom) from 5 EBV-seronegative (N1-N5) and 5 EBV-seropositive (P1-P5) healthy donors of known HLA type. PBMCs were separated by Ficoll-Hypaque centrifugation into RPMI 1640 medium (Invitrogen) supplemented with 2 mM glutamine, 100 IU penicillin/mL, 100 μg streptavidin/mL (standard medium) and 5% human serum (HuS). When specified, PBMCs were depleted of CD56+ natural killer (NK) cells and either CD8+ or CD4+ T cells with the use of magnetic-activated cell sorting beads (Miltenyi) or Dynabeads (Dynal) as described.20
Target cell lines
LCLs were generated from donor B cells with the use of B95.8 EBV (B95.8 LCLs) or a replication-defective B95.8 recombinant lacking the immediate early gene BZLF1 (BZ k/o LCLs).24 The human lymphoma-derived lines studied, their EBV genome status, and HLA II type are shown in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). All lines were cultured in standard medium with 8% fetal calf serum (FCS) or 5% HuS as specified. B lymphoblasts (B blasts) were generated by culturing PBMCs on CD40 ligand–expressing mouse L-cells in medium supplemented with interleukin-4 (IL-4), cyclosporin A, and either HuS or FCS as described20,25 and maintained by twice-weekly transfer onto fresh L-cells. T lymphoblasts (T blasts) were generated from PBMCs cultured in 1 μg/mL phytohemagglutinin (PHA) and expanded in IL-2–supplemented medium. Dendritic cells were prepared by 6-day culture of adherent PBMCs in medium supplemented with granulocyte-macrophage colony-stimulating factor and IL-4 as described,26 then 24 hours in 50 ng/mL tumor necrosis factor α.
Enzyme-linked immunospot assays
PBMCs, depleted of CD56+ NK and either CD4+ or CD8+ T cells as described,20 were tested in enzyme-linked immunospot (ELISpot) assays of IFNγ release22 at 40:1 responder-to-stimulator ratio against B95.8 or BZ k/o LCLs grown for at least 1 month in HuS. Spot-forming cells were counted with the use of an automated AID ELISpot reader (Autoimmun Diagnostika). Results are expressed as the number of spot-forming cells per 2 × 105 depleted PBMCs.
In vitro reactivation protocols
CD8-depleted PBMCs were stimulated with autologous BZ k/o LCL at 40:1 responder-to-stimulator ratio in standard medium and 5% HuS either for 4 hours, after which responding cells were isolated by IFNγ-secreting cell enrichment (Miltenyi), or for up to 5 weeks with weekly restimulation. T-cell clones were then isolated from the above-mentioned preparations by limited dilution seeding in IL-2–supplemented medium with 5% HuS, and growing microcultures reactive against autologous LCLs in IFNγ enzyme-linked immunoabsorbent assays (ELISAs), examined for CD4, CD8, CD27, CD28, CD45RO, CD45RA, and CD57 status by fluorescence-activated cell sorting (FACS) staining,27 and further expanded as described.20 In some cases the LCL-restimulated bulk cultures were screened as above for ELISpot reactivity against a range of target lines.
IFNγ ELISAs
T cells (103-104/triplicate microtest well) were cocultured overnight with 5 × 104 target cells and supernatant medium, harvested after 18 hours, and screened for IFNγ release by ELISA (Perbio) against internal standards (Sigma-Aldrich) as described.22 In blocking assays, LCLs were pr-incubated for 1 hour with 1 μg/mL mAb specific for HLA-DR (L243; ATCC clone HB-55), HLA-DQ (SPV-L3; Serotech), or HLA-DP (B7.21; kindly provided by A. M. de Jong, Leiden University, Leiden, The Netherlands) before the addition of T cells in the continued presence of antibody. In ELISAs that used Modified Vaccinia Ankara (MVA) recombinants, autologous B blasts were either unmanipulated, exposed for 1 hour to 5 MOI MVA recombinants carrying invariant chain (Ii)–targeted EBNA-1(lacking the gly-ala repeat), EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, or LMP2 at a MOI of 5, and then washed before being used as targets. For LMP-1 and EBNA-LP, T cells were tested against autologous B blasts coated with pools of overlapping 20mer peptides (5 peptides/pool) covering the primary sequences of the proteins.
Cytotoxicity assays
CD4+ T-cell clones were tested at effector-to-target ratios of 10:1, 5:1, and 2.5:1 in 12-hour chromium (51Cr) release assays, and results were expressed as the percentage of specific lysis. In some assays, targets were preexposed to 100 nM concanamycin A (Con A) for 1 hour after51Cr labeling, or T cells were preincubated for 2 hours with 100 ng/mL anti-Fas blocking mAb ZB4 (Immunotech); blocking of Fas-mediated lysis by ZB4 was checked on targets exposed to 1 μg/mL anti-Fas agonistic mAb CH11 (Beckman Coulter).
Outgrowth assays
Target cells were cultured at doubling dilutions in triplicate microtest wells (300 × 104 cells/well) with and without 104 T cells. Target cell outgrowth, confirmed by staining for CD19 (LCL, BL, and B lymphoma), CD138 (multiple myeloma), or CD30 (HL), was scored at 4 weeks as described.22 Results are expressed as the minimum target cell seeding required for successful outgrowth.
Results
LCL-reactive CD4+ T cells are detectable in EBV-seropositive and -seronegative donors
We first asked whether LCL-reactive T cells could be detected in ex vivo ELISpot assays of IFNγ secretion. Fresh PBMCs from EBV-seropositive and -seronegative donors were first depleted of CD56+ NK cells and then of CD4+ or CD8+ T cells. The resultant CD8-enriched and CD4-enriched preparations were then challenged with an autologous LCL transformed either by wild-type B95.8 EBV, therefore containing a small fraction of cells in lytic cycle, or by replication-defective BZLF1 k/o (BZ k/o) virus and therefore 100% latently infected.24 All LCLs had been grown in media supplemented with HuS to avoid FCS reactivities.
As shown in Figure 1, all 5 seropositive donors tested (P1-P5) possess easily detectable numbers of LCL-reactive T cells in both the CD8-enriched and CD4-enriched fractions, testifying to the strength of virus-specific T-cell memory in the blood of immune donors. These responses were greater against the B95.8 LCL than against the BZ k/o LCL, reflecting the additional increment of lytic epitope specificities detected by the B95.8 line. Among the 5 seronegative donors tested (N1-N5), none gave a significant CD8+ T-cell response, but 3 (N1, N3, N5) had small but significant numbers of LCL-reactive cells in the CD4-enriched population. These latter responses were repeatable and equal in size whether using the B95.8 LCL or the BZ k/o LCL.
Analysis of LCL-reactive T cells in peripheral blood using the ELISpot assay of IFNγ release. Ex vivo PBMCs from EBV-seropositive donors (P1-P5) or EBV-seronegative donors (N1-N5) were CD56-depleted and then either (A) CD8-enriched by CD4+ T-cell depletion or (B) CD4-enriched by CD8+ T-cell depletion. These preparations were seeded at 1 × 105 and 2 × 105 cells/well in duplicate wells and cocultured with autologous B95.8 and BZ k/o LCLs (grown for at least 1 month in HuS) at an effector-to-target ratio of 40:1. Results from one representative experiment are expressed as mean ± 1 SD spot-forming cells (SFCs) per 2 × 105 cells; background responses from nonstimulated cells are also shown (control). *P < .05 (Wilcoxon-Mann-Whitney test).
Analysis of LCL-reactive T cells in peripheral blood using the ELISpot assay of IFNγ release. Ex vivo PBMCs from EBV-seropositive donors (P1-P5) or EBV-seronegative donors (N1-N5) were CD56-depleted and then either (A) CD8-enriched by CD4+ T-cell depletion or (B) CD4-enriched by CD8+ T-cell depletion. These preparations were seeded at 1 × 105 and 2 × 105 cells/well in duplicate wells and cocultured with autologous B95.8 and BZ k/o LCLs (grown for at least 1 month in HuS) at an effector-to-target ratio of 40:1. Results from one representative experiment are expressed as mean ± 1 SD spot-forming cells (SFCs) per 2 × 105 cells; background responses from nonstimulated cells are also shown (control). *P < .05 (Wilcoxon-Mann-Whitney test).
Characterization of LCL-reactive CD4+ T-cell clones
We then isolated LCL-reactive CD4+ cells from both types of donor (1) by stimulating CD8-depleted PBMCs with γ-irradiated autologous BZ k/o LCL cells at a 40:1 PBMC-to-LCL ratio for 10 days and then expanding by further LCL stimulation, or (2) by exposing CD8-depleted PBMCs ex vivo to autologous BZ k/o LCL cells for 3 hours as in the ELISpot assays, before capturing the IFNγ-secreting cells. In both cases, the reactive cells were then cloned onto γ-irradiated feeder cells in IL-2–conditioned medium supplemented with HuS. T-cell clones showing recognition of the autologous BZ k/o LCL but not CD40-activated B blasts in IFNγ ELISA screening assays were isolated from all 4 seropositive donors (P1-P4) and from 3 of 5 seronegative donors (N1-N3) tested. When present, LCL-reactive clones represented approximately 10% of all proliferating microcultures from both types of donor; all displayed a CD4+, CD8−, CD45RO+, CD45RA−, CD28+, CD27−, CD57− phenotype by FACS analysis. Other microcultures showed no reactivity to either LCL or B-blast targets.
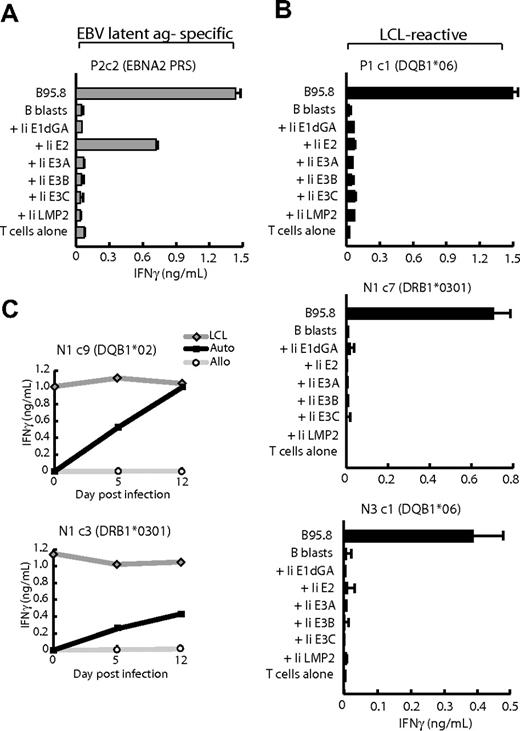
All LCL-reactive clones were then screened in IFNγ release assays against (1) a wider panel of autologous nontransformed cells, (2) the autologous LCL in the presence of anti-HLA blocking mAbs, and (3) a panel of allogeneic HLA-typed LCLs to identify the HLA II–restricting allele. Figure 2 shows representative results for LCL-reactive clones from seropositive donor P1 and seronegative donors N1 and N3. In each case, the CD4+ T-cell clones recognized the B95.8 and BZ k/o autologous LCLs but not the autologous CD40-activated B blasts, PHA-activated T blasts, or dendritic cells (Figure 2A). Furthermore, autologous LCL recognition could be specifically blocked with mAbs against HLA-DR, -DP, or -DQ molecules. From Figure 2B, recognition was restricted through an HLA-DQ allele for clones P1c2 and N3c1 and through an HLA-DR allele for clone N1c10. Data from subsequent assays on a large panel of allogeneic LCL targets, some of which are shown in Figure 2C, identified the restricting alleles for these particular clones as HLA-DQB1*06 for P1c2, HLA-DRB1*0101 for N1c10, and HLA-DQB1*06 for N3c1.
Functional analysis of LCL-reactive CD4+ T-cell clones isolated from EBV-seropositive (P1) and EBV-seronegative (N1 and N3) donors. (A) T cells (104 cells/well) were cocultured overnight with standard numbers (5 × 104 cells/well) of autologous B95.8 and BZ k/o LCLs, CD40L-activated B lymphoblasts (B blasts), PHA-activated T lymphoblasts (T blasts), and dendritic cells (DCs) all grown in human serum. (B) Autologous LCLs were incubated for 1 hour in the presence of mAb to HLA class I (aHLA I), HLA-DP (aDP), HLA-DQ (aDQ), HLA-DR (aDR), or medium as a control (B95 LCL) before the addition of T cells to the assay. (C) T cells were cocultured overnight (as in panel A) with cells of the autologous B95.8 LCL (Auto) and of partially HLA class II–matched allogeneic LCLs (Allo LCLs), for which the matched allele is shown in each case. All results shown are the mean ± 1 SD of triplicate wells in which IFNγ release into the supernatant medium was determined by ELISA (in ng/mL). Results are representative of those seen on 3 occasions of testing and include only a subset of the total number of target LCLs tested.
Functional analysis of LCL-reactive CD4+ T-cell clones isolated from EBV-seropositive (P1) and EBV-seronegative (N1 and N3) donors. (A) T cells (104 cells/well) were cocultured overnight with standard numbers (5 × 104 cells/well) of autologous B95.8 and BZ k/o LCLs, CD40L-activated B lymphoblasts (B blasts), PHA-activated T lymphoblasts (T blasts), and dendritic cells (DCs) all grown in human serum. (B) Autologous LCLs were incubated for 1 hour in the presence of mAb to HLA class I (aHLA I), HLA-DP (aDP), HLA-DQ (aDQ), HLA-DR (aDR), or medium as a control (B95 LCL) before the addition of T cells to the assay. (C) T cells were cocultured overnight (as in panel A) with cells of the autologous B95.8 LCL (Auto) and of partially HLA class II–matched allogeneic LCLs (Allo LCLs), for which the matched allele is shown in each case. All results shown are the mean ± 1 SD of triplicate wells in which IFNγ release into the supernatant medium was determined by ELISA (in ng/mL). Results are representative of those seen on 3 occasions of testing and include only a subset of the total number of target LCLs tested.
Although reactivity against EBV-lytic antigens could be ruled out because all clones recognized the autologous BZ k/o LCL, reactivity against EBV latent antigens remained a possibility. We therefore screened each clone against (1) a panel of recombinant MVAs encoding 6 of the 8 EBV latent antigens (B95.8 strain) fused to the invariant chain (Ii) target sequence that mediates delivery of the antigens directly into the HLA class II–processing pathway,28 and (2) pools of overlapping peptides covering the sequences of EBNA-LP and LMP-1, the 2 latent proteins not available as MVA constructs. Of LCL-reactive clones derived from seropositive donors, typically 50% to 60% proved to be EBV latent antigen specific and skewed toward those epitopes that are best presented on the LCL surface. One example is clone P2c2 which recognized B blasts expressing the EBNA-2 antigen (Figure 3A) and was subsequently mapped (data not shown) to the DRB3*0201-restricted PRS epitope (EBNA-2 276-295).22 However, other LCL-reactive clones from seropositive donors (eg, P1c1) and all LCL-reactive clones from seronegative donors (eg, N1c7, N3c1) failed to recognize any of the Ii-targeted EBV antigens or any of the EBNA-LP or LMP-1 peptides (Figure 3B). Such clones, termed “LCL-specific,” therefore appear to recognize as-yet-undefined antigens, probably to be of cellular origin, expressed by EBV-transformed B-cell lines. Importantly, these antigens are up-regulated as an early event in EBV-induced transformation and not as secondary consequences of long-term LCL culture. Thus, in Figure 3C, LCL-specific clones N1c9 and N1c3, restricted through different HLA II alleles, showed recognition of autologous EBV-infected B-cell cultures as early as 5 days after infection, increasing further by day 12 as transformed foci began to dominate the culture.
Antigen mapping assays. (A) CD4+ T-cell clone P2c2 specific for the EBNA2276-295 PRS epitope and (B) LCL-reactive clones P1 c1, N1 c7, and N3 c1 were cocultured overnight with HLA class II–matched B95.8 LCL cells and HLA class II–matched CD40L-activated B lymphoblasts either uninfected (B blasts) or infected with a panel of recombinant MVA vectors each expressing an invariant chain (Ii)–targeted form of one of the EBV latent proteins EBNA-1 (glycine-alanine repeat-deleted, Ii E1dGA), EBNA-2 (Ii E2), EBNA-3A (Ii E3A), EBNA-3B (Ii E3B), EBNA-3C (Ii E3C), or LMP-2 (Ii LMP2); parallel assays on EBNA-LP and LMP-1 peptide panels gave uniformly negative results (data not shown). (C) B cells isolated from autologous PBMCs (Auto) and from HLA mismatched PBMCs (Allo) were infected with B95.8 EBV. At day 5 and 12 after infection, 5 × 104 target B cells/well were cocultured with CD4+ T-cell clones N1c9 and N1c3 in an IFNγ ELISA. In each case, the autologous B95.8 LCL was used as a positive control target. All results shown are the mean ± 1 SD of triplicate wells in which IFNγ release into the supernatant medium was determined by ELISA (in ng/mL). Results are representative of those seen on 3 occasions of testing.
Antigen mapping assays. (A) CD4+ T-cell clone P2c2 specific for the EBNA2276-295 PRS epitope and (B) LCL-reactive clones P1 c1, N1 c7, and N3 c1 were cocultured overnight with HLA class II–matched B95.8 LCL cells and HLA class II–matched CD40L-activated B lymphoblasts either uninfected (B blasts) or infected with a panel of recombinant MVA vectors each expressing an invariant chain (Ii)–targeted form of one of the EBV latent proteins EBNA-1 (glycine-alanine repeat-deleted, Ii E1dGA), EBNA-2 (Ii E2), EBNA-3A (Ii E3A), EBNA-3B (Ii E3B), EBNA-3C (Ii E3C), or LMP-2 (Ii LMP2); parallel assays on EBNA-LP and LMP-1 peptide panels gave uniformly negative results (data not shown). (C) B cells isolated from autologous PBMCs (Auto) and from HLA mismatched PBMCs (Allo) were infected with B95.8 EBV. At day 5 and 12 after infection, 5 × 104 target B cells/well were cocultured with CD4+ T-cell clones N1c9 and N1c3 in an IFNγ ELISA. In each case, the autologous B95.8 LCL was used as a positive control target. All results shown are the mean ± 1 SD of triplicate wells in which IFNγ release into the supernatant medium was determined by ELISA (in ng/mL). Results are representative of those seen on 3 occasions of testing.
We selected 24 LCL-specific clones derived from 4 seropositive and 3 seronegative donors for detailed study. Table 1 lists these clones and their defined HLA II–restricting alleles. Among these were clones derived from the same donor but restricted through different HLA alleles, as well as clones derived from different persons but restricted through the same HLA allele. On the basis of restrictions, the panel of clones contained 14 potentially distinct reactivities restricted through 10 different HLA alleles.
CD4+ T-cell clones and HLA class II restrictions
| Donor . | Clone . | HLA restriction . |
|---|---|---|
| P1 | c1,c2 | DQB1*06 |
| c3 | DRB1*0301 | |
| c4 | DRB1*1302 | |
| P2 | c1 | DRB1*1301 |
| P3 | c1,c2 | DRB1*0701 |
| P4 | c1,c2 | DRB3*0201/0301 |
| c3 | DQB1*02 | |
| N1 | c1,c2 | DPB1*0402/0602 |
| c3–8 | DRB1*0301 | |
| c9 | DQB1*02 | |
| c10 | DRB1*0101 | |
| N2 | c1 | DQB1*02 |
| N3 | c1 | DQB1*06 |
| c2,c3 | DRB1*0103 |
| Donor . | Clone . | HLA restriction . |
|---|---|---|
| P1 | c1,c2 | DQB1*06 |
| c3 | DRB1*0301 | |
| c4 | DRB1*1302 | |
| P2 | c1 | DRB1*1301 |
| P3 | c1,c2 | DRB1*0701 |
| P4 | c1,c2 | DRB3*0201/0301 |
| c3 | DQB1*02 | |
| N1 | c1,c2 | DPB1*0402/0602 |
| c3–8 | DRB1*0301 | |
| c9 | DQB1*02 | |
| c10 | DRB1*0101 | |
| N2 | c1 | DQB1*02 |
| N3 | c1 | DQB1*06 |
| c2,c3 | DRB1*0103 |
CD4+ T-cell recognition of EBV-positive and EBV-negative lines of BL origin
We then assayed such LCL-specific CD4+ effectors for recognition of BL lines of known HLA type.29 Figure 4A shows results from EBV-positive targets. The HLA-DP B1*0402/0602-restricted clone N1c1 recognized the DP-matched Kem-BL line and the Kem LCL derived from normal B cells of the same person. Similarly, clone P1c3 (HLA DRB1*0301-restricted) recognized 2 DR-matched BL lines, BL37 and Isa-BL, at levels even greater than seen against the autologous LCL. In further assays, we observed equally good recognition of EBV-negative BL lines (Figure 4B). For example clone N1c9 (HLA DQ B1*02-restricted) recognized both EBV-positive and EBV-loss subclones of the HLA DQ-matched Mutu BL line, and clone N1c10 (DRB1*0101-restricted) recognized both HLA-matched EBV-positive Isa-BL and EBV-negative BL40 targets.
LCL-specific CD4+ T-cell recognition of HLA class II–matched BL lines. (A) EBV-positive lines and (B) EBV-positive and -negative lines. T cells (104 cells/well) were cocultured overnight with standard numbers (5 × 104 cells/well) of LCLs and BL lines whose EBV status and matching for the relevant HLA class II–restricting allele are shown. In each case, the lines that had been preincubated either with medium alone or in the presence of the relevant HLA class II–blocking mAb (as described in the legend for Figure 2). All responses are shown as the mean − 1 SD of triplicate wells in which IFNγ release into the supernatant medium was determined by ELISA (in ng/mL). Results are representative of those seen on 3 occasions of testing.
LCL-specific CD4+ T-cell recognition of HLA class II–matched BL lines. (A) EBV-positive lines and (B) EBV-positive and -negative lines. T cells (104 cells/well) were cocultured overnight with standard numbers (5 × 104 cells/well) of LCLs and BL lines whose EBV status and matching for the relevant HLA class II–restricting allele are shown. In each case, the lines that had been preincubated either with medium alone or in the presence of the relevant HLA class II–blocking mAb (as described in the legend for Figure 2). All responses are shown as the mean − 1 SD of triplicate wells in which IFNγ release into the supernatant medium was determined by ELISA (in ng/mL). Results are representative of those seen on 3 occasions of testing.
Overall, clones representing 10 of the potentially different reactivities in Table 1 were tested against BL targets and reproducibly recognized most HLA-matched BL lines, whether EBV positive or negative. Recognition appeared to be specific because it could always be blocked by the relevant anti-HLA II mAb and was never seen on HLA-mismatched targets. However, it is important to note that, for some clones, not all HLA-matched BL lines (EBV positive or negative) were recognized. For example in supplemental Figure 1, the DRB1*0301-restricted clone N1c3 recognized 3 of 4 allele-matched BL lines (Mutu-BL, BL2, and Joy-BL, but not BL37), whereas clone P2c1, from a different donor but with the same restriction, recognized Mutu-BL and BL37, but not BL2 or Joy-BL. These individual patterns of clonal recognition were reproducible on repeated testing.
CD4+ T-cell recognition of other B- and T-lymphoma cell lines
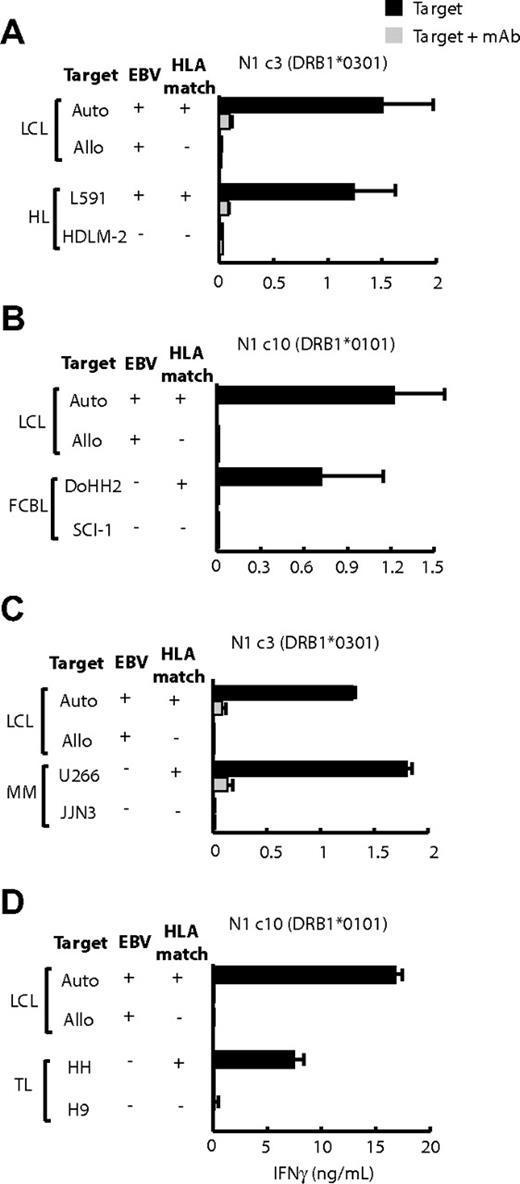
Among the few HL-derived lines available, only L591 (EBV positive) and HDLM-2 (EBV negative) could be tested against appropriately HLA-matched CD4+ T-cell clones, and both were recognized. For example in Figure 5A, the DRB1*0301-restricted clone N1c3 recognized the DR-matched L591 cells but not mismatched HDLM-2 cells. The converse result was seen with the use of other effectors with which HDLM2 but not L591 was matched (see later, supplemental Figure 2B).
LCL-specific CD4+ T-cell recognition of other B- and T-lymphoma cell lines. T cells (104 cells/well) were cocultured overnight with standard numbers (5 × 104 cells/well) of HLA-matched and -mismatched LCLs and (A) Hodgkin lymphoma (HL) lines, (B) follicular cell B lymphoma (FCBL) lines, (C) multiple myeloma (MM) lines, and (D) T-cell lymphoma (TL) lines. The experimental design and expression of results are the same as in Figure 4.
LCL-specific CD4+ T-cell recognition of other B- and T-lymphoma cell lines. T cells (104 cells/well) were cocultured overnight with standard numbers (5 × 104 cells/well) of HLA-matched and -mismatched LCLs and (A) Hodgkin lymphoma (HL) lines, (B) follicular cell B lymphoma (FCBL) lines, (C) multiple myeloma (MM) lines, and (D) T-cell lymphoma (TL) lines. The experimental design and expression of results are the same as in Figure 4.
Further studies used HLA II–positive target lines from lymphomas that had no EBV association. These assays were conducted without prior knowledge of the target lines' HLA II type, and so every effector clone was tested on the full panel of targets. We noted many cases in which individual follicular lymphoma and multiple myeloma lines, the B-lymphoma line BJAB, and even T-lymphoma lines were recognized by particular effector clones. Subsequent HLA II typing showed that, in each case, the targets did indeed express the appropriate restricting allele. Thus, Figure 5B shows that the HLA DRB1*0101-restricted clone N1c10 recognized the DR-matched follicular B-cell lymphoma line DoHH2 but not a mismatched follicular line SCI-1. Likewise, from Figure 5C, the HLA-DQB1*0301-restricted clone N1c3 reacted strongly to the DQ-matched myeloma line U266 but not to the mismatched myeloma line JJN3. In both instances, recognition was blocked by the appropriate anti-DR or anti-DQ mAb (Figure 5B-C), and there was no recognition of many other mismatched targets (data not shown). Informative assays on T lymphoma–derived target lines were limited by a paucity of appropriate HLA matches, but nevertheless we did see evidence of reactivity. Thus, the DQB1*06-restricted clone N3c1 recognized the DQ-matched HH line, but not a mismatched T-cell line, and this was blocked by the anti-DQ mAb (Figure 5D).
Again, not all HLA-matched effector/target combinations led to IFNγ release. As detailed in supplemental Figure 2, clones N1c9 showed clear recognition of 2 HLA DQB1*02-matched target lines (L591 and U266), weaker recognition of a third matched line (JJN3) but no reactivity against 2 other matched lines (BC-2 and BC-3); another clone, N3c1, recognized 2 DQB1*06-matched lines (DoHH2 and HDLM2) but not a third matched line (SCI-1). Once again, when responses were observed, they could always be specifically blocked by the relevant anti-DR, -DQ, or -DP mAb. Overall, these findings suggest that such recognition is immunologically specific, and the target range of LCL-specific effectors extends to other non-EBV–associated lymphoma types.
To confirm that such reactivities are indeed detectable within bulk LCL-stimulated CD4+ T-cell preparations and are not just rare clonal entities, we followed bulk cultures from selected donors from days 14 to 28 of their in vitro expansion, screening for ELISpot reactivity against the autologous LCL and against a selection of lymphoma cell lines. As shown in supplemental Figure 3, bulk cultures from seropositive donor P1 recognized the autologous LCL strongly but also contained significant reactivity against the DRB1*1302-matched BJAB cell line, similar to that which had been rescued from the same donor as clone P1c4 (see Table 1). Bulk cultures from seronegative donor N3 showed an early peak of reactivity against both the autologous LCL and the DQB1*06-matched DoHH2 cell line, a target also seen by the donor N3-derived clone N3c1 (see supplemental Figure 2). In such experiments, bulk culture reactivities against HLA-mismatched lymphoma lines and against K562 cells included as an index of NK cell–like activity, were generally low.
Fas-mediated lysis and outgrowth control of lymphoma lines by CD4+ T cells
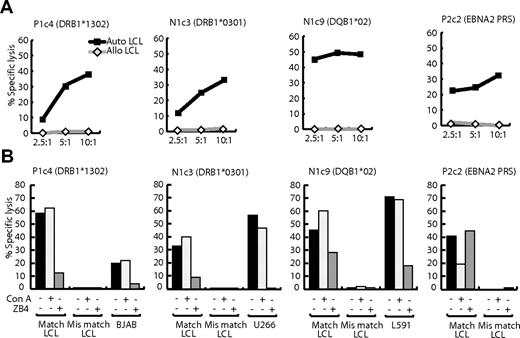
We found that LCL-specific T-cell recognition also led to target cell lysis. Figure 6A shows the results of 12-hour chromium release assays in which clones P1c4, N1c3, and N1c9, whose restricting alleles are shown, were tested as effectors against target LCLs. All clones killed HLA-matched (but not HLA-mismatched) LCLs at levels at least the equal of an EBNA-2–specific CD4+ T-cell clone; the latter was included for reference because it recognizes one of the best displayed EBV-coded epitopes, PRS, on the LCL surface.22 Furthermore, HLA-matched lymphoma targets (respectively BJAB, U266, and L591, chosen because they had been recognized in IFNγ release assays) were also killed (Figure 6B). As to the mechanism of killing, unlike EBV-specific CD4+ T-cell clones, LCL-specific clones lacked perforin and expressed only very low levels of granzyme B. Accordingly, as shown in Figure 6B, the vacuolar type H+-ATPase inhibitor Con A, which prevents perforin and granzyme-mediated cytolysis,30 blocked LCL lysis by the EBV-specific clone but did not affect the killing of LCL or lymphoma cell lines by LCL-specific effectors. By contrast, preincubation with blocking antibody against Fas-L (ZB4) did not affect the EBV-specific clone but blocked LCL-specific cytolytic function almost completely (Figure 6B). Thus, LCL-specific CD4+ T cells kill their targets preferentially, if not exclusively, through the Fas/Fas-L pathway.
Lysis of LCL and B-lymphoma targets by LCL-specific clones P1c4, N1c3, N1c9 and by clone P2c2 specific for the EBNA2276-295 PRS epitope. (A) Twelve-hour chromium release assays were conducted with HLA class II–matched (auto) and mismatched (allo) LCL targets. Results are expressed as the percentage of specific chromium release from the target cells at effector-to-target ratios of 2.5:1, 5:1, and 10:1. (B) Results of similar assays conducted at an effector-to-target ratio of 10:1, either when both effector and targets were unmanipulated, or when target cells were preexposed to 100 nM Con A for 1 hour after labeling with 51Cr, or when effector cells were preincubated with 100 ng/mL CD95 Fas (blocking) mAb ZB4 (Immunotech) for 2 hours before the assay. These results are representative of those seen on 3 occasions of testing.
Lysis of LCL and B-lymphoma targets by LCL-specific clones P1c4, N1c3, N1c9 and by clone P2c2 specific for the EBNA2276-295 PRS epitope. (A) Twelve-hour chromium release assays were conducted with HLA class II–matched (auto) and mismatched (allo) LCL targets. Results are expressed as the percentage of specific chromium release from the target cells at effector-to-target ratios of 2.5:1, 5:1, and 10:1. (B) Results of similar assays conducted at an effector-to-target ratio of 10:1, either when both effector and targets were unmanipulated, or when target cells were preexposed to 100 nM Con A for 1 hour after labeling with 51Cr, or when effector cells were preincubated with 100 ng/mL CD95 Fas (blocking) mAb ZB4 (Immunotech) for 2 hours before the assay. These results are representative of those seen on 3 occasions of testing.
Finally, we conducted outgrowth assays in which replicate cultures of target lines were seeded across a range of doubling dilutions in the presence and absence of a standard number of LCL-specific CD4+ T cells, and the cocultures were scored after 4 weeks for successful target cell outgrowth. Each experiment included HLA-matched and HLA-mismatched LCL targets as well as HLA-matched lymphoma lines known to have been recognized in the earlier IFNγ release assays. Figure 7 shows typical results of assays involving 3 such effector/target combinations (clone N1c3 with U266, clone N1c9 with L591, and clone N1c1 with BJAB). In each case, the results are expressed as the minimum number of target cells required for successful outgrowth in the presence (filled symbols) or absence (dotted lines) of CD4+ T cells. Clearly, the LCL-specific clones strongly inhibited outgrowth not just of the HLA-matched LCL but also of the HLA-matched lymphoma lines.
CD4+ T-cell inhibition of LCL and lymphoma cell line outgrowth. HLA class II–matched and –mismatched LCLs and HLA-matched lymphoma lines L591, U266, and BJAB were seeded at doubling dilutions of 104 to 300 cells/well either alone or with the addition of 104 CD4+ T cells of LCL-specific clones N1c3, N1c9, and N1c1, respectively. In each case the efficiency of target cell outgrowth, scored at 3 to 4 weeks, is expressed as the minimum target cell seeding required for successful outgrowth from T cell–target cocultures (■) compared with target cells cultured alone ( ). Results are shown from a single experiment representative of several such assays.
). Results are shown from a single experiment representative of several such assays.
CD4+ T-cell inhibition of LCL and lymphoma cell line outgrowth. HLA class II–matched and –mismatched LCLs and HLA-matched lymphoma lines L591, U266, and BJAB were seeded at doubling dilutions of 104 to 300 cells/well either alone or with the addition of 104 CD4+ T cells of LCL-specific clones N1c3, N1c9, and N1c1, respectively. In each case the efficiency of target cell outgrowth, scored at 3 to 4 weeks, is expressed as the minimum target cell seeding required for successful outgrowth from T cell–target cocultures (■) compared with target cells cultured alone ( ). Results are shown from a single experiment representative of several such assays.
). Results are shown from a single experiment representative of several such assays.
Discussion
LCL-stimulated T-cell preparations from virus-immune donors, although dominated by EBV latent antigen-specific CD8+ T cells, contain a smaller component of LCL-reactive CD4+ T cells8,14 that are often assumed to be similarly EBV specific. However, in an earlier work we noted that many components of latent antigen-specific CD4 memory are not efficiently reactivated by LCL stimulation in vitro.20,21 This reflects the fact that, when processed from endogenously expressed antigen, many CD4 epitopes within latent cycle proteins are poorly displayed on the LCL surface.22,23 Here, we show that a significant component of the LCL-stimulated CD4+ T response from virus-immune donors, although recognizing LCLs in a classically HLA II–restricted manner, is not EBV antigen specific. Rather, these LCL-specific CD4+ T cells appear to recognize cellular targets whose expression is specifically up-regulated during EBV-induced B-cell transformation. Indeed, such effectors can also be isolated from the blood of EBV-naive donors, in the absence of any accompanying virus-specific response. We also show that such reactivities may have wider-than-expected therapeutic potential because their targets are also expressed in a range of human lymphoma lines, irrespective of EBV status.
We first screened CD4-enriched and CD8-enriched PBMC preparations, isolated from EBV-seropositive and -seronegative donors, for recognition of autologous LCL cells in ex vivo ELISpot assays of IFNγ release. Note that all assays were conducted on LCLs grown in HuS to avoid spurious cross-reactivities with FCS proteins and used a PBMC-to-LCL ratio (40:1) that we have already shown is optimal for the induction of antigen-specific rather than anomalous responses to LCL stimulation.31 As others have found,32,33 seropositive donors show clear evidence of both CD4 and CD8 reactivity in such assays. However, because conventional LCLs transformed with wild-type EBV contain variable numbers of cells in lytic cycle, the observed reactivities include both latent and lytic antigen responses. The latter responses can be numerically dominant and are directed, in the case of CD4+ T cells, mainly against reprocessed virion structural proteins21 and, in the case of CD8+ T cells, mainly against immediate early and early nonstructural proteins.34 Using LCLs transformed with a virus deleted for BZLF1, the key initiator of EBV lytic cycle, allows one to exclude lytic cycle proteins as an antigen source. As anticipated, we found that seropositive donors mounted ex vivo CD8 responses that were stronger to the wild-type than to the BZ k/o virus-transformed LCL, whereas seronegative donors responded to neither stimulus. In the CD4+ T-cell assays, seropositive donors gave ex vivo responses of different magnitudes but again always stronger to the wild-type LCL; here, however, we also detected small but significant responses from some seronegative donors that were directed equally against the wild-type and the BZ k/o LCL. We were aware, as others have shown,35 that not all persons who are seronegative by conventional EBV antibody screening are truly EBV negative. However, we confirmed that our 5 seronegative persons were indeed EBV naive, because they were negative in several different antibody assays, in regression assays of EBV-specific T-cell memory,20 and in sensitive quantitative polymerase chain reaction assays for EBV DNA in peripheral blood B cells (data not shown). We note that, in a recent study, others also detected CD4 but not CD8 ELISpot reactivity to ex vivo LCL challenge in some seronegative persons, but they did not pursue the finding.33
The significance of these results became clearer when the LCL-reactive CD4+ T cells were either selected from ex vivo assays by IFNγ capture and expanded in vitro or isolated by limiting dilution cloning from LCL-stimulated bulk cultures. Both seropositive and seronegative donors yielded LCL-specific clones, including one clone isolated from LCL-stimulated bulk cultures of a seronegative donor, N2, who did not give a detectable CD4 response in the ex vivo ELISpot assay. All such clones were classically HLA-DR, -DQ, or -DP restricted, recognized the autologous BZ k/o virus–transformed LCL but not autologous mitogen-activated B blasts, T blasts, or dendritic cells, and yet they could not be mapped to any known EBV antigen or epitope peptide. We infer that each distinct LCL-specific reactivity in Table 1 is recognizing its own unique HLA II/epitope complex, but that all such epitopes are derived from cellular target antigens preferentially expressed in LCL cells. Indeed, these target antigens appear to be directly EBV induced (rather than accompaniments of long-term LCL passage) because B cells become sensitive to LCL-specific T-cell recognition within a few days of EBV infection. This rapidity of target antigen induction helps to explain why, in an earlier study, we detected the activation of similar LCL-specific CD4+ T cells in short-term cultures of EBV-infected PBMCs.20 In that work, however, such cells were not detected in cultures from the one seronegative donor tested, leaving the relationship of these CD4 responses to EBV-immune status unresolved. Here, we make clear that LCL-specific CD4+ T cells can be reactivated from the T-cell repertoire of healthy adults irrespective of their EBV status.
A review of earlier literature shows that CD4+ T cells capable of recognizing autologous LCL cells have been detected in various in vitro settings, usually after LCL stimulation, where the effector cells were derived from either fetal cord blood,36,37 EBV-seronegative donors,35,38 EBV-seropositive donors,39-43 or, most recently, patients with infectious mononucleosis undergoing primary EBV infection.21 In some cases, further analysis at the clonal level showed these cells to be HLA II restricted or to recognize LCL cells but not mitogen-activated lymphoblasts.21,38,40-42 However, most of these studies did not look for reactivity against individual EBV antigens and so never showed EBV specificity. We suggest that many of these responses were, as described here, LCL specific. Interestingly, in the one study in which EBV antigen specificity was examined,21 clones that did not map to known antigens were identified and described as “autoreactive.”
Because the targets antigens being recognized by LCL-specific CD4+ T cells appeared to be of cellular origin, we asked whether such antigens might also be up-regulated in other EBV-associated B lymphomas such as BL and HL. The availability of a wide panel of well-characterized EBV-positive and EBV-negative BL cell lines, all HLA-typed, allowed us to investigate the point in some detail. When matched for the relevant restricting allele, both EBV-positive and EBV-negative BL target lines could be recognized by LCL-specific effector cells, often just as strongly as matched LCL targets included as controls in the assay. The same held true for the one EBV-positive and one EBV-negative HL line whose HLA types allowed matching with individual CD4+ clones. We then extended the target cell panel to include HLA II–expressing cell lines derived from non–EBV-associated lymphomas and observed recognition of follicular lymphoma, multiple myeloma, and T-lymphoma cell lines. Importantly, however, not all HLA-matched effector/target combinations in the lymphoma cell line assays led to recognition. Each CD4 effector clone had its own unique pattern of reactivity with some HLA-matched lines consistently recognized and others repeatedly not recognized. This is not unexpected if, as we propose, each clone is reactive to a unique peptide epitope derived from a cellular protein that is up-regulated in response to EBV infection in normal B cells but may or may not be expressed in particular lymphomas. Identifying these cellular targets and determining their in vivo pattern of expression, both in malignant and normal cell types, are important long-term goals, if the therapeutic potential of LCL-specific CD4+ T cells is to be properly assessed.
Our immediate goal in this study was to test these CD4+ T cells in vitro for attributes probably required of therapeutic effectors in vivo, namely an ability to kill specific target cells in short-term assays or to inhibit target cell growth in longer-term assays or both. Although tumor-specific CD4+ T-cell responses are generally less well characterized than their CD8+ counterparts, some studies in other systems have shown direct killing of malignant cells by cytotoxic CD4+ T-cell effectors.44-46 Almost all the CD4+ T-cell clones isolated in the present work were cytotoxic against HLA-matched targets, killing by Fas/Fas-L mediated lysis. Interestingly, they differ in this respect from other types of CD4+ effectors (including EBV antigen-specific effectors) which have been reported to use Fas/Fas-L–independent mechanisms, including perforin/granzyme,18,42,43,47 granulysin,42,48 or TRAIL.49 Furthermore, the LCL-specific CD4+ T cells that were cytotoxic against HLA class II–matched LCLs and B-lymphoma cell lines were also able to inhibit their outgrowth in longer-term assays.
Although these properties support the notion of a therapeutic potential for LCL-specific CD4+ T cells, the safety and efficacy of such cells for in vivo administration remain to be determined. However, we infer that CD4+ T cells of this type are probably present in the LCL-stimulated bulk T-cell preparations that, after adoptive transfer into patients who received a transplant, have been well tolerated without autoimmune effects6,8,9,35,50 and indeed have successfully targeted PTLD35 and, in rare cases, other EBV-positive lymphomas such as BL and HL.15 If such LCL-specific CD4+ T cells indeed proved safe to administer, the relative ease with which they can be generated from most EBV-naive as well as EBV-immune donors, and their ability to recognize cells derived from a range of human lymphomas, irrespective of EBV association, dramatically widens their potential for therapeutic use.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Leukemia Research Fund (London, United Kingdom; project grant 08012).
Authorship
Contribution: H.M.L. designed and performed the research, analyzed data, and wrote the paper; J.Z. designed and performed research; A.M.L., N.H.G., and H.J. performed research; G.S.T. analyzed data; and A.B.R. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan B. Rickinson, CRUK Institute for Cancer Studies, School of Cancer Sciences, The University of Birmingham, Vincent Dr, Birmingham B15 2TT, United Kingdom; e-mail: a.b.rickinson@bham.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal