Abstract
This phase 3, multicenter, open-label study evaluated the efficacy and safety of tipifarnib compared with best supportive care (BSC), including hydroxyurea, as first-line therapy in elderly patients (≥70 years) with newly diagnosed, de novo, or secondary acute myeloid leukemia. A total of 457 patients were enrolled with 24% 80 years of age or older. Tipifarnib 600 mg orally twice a day was administered for the first 21 consecutive days, in 28-day cycles. The primary endpoint was overall survival. The median survival was 107 days for the tipifarnib arm and 109 days for the BSC arm. The hazard ratio (tipifarnib vs BSC) for overall survival was 1.02 (P value by stratified log-rank test, .843). The complete response rate for tipifarnib in this study (8%) was lower than that observed previously, but with a similar median duration of 8 months. The most frequent grade 3 or 4 adverse events were cytopenias in both arms, slightly more infections (39% vs 33%), and febrile neutropenia (16% vs 10%) seen in the tipifarnib arm. The results of this randomized study showed that tipifarnib treatment did not result in an increased survival compared with BSC, including hydroxyurea. This trial was registered at www.clinicaltrials.gov as #NCT00093990.
Introduction
The incidence of acute myeloid leukemia (AML) increases exponentially with age.1,2 Approximately 55% of all cases occur in patients more than 65 years of age.1,3 There is no established standard of care for treating elderly patients with AML, and those who receive treatment have a median survival time of less than 1 year
When possible, it is recommended to give induction combination chemotherapy in patients with AML. However, for the patients with disease-related risk factors (secondary AML, prior myelodysplastic syndrome [MDS], unfavorable karyotype) associated with poor treatment outcome, or patient-related risk factors (comorbidities, impaired performance status, or increased age) associated with diminished ability to tolerate adverse effects,3-5 treatment with best supportive care (BSC), including hydroxyurea, single agents, or treatment in clinical studies is recommended.6-9 For those elderly patients who are fit to receive induction chemotherapy, significant toxicity has been reported,10 including a therapy-related mortality rate of approximately 25%.11 In addition, median survival and remission rates decrease with increasing age. In a retrospective study using data from the Surveillance, Epidemiology, and End Results registries and Medicare claims in the United States, median survival was 3.9 months among those 65 to 74 years of age, 2.2 months in those 75 to 84 years of age, and 1.4 months in those 85 years of age or older.12 In the Medical Research Council (United Kingdom) AML-8 study, an induction therapy consisting of daunorubicin, cytarabine, and 6-thioguanine yielded a remission rate of only 26% for those more than 70 years of age compared with 52% for those between 60 and 69 years of age and 70% for those less than 50 years of age.10 For these reasons, induction chemotherapy is often not a viable treatment option for elderly patients with AML, and many patients receive palliative chemotherapy or supportive care alone.13-15
Tipifarnib is a selective, nonpeptidomimetic, orally active inhibitor of the enzyme farnesyltransferase. Tipifarnib has been tested in a wide array of solid tumors and hematologic malignancies, with antitumor activity seen in several tumor types, including MDS16,17 and AML.18,19 Tipifarnib was the first farnesyltransferase inhibitor to induce, in the phase 1 setting, complete remissions in AML.18 The phase 2 study, CTEP-20, confirmed the early report with a complete remission rate of 14%, and a median complete remission duration of 7.3 months.19 Tipifarnib was found to have an acceptable safety profile in elderly patients with AML, with the most common adverse events related to myelosuppression or gastrointestinal disorders. The current study constitutes the largest prospectively studied cohort of elderly subjects with newly diagnosed AML to date. This phase 3 study was designed to evaluate safety and to establish the effect on survival of tipifarnib compared with best supportive care in elderly subjects with newly diagnosed AML who are not eligible for induction chemotherapy.
Methods
Patients
Patients 70 years of age or older with newly diagnosed de novo or secondary AML were eligible for enrollment. The main criteria for inclusion were: pathologic confirmation of AML (≥ 20% bone marrow leukemic blasts), not medically fit, or did not wish to be treated with induction chemotherapy, and Eastern Cooperative Oncology Group (ECOG) performance scores of 0, 1, or 2. Patients with previous cytotoxic or biologic treatment for AML were excluded. Additional exclusion criteria included known central nervous system leukemia, acute promyelocytic leukemia, absolute peripheral blast count greater than 30 000/mm3, uncontrolled systemic infection, and symptomatic neuropathy of grade 2 or worse. Review boards at participating institutions approved the study, which was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and Guidelines for Good Clinical Practice. All patients provided written informed consent to their study participation.
Study design and treatment
This was a phase 3, randomized, multicenter, multinational, open-label study comparing tipifarnib with BSC, for the treatment of 457 elderly patients (≥ 70 years of age) with newly diagnosed AML who either were not fit for or not willing to receive induction chemotherapy. Patients were randomly assigned in a 1:1 ratio to either BSC or tipifarnib treatment using a central interactive voice response system. Patients were stratified at randomization based on ECOG performance status (performance score: 0 or 1 vs 2) and age group (< 75 years vs ≥ 75 years). Randomization was to occur no later than 3 weeks after diagnosis and no more than 1 day before the start of treatment. Tipifarnib treatment consisted of 28-day cycles with 21 days of consecutive treatment followed by a mandatory 7-day rest period. Adverse events, concomitant medications, and clinical laboratory analyses were recorded weekly. Specific dose modifications were allowed as defined in the protocol. The treatment continued until disease progression, intolerable toxicity, death, loss to follow-up, investigator decision, or withdrawal of consent to further treatment. All patients received supportive care, which included blood product transfusions, prophylactic or symptomatic use of anti-infectives and cytokines, according to institutional practices and other therapy appropriate for the symptomatic treatment of AML and its complications. Hydroxyurea was permitted on the BSC arm only.
The primary endpoint was to compare overall survival of patients treated with tipifarnib and patients treated with BSC, including hydroxyurea. The primary efficacy comparison was performed for all randomized subjects, and a secondary comparison was performed on the subgroup of subjects with “AML with myelodysplasia,” which included subjects classified as per the World Health Organization classifications “AML with multilineage dysplasia” and “AML and myelodysplasic syndromes, treatment-related.” Secondary endpoints included progression-free survival (PFS), complete remission (CR) rate, CR duration, rate of morphologic leukemia-free state, and 1-year survival. CR required bone marrow (BM) aspiration showing less than 5% leukemic blasts and an absence of Auer rods, peripheral blood counts showing absolute neutrophil count more than or equal to 1000/mm3, platelet count more than or equal to 100 000/mm3, no peripheral leukemic blasts, blood-product transfusion independence, and an absence of extramedullary leukemia. Morphologic leukemia-free state required BM aspiration showing less than 5% leukemic blasts, Auer rods not detected, and absence of extramedullary leukemia. Progression was defined as: more than 50% rise in bone marrow blast count, confirmed unequivocal rise in peripheral blasts in presence of other peripheral blood counts consistent with leukemic infiltration of bone marrow, and new appearance of extramedullary disease or circulating blasts, confirmed 1 week later.
Adverse events were collected and reported from the day of informed consent until 30 days after completion of the treatment phase, or until start of another antileukemic therapy. Clinical laboratory tests (serum chemistry and hematology) were performed during the prerandomization phase, within 48 hours of randomization, weekly thereafter during treatment, and on study termination.
Statistical analyses
A total of 450 patients were to be randomized in a 1:1 ratio to provide the required 394 events (deaths) to detect a 33% improvement in median survival when tipifarnib (16 weeks) was compared with BSC (12 weeks), with 80% power given a 2-sided significance level of 4.3%.
Hypothesis testing on overall survival involved a 2-step testing procedure (as outlined in the protocol and the statistical analysis plan) with a prespecified significance level of .043 for all randomized patients for testing the composite hypotheses (all randomized group and subgroup) and prespecified significance level of .10 for testing the subgroup of patients having AML with myelodysplasia.
The Kaplan-Meier method was used to estimate all time-to-event efficacy variables (overall survival, duration of CR, PFS, overall survival at 1 year, time to first hospitalization, duration of hospitalization, and time to first transfusion). PFS was defined as time from randomization to progression or death from any cause. All time-to-event variables were compared between the groups using a stratified log-rank test adjusting for stratification factors (ECOG performance status and age group). Cox regression analysis was used to test for the effects of treatment on survival while adjusting for potential baseline adverse risk factors (age, ECOG, baseline bone marrow blast counts, AML with myelodysplasia, unfavorable karyotype, baseline lactate dehydrogenase [LDH], and baseline white blood cell count [WBC]). The CR and morphologic leukemia-free state rates were compared between the groups using the Cochran-Mantel-Haenszel test while adjusting for potential baseline adverse risk factors.
Adverse events were tabulated by Medical Dictionary for Regulatory Activities, Version 9.0, body system and preferred term, according to frequency and toxicity grade per the National Cancer Institute, Common Toxicity Criteria, Version 2.0, relationship to study medication, action taken, outcome, and type (hematologic vs nonhematologic).
Results
Patient characteristics
From October 2004 to May 2007, 457 patients were randomized at 115 sites in 23 countries. There were more males (54%) than females (46%; Table 1). The median age was 76 years. Twenty-four percent of the patients were 80 years or older. One-third of the patients had unfavorable cytogenetics, and 20% had prior MDS (defined as a history of at least 3 months of MDS diagnosed by BM examination). The median duration of prior MDS was 10.7 months (range, 2.8-126 months) in the BSC arm and 7.2 months (range, 0.9-178 months) in the tipifarnib arm. Twenty-three (5%) patients had received prior therapy for MDS, which was mainly biologic or immunotherapy; 3 patients had received prior hydroxyurea, 3 had prior low-dose cytarabine, and 2 had prior treatment with demethylating agents. Twenty-four percent of patients entered the study with baseline BM blast counts of 20% to 30% (formerly classified as MDS by French-American-British classification; Table 1). Reasons provided for ineligibility for induction chemotherapy were subject choice (13%), physician assessment (65%), or both (22%). Factors contributing to the physician assessment of ineligibility were age alone (32%); comorbidities, especially cardiovascular, or poor general health (24%); disease factors, such as poor karyotype, prior MDS, or treatment-related AML (3%); or a combination of 2 or more of these factors (28%). Baseline characteristics (Table 1) were generally well balanced between the 2 treatment arms, although the tipifarnib group had more patients who started the study with grade 3 or 4 thrombocytopenia (53% vs 44%).
Baseline characteristics for all randomized patients
| Characteristic . | BSC group (n = 229) . | Tipifarnib group (n = 228) . | Total (n = 457) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 128 (56) | 120 (53) | 248 (54) |
| Female | 101 (44) | 108 (47) | 209 (46) |
| Race, n (%)* | |||
| White | 217 (95) | 215 (95) | 432 (95) |
| Black | 0 | 1 (<1) | 1 (<1) |
| Asian | 5 (2) | 7 (3) | 12 (3) |
| Other | 6 (3) | 4 (2) | 10 (2) |
| Age, y, n (%) | |||
| ≤ 69 | 1 (< 1) | 2 (1) | 3 (1) |
| 70-74 | 92 (40) | 90 (39) | 182 (40) |
| 75-79 | 77 (34) | 87 (38) | 164 (36) |
| 80-84 | 53 (23) | 38 (17) | 91 (20) |
| ≥ 85 | 6 (3) | 11 (5) | 17 (4) |
| Mean age (SD) | 76.2 (4.43) | 76.2 (4.37) | 76.2 (4.39) |
| Median age | 76.0 | 76.0 | 76.0 |
| Range | 69-89 | 69-90 | 69-90 |
| Weight, kg† | |||
| Mean (SD) | 70.89 (12.858) | 70.59 (13.572) | 70.74 (13.202) |
| Median | 70.00 | 70.00 | 70.00 |
| Range | 44.0-113.1 | 39.0-106.0 | 39.0-113.1 |
| ECOG performance status, n (%) | |||
| 0 | 47 (21) | 46 (20) | 93 (20) |
| 1 | 118 (52) | 119 (52) | 237 (52) |
| 2 | 64 (28) | 63 (28) | 127 (28) |
| AML diagnosis to randomization, days, n (%) | |||
| ≤ 14 days | 161 (70) | 163 (71) | 324 (71) |
| 15-21 | 53 (23) | 50 (22) | 103 (23) |
| > 21 | 15 (7) | 15 (7) | 30 (7) |
| Mean (SD) | 11.13 (6.408) | 11.30 (11.786) | 11.21 (9.470) |
| Median | 10.00 | 9.00 | 9.00 |
| Range | 1.0-32.0 | 1.0-157.0 | 1.0-157.0 |
| Unfavorable cytogenetics, n (%) | |||
| Yes | 76 (33) | 68 (30) | 144 (32) |
| No | 116 (51) | 123 (54) | 239 (52) |
| Not done/not available | 37 (16) | 37 (16) | 74 (16) |
| WHO classification, n (%) | |||
| Recurrent cytogenetic translocations | 12 (5) | 6 (3) | 18 (4) |
| AML with t(8;21)(q22;q22) AML1/CBFalpha/ETO | 2 (1) | 3 (1) | 5 (1) |
| AML with abnormal bone marrow eosinophils Inv(16) (p13;q22) or t(16;16)(p13;q22) CBFbeta/MYH11 | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| AML with 11q23 MLL abnormalities | 9 (4) | 2 (1) | 11 (2) |
| Multilineage dysplasia, n (%) | 80 (35) | 85 (37) | 165 (36) |
| With prior MDS | 45 (20) | 45 (20) | 90 (20) |
| Without prior MDS | 35 (15) | 40 (18) | 75 (16) |
| MDS, therapy-related, n (%) | 3 (1) | 4 (2) | 7 (2) |
| Alkylating agent | 2 (1) | 1 (< 1) | 3 (1) |
| Other types | 1 (< 1) | 3 (1) | 4 (1) |
| Baseline blasts in marrow, n (%) | |||
| 0%-20% | 3 (1) | 0 | 3 (1) |
| 20%-30% | 47 (21) | 63 (28) | 110 (24) |
| 30%-100% | 179 (78) | 165 (72) | 344 (75) |
| Mean (SD) percentage | 49.96 (22.247) | 48.51 (23.383) | 49.24 (22.807) |
| Median percentage | 44.80 | 41.00 | 43.50 |
| Baseline ANC grade, n (%)‡ | |||
| Less than grade 3 | 103 (45) | 98 (44) | 201 (45) |
| Grade 3 | 36 (16) | 47 (21) | 83 (18) |
| Grade 4 | 89 (39) | 78 (35) | 167 (37) |
| Baseline platelet count grade, n (%) | |||
| Less than grade 3 | 129 (56) | 108 (47) | 237 (52) |
| Grade 3 | 96 (42) | 109 (48) | 205 (45) |
| Grade 4 | 4 (2) | 11 (5) | 15 (3) |
| Characteristic . | BSC group (n = 229) . | Tipifarnib group (n = 228) . | Total (n = 457) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 128 (56) | 120 (53) | 248 (54) |
| Female | 101 (44) | 108 (47) | 209 (46) |
| Race, n (%)* | |||
| White | 217 (95) | 215 (95) | 432 (95) |
| Black | 0 | 1 (<1) | 1 (<1) |
| Asian | 5 (2) | 7 (3) | 12 (3) |
| Other | 6 (3) | 4 (2) | 10 (2) |
| Age, y, n (%) | |||
| ≤ 69 | 1 (< 1) | 2 (1) | 3 (1) |
| 70-74 | 92 (40) | 90 (39) | 182 (40) |
| 75-79 | 77 (34) | 87 (38) | 164 (36) |
| 80-84 | 53 (23) | 38 (17) | 91 (20) |
| ≥ 85 | 6 (3) | 11 (5) | 17 (4) |
| Mean age (SD) | 76.2 (4.43) | 76.2 (4.37) | 76.2 (4.39) |
| Median age | 76.0 | 76.0 | 76.0 |
| Range | 69-89 | 69-90 | 69-90 |
| Weight, kg† | |||
| Mean (SD) | 70.89 (12.858) | 70.59 (13.572) | 70.74 (13.202) |
| Median | 70.00 | 70.00 | 70.00 |
| Range | 44.0-113.1 | 39.0-106.0 | 39.0-113.1 |
| ECOG performance status, n (%) | |||
| 0 | 47 (21) | 46 (20) | 93 (20) |
| 1 | 118 (52) | 119 (52) | 237 (52) |
| 2 | 64 (28) | 63 (28) | 127 (28) |
| AML diagnosis to randomization, days, n (%) | |||
| ≤ 14 days | 161 (70) | 163 (71) | 324 (71) |
| 15-21 | 53 (23) | 50 (22) | 103 (23) |
| > 21 | 15 (7) | 15 (7) | 30 (7) |
| Mean (SD) | 11.13 (6.408) | 11.30 (11.786) | 11.21 (9.470) |
| Median | 10.00 | 9.00 | 9.00 |
| Range | 1.0-32.0 | 1.0-157.0 | 1.0-157.0 |
| Unfavorable cytogenetics, n (%) | |||
| Yes | 76 (33) | 68 (30) | 144 (32) |
| No | 116 (51) | 123 (54) | 239 (52) |
| Not done/not available | 37 (16) | 37 (16) | 74 (16) |
| WHO classification, n (%) | |||
| Recurrent cytogenetic translocations | 12 (5) | 6 (3) | 18 (4) |
| AML with t(8;21)(q22;q22) AML1/CBFalpha/ETO | 2 (1) | 3 (1) | 5 (1) |
| AML with abnormal bone marrow eosinophils Inv(16) (p13;q22) or t(16;16)(p13;q22) CBFbeta/MYH11 | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| AML with 11q23 MLL abnormalities | 9 (4) | 2 (1) | 11 (2) |
| Multilineage dysplasia, n (%) | 80 (35) | 85 (37) | 165 (36) |
| With prior MDS | 45 (20) | 45 (20) | 90 (20) |
| Without prior MDS | 35 (15) | 40 (18) | 75 (16) |
| MDS, therapy-related, n (%) | 3 (1) | 4 (2) | 7 (2) |
| Alkylating agent | 2 (1) | 1 (< 1) | 3 (1) |
| Other types | 1 (< 1) | 3 (1) | 4 (1) |
| Baseline blasts in marrow, n (%) | |||
| 0%-20% | 3 (1) | 0 | 3 (1) |
| 20%-30% | 47 (21) | 63 (28) | 110 (24) |
| 30%-100% | 179 (78) | 165 (72) | 344 (75) |
| Mean (SD) percentage | 49.96 (22.247) | 48.51 (23.383) | 49.24 (22.807) |
| Median percentage | 44.80 | 41.00 | 43.50 |
| Baseline ANC grade, n (%)‡ | |||
| Less than grade 3 | 103 (45) | 98 (44) | 201 (45) |
| Grade 3 | 36 (16) | 47 (21) | 83 (18) |
| Grade 4 | 89 (39) | 78 (35) | 167 (37) |
| Baseline platelet count grade, n (%) | |||
| Less than grade 3 | 129 (56) | 108 (47) | 237 (52) |
| Grade 3 | 96 (42) | 109 (48) | 205 (45) |
| Grade 4 | 4 (2) | 11 (5) | 15 (3) |
Percentages were calculated with the number of subjects in each group as denominator. Except where noted, numbers equaled the numbers in each of the 2 trial groups and in the entire cohort. AML indicates acute myeloid leukemia; ANC, absolute neutrophil count; ECOG, Eastern Cooperative Oncology Group; and MDS, myelodysplastic syndrome .
Race data were available for 228 in the BSC group and 227 in the tipifarnib group (total, 455).
Weight data were available for 229 in the BSC group and 224 in the tipifarnib group (total, 453).
Baseline ANC grade data were available for 228 in the BSC group and 223 in the tipifarnib group (total, 451).
Efficacy results
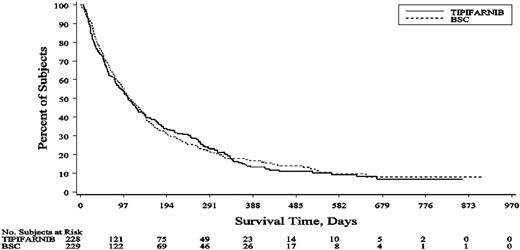
The number of deaths at the time of clinical cutoff (May 21, 2007) was 195 (89%) of 229 patients in the BSC group and 201 (88%) of 228 patients in the tipifarnib group. With a median follow-up of 574 days for tipifarnib and 539 days for BSC, the median overall survival was 107 days (95% confidence interval [CI], 85-129 days) for the tipifarnib group and 109 days (95% CI, 93-136 days) for the BSC group (Figure 1). The hazard ratio (tipifarnib vs BSC) for overall survival was 1.02 (95% CI, 0.84-1.24). The stratified log-rank test (P value) for overall survival was .843, and the P value from the unstratified log-rank test was .847. There was no statistically significant difference in the 1-year overall survival rate (14.9% for tipifarnib vs 17.7% for BSC). The lack of treatment effect on survival was shown across the different prognostic factors (Figure 2). A Cox proportional hazards model was used to estimate the effect of prognostic factors on overall survival (Table 2). The model suggested that age (≥ 75 years), an ECOG performance score of 2, unfavorable cytogenetics, or baseline BM blast count greater than 50% may lead to a higher risk of death. AML with or without myelodysplasia, baseline LDH more than or less than or equal to 1500 U/L, and baseline WBC more than or less than or equal to 25 giga/L did not significantly affect overall survival.
Kaplan-Meier plot of the overall survival for the BSC and tipifarnib groups.
Hazard ratio estimates for overall survival (BSC and tipifarnib groups) for all randomized patients and various subgroups.
Hazard ratio estimates for overall survival (BSC and tipifarnib groups) for all randomized patients and various subgroups.
Multivariate analysis on overall survival
| Prognostic factor . | Parameter estimate . | SE . | Hazard ratio . | 95% CI of hazard ratio . | P . |
|---|---|---|---|---|---|
| Treatment group: tipifarnib vs BSC | 0.049 | 0.114 | 1.050 | (0.84-1.314) | .667 |
| Age: ≥ 75 vs < 75 | 0.252 | 0.116 | 1.287 | (1.025-1.616) | .030 |
| ECOG: 2 vs 0 and 1 | 0.832 | 0.131 | 2.298 | (1.778-2.97) | .000 |
| Unfavorable cytogenetics: yes vs no | 0.564 | 0.120 | 1.758 | (1.39-2.223) | .000 |
| AML with myelodysplasia vs AML without myelodysplasia | −0.089 | 0.124 | 0.915 | (0.718-1.166) | .473 |
| LDH U/L: > 1500 vs ≤ 1500 | 0.081 | 0.271 | 1.085 | (0.638-1.845) | .764 |
| WBC count: > 25 giga/L vs ≤ 25 giga/L | 0.214 | 0.172 | 1.239 | (0.884-1.736) | .213 |
| Bone marrow blasts: > 50% vs ≤ 50% | 0.542 | 0.124 | 1.719 | (1.347-2.194) | .000 |
| Prognostic factor . | Parameter estimate . | SE . | Hazard ratio . | 95% CI of hazard ratio . | P . |
|---|---|---|---|---|---|
| Treatment group: tipifarnib vs BSC | 0.049 | 0.114 | 1.050 | (0.84-1.314) | .667 |
| Age: ≥ 75 vs < 75 | 0.252 | 0.116 | 1.287 | (1.025-1.616) | .030 |
| ECOG: 2 vs 0 and 1 | 0.832 | 0.131 | 2.298 | (1.778-2.97) | .000 |
| Unfavorable cytogenetics: yes vs no | 0.564 | 0.120 | 1.758 | (1.39-2.223) | .000 |
| AML with myelodysplasia vs AML without myelodysplasia | −0.089 | 0.124 | 0.915 | (0.718-1.166) | .473 |
| LDH U/L: > 1500 vs ≤ 1500 | 0.081 | 0.271 | 1.085 | (0.638-1.845) | .764 |
| WBC count: > 25 giga/L vs ≤ 25 giga/L | 0.214 | 0.172 | 1.239 | (0.884-1.736) | .213 |
| Bone marrow blasts: > 50% vs ≤ 50% | 0.542 | 0.124 | 1.719 | (1.347-2.194) | .000 |
Regression analysis of survival data (number of observations used = 362) was based on Cox proportional hazards model.
In the tipifarnib group, 18 (8%) patients achieved a CR. The median duration of the CR was 240 days, and the median overall survival of these patients was 666 days (Table 3). Most patients who reached CR did so by the end of the second cycle of treatment; median time to CR was 58 days (range, 28-107 days). Table 4 gives the baseline characteristics of the patients with CR. There were no CRs in the BSC group.
Objective response rate for all randomized patients
| Response . | BSC (n = 229) . | Tipifarnib (n = 228) . |
|---|---|---|
| Complete response | 0 | 18 (8%) |
| Partial response | 1 (< 1%) | 6 (3%) |
| Hematologic improvement | 2 (1%) | 14 (6%) |
| Stable disease | 130 (57%) | 105 (46%) |
| Progressive disease | 46 (20%) | 36 (16%) |
| Not done/not evaluable | 50 (22%) | 49 (21%) |
| Response . | BSC (n = 229) . | Tipifarnib (n = 228) . |
|---|---|---|
| Complete response | 0 | 18 (8%) |
| Partial response | 1 (< 1%) | 6 (3%) |
| Hematologic improvement | 2 (1%) | 14 (6%) |
| Stable disease | 130 (57%) | 105 (46%) |
| Progressive disease | 46 (20%) | 36 (16%) |
| Not done/not evaluable | 50 (22%) | 49 (21%) |
Baseline characteristics of patients with complete response
| Characteristic . | Proportion of tipifarnib patients with CR (%) . |
|---|---|
| Age, y | |
| Less than 75 | 7/92 (8) |
| 75 or more | 11/136 (8) |
| AML with myelodysplasia | |
| Yes | 8/90 (9) |
| No | 10/138 (7) |
| Unfavorable cytogenetics | |
| Yes | 3/68 (4) |
| No | 12/123 (10) |
| Not done | 3/37 (8) |
| ECOG performance status | |
| 0 | 5/46 (11) |
| 1 | 9/119 (8) |
| 2 | 4/63 (6) |
| Baseline blasts in marrow, range | 21%-98% |
| Characteristic . | Proportion of tipifarnib patients with CR (%) . |
|---|---|
| Age, y | |
| Less than 75 | 7/92 (8) |
| 75 or more | 11/136 (8) |
| AML with myelodysplasia | |
| Yes | 8/90 (9) |
| No | 10/138 (7) |
| Unfavorable cytogenetics | |
| Yes | 3/68 (4) |
| No | 12/123 (10) |
| Not done | 3/37 (8) |
| ECOG performance status | |
| 0 | 5/46 (11) |
| 1 | 9/119 (8) |
| 2 | 4/63 (6) |
| Baseline blasts in marrow, range | 21%-98% |
The median PFS was similar for the tipifarnib (64 days) and BSC groups (68 days). A Cox proportional hazards model used to estimate the effect of prognostic factors on PFS showed that, although a history of prior MDS had no significant effect, an ECOG performance score of 2, unfavorable cytogenetics, and a baseline BM blast percentage of greater than 50% may have adversely influenced PFS.
Safety results
The most common grade 3 or 4 adverse events were related to myelosuppression (44% for BSC and 62% for tipifarnib) or infections (33% for BSC and 39% for tipifarnib; Table 5). Tipifarnib is known to have myelosuppressive effects, but AML itself is also associated with profound and persistent cytopenias. This was illustrated by the high proportion of patients starting the study with grade 3 or 4 cytopenias according to laboratory tests; 80% in BSC group and 84% in tipifarnib group had at least one grade 3 or 4 cytopenia at baseline. During treatment, neutropenia and thrombocytopenia were the most common hematologic abnormalities with grade 4 abnormalities occurring more often in the tipifarnib group (neutropenia, 72% vs 60%; thrombocytopenia, 43% vs 28%). There was no cumulative effect on absolute neutrophil count, platelets, or hemoglobin over the cycles. The incidence of grade 3 or 4 hypokalemia was higher with tipifarnib treatment compared with BSC (16% vs 6%). Grade 3 or 4 diarrhea was reported in 7% of patients treated with tipifarnib, but there were no occurrences in the BSC group. The incidence of other grade 3 or 4 adverse events (fatigue, pyrexia, dyspnea, and cardiac failure) was similar in both groups.
Drug-related grade 3 or 4 adverse events in at least 5% of the patients
| Body system (dictionary-derived term) . | BSC (n = 229) . | Tipifarnib (n = 225) . | ||||
|---|---|---|---|---|---|---|
| Total, n (%) . | Toxicity grade, n (%) . | Total, n (%) . | Toxicity grade, n (%) . | |||
| 3 . | 4 . | 3 . | 4 . | |||
| Patients with an adverse event | 172 (75) | 202 (90) | ||||
| Blood and lymphatic system disorders | 100 (44) | 36 (16) | 64 (28) | 140 (62) | 41 (18) | 99 (44) |
| Thrombocytopenia | 61 (27) | 30 (13) | 31 (14) | 88 (39) | 35 (16) | 53 (24) |
| Anemia | 58 (25) | 39 (17) | 19 (8) | 75 (33) | 47 (21) | 28 (12) |
| Neutropenia | 35 (15) | 10 (4) | 25 (11) | 56 (25) | 10 (4) | 46 (20) |
| Febrile neutropenia | 24 (10) | 19 (8) | 5 (2) | 37 (16) | 28 (12) | 9 (4) |
| Leukopenia | 19 (8) | 8 (3) | 11 (5) | 25 (11) | 6 (3) | 19 (8) |
| Infections and infestations | 76 (33) | 55 (24) | 21 (9) | 87 (39) | 61 (27) | 26 (12) |
| Pneumonia | 43 (19) | 33 (14) | 10 (4) | 38 (17) | 29 (13) | 9 (4) |
| Sepsis | 19 (8) | 8 (3) | 11 (5) | 32 (14) | 18 (8) | 14 (6) |
| Metabolism and nutrition disorders | 22 (10) | 15 (7) | 7 (3) | 54 (24) | 40 (18) | 14 (6) |
| Hypokalemia | 13 (6) | 10 (4) | 3 (1) | 37 (16) | 28 (12) | 9 (4) |
| General disorders and administration site conditions | 46 (20) | 40 (17) | 6 (3) | 48 (21) | 42 (19) | 6 (3) |
| Fatigue | 29 (13) | 25 (11) | 4 (2) | 31 (14) | 28 (12) | 3 (1) |
| Pyrexia | 15 (7) | 14 (6) | 1 (< 1) | 11 (5) | 11 (5) | 0 |
| Gastrointestinal disorders | 20 (9) | 18 (8) | 2 (1) | 42 (19) | 39 (17) | 3 (1) |
| Diarrhea | 0 | 0 | 0 | 16 (7) | 16 (7) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 27 (12) | 15 (7) | 12 (5) | 28 (12) | 20 (9) | 8 (4) |
| Dyspnea | 12 (5) | 8 (3) | 4 (2) | 9 (4) | 7 (3) | 2 (1) |
| Cardiac disorders | 33 (14) | 13 (6) | 20 (9) | 25 (11) | 7 (3) | 18 (8) |
| Cardiac failure | 17 (7) | 4 (2) | 13 (6) | 12 (5) | 2 (1) | 10 (4) |
| Body system (dictionary-derived term) . | BSC (n = 229) . | Tipifarnib (n = 225) . | ||||
|---|---|---|---|---|---|---|
| Total, n (%) . | Toxicity grade, n (%) . | Total, n (%) . | Toxicity grade, n (%) . | |||
| 3 . | 4 . | 3 . | 4 . | |||
| Patients with an adverse event | 172 (75) | 202 (90) | ||||
| Blood and lymphatic system disorders | 100 (44) | 36 (16) | 64 (28) | 140 (62) | 41 (18) | 99 (44) |
| Thrombocytopenia | 61 (27) | 30 (13) | 31 (14) | 88 (39) | 35 (16) | 53 (24) |
| Anemia | 58 (25) | 39 (17) | 19 (8) | 75 (33) | 47 (21) | 28 (12) |
| Neutropenia | 35 (15) | 10 (4) | 25 (11) | 56 (25) | 10 (4) | 46 (20) |
| Febrile neutropenia | 24 (10) | 19 (8) | 5 (2) | 37 (16) | 28 (12) | 9 (4) |
| Leukopenia | 19 (8) | 8 (3) | 11 (5) | 25 (11) | 6 (3) | 19 (8) |
| Infections and infestations | 76 (33) | 55 (24) | 21 (9) | 87 (39) | 61 (27) | 26 (12) |
| Pneumonia | 43 (19) | 33 (14) | 10 (4) | 38 (17) | 29 (13) | 9 (4) |
| Sepsis | 19 (8) | 8 (3) | 11 (5) | 32 (14) | 18 (8) | 14 (6) |
| Metabolism and nutrition disorders | 22 (10) | 15 (7) | 7 (3) | 54 (24) | 40 (18) | 14 (6) |
| Hypokalemia | 13 (6) | 10 (4) | 3 (1) | 37 (16) | 28 (12) | 9 (4) |
| General disorders and administration site conditions | 46 (20) | 40 (17) | 6 (3) | 48 (21) | 42 (19) | 6 (3) |
| Fatigue | 29 (13) | 25 (11) | 4 (2) | 31 (14) | 28 (12) | 3 (1) |
| Pyrexia | 15 (7) | 14 (6) | 1 (< 1) | 11 (5) | 11 (5) | 0 |
| Gastrointestinal disorders | 20 (9) | 18 (8) | 2 (1) | 42 (19) | 39 (17) | 3 (1) |
| Diarrhea | 0 | 0 | 0 | 16 (7) | 16 (7) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 27 (12) | 15 (7) | 12 (5) | 28 (12) | 20 (9) | 8 (4) |
| Dyspnea | 12 (5) | 8 (3) | 4 (2) | 9 (4) | 7 (3) | 2 (1) |
| Cardiac disorders | 33 (14) | 13 (6) | 20 (9) | 25 (11) | 7 (3) | 18 (8) |
| Cardiac failure | 17 (7) | 4 (2) | 13 (6) | 12 (5) | 2 (1) | 10 (4) |
Percentages in ″Total″ column for each group are calculated with the number of subjects in each group as the denominator. Percentages of toxicity grade subgroups are calculated with the number of subjects in each group as the denominator. Incidence is based on the number of patients, not the number of events.
More patients (72% vs 62%) were hospitalized on or after randomization in the tipifarnib group, although the incidence of infections leading to hospitalization was similar (34% vs 30%). The most common tipifarnib-related serious adverse events were febrile neutropenia and thrombocytopenia. Tipifarnib-related adverse events led to treatment termination in 25 (11%) of the patients.
The incidence of deaths on study (during treatment and up to 30 days after treatment termination) was similar in the 2 groups (BSC 43%; tipifarnib 40%; Table 6). There were more deaths of progressive disease with BSC compared with tipifarnib (26% vs 18%, respectively). Four (2%) deaths resulting from adverse events were considered by the investigator to be related to tipifarnib treatment: cerebral hemorrhage, febrile neutropenia, pneumonia, and sepsis. There were more early deaths (within 30 days from randomization) in the tipifarnib group (21% vs 17%), which were accounted for by the increased number of early deaths resulting from adverse events (11% vs 7%; for tipifarnib 1% were drug-related). The most common adverse events leading to early death were cardiac events, infections, or bleeding events. The most common reasons for treatment termination are provided in Table 7.
Deaths during study
| . | BSC (n = 229) . | Tipifarnib (n = 225) . |
|---|---|---|
| Deaths, n (%)* | 98 (43) | 91 (40) |
| Cause of death | ||
| Progressive disease, n (%) | 59 (26) | 41 (18) |
| Adverse events, n (%) | 35 (15) | 43 (19) |
| Drug-related adverse events, n (%)† | 0 | 4 (2) |
| Other, n (%)‡ | 4 (2) | 7 (3) |
| . | BSC (n = 229) . | Tipifarnib (n = 225) . |
|---|---|---|
| Deaths, n (%)* | 98 (43) | 91 (40) |
| Cause of death | ||
| Progressive disease, n (%) | 59 (26) | 41 (18) |
| Adverse events, n (%) | 35 (15) | 43 (19) |
| Drug-related adverse events, n (%)† | 0 | 4 (2) |
| Other, n (%)‡ | 4 (2) | 7 (3) |
Includes deaths at any time during treatment, within 30 days after treatment termination, or before subsequent treatment, whichever was earlier.
Drug-related indicates possible, probable, or very likely related to trial medication as assessed by the investigator.
Other refers to deaths where the cause was unknown, probable cardiac failure, suspected infection, or probable gastrointestinal hemorrhage.
Primary reason for treatment termination
| . | BSC (n = 229), n (%) . | Tipifarnib (n = 228), n (%) . | Total (n = 457), n (%) . |
|---|---|---|---|
| Patients who terminated treatment | 221 (97) | 227 (> 99) | 448 (98) |
| Reason for termination | |||
| Progressive AML/relapse | 97 (42) | 98 (43) | 195 (43) |
| Peripheral blood counts | 37 (16) | 52 (23) | 89 (19) |
| Bone marrow examination | 50 (22) | 37 (16) | 87 (19) |
| Clinical examination | 10 (4) | 9 (4) | 19 (4) |
| Death | 70 (31) | 48 (21) | 118 (26) |
| Adverse events | 30 (13) | 25 (11) | 55 (12) |
| Progressive disease | 37 (16) | 18 (8) | 55 (12) |
| Other | 3 (1) | 5 (2) | 8 (2) |
| Subject choice | 33 (14) | 35 (15) | 68 (15) |
| Investigator decision | 16 (7) | 11 (5) | 27 (6) |
| Adverse event | 4 (2) | 26 (11) | 30 (7) |
| Complete remission | 0 | 9 (4) | 9 (2) |
| Lost to follow-up | 1 (< 1) | 0 | 1 (< 1) |
| Ongoing | 8 (3) | 1 (< 1) | 9 (2) |
| . | BSC (n = 229), n (%) . | Tipifarnib (n = 228), n (%) . | Total (n = 457), n (%) . |
|---|---|---|---|
| Patients who terminated treatment | 221 (97) | 227 (> 99) | 448 (98) |
| Reason for termination | |||
| Progressive AML/relapse | 97 (42) | 98 (43) | 195 (43) |
| Peripheral blood counts | 37 (16) | 52 (23) | 89 (19) |
| Bone marrow examination | 50 (22) | 37 (16) | 87 (19) |
| Clinical examination | 10 (4) | 9 (4) | 19 (4) |
| Death | 70 (31) | 48 (21) | 118 (26) |
| Adverse events | 30 (13) | 25 (11) | 55 (12) |
| Progressive disease | 37 (16) | 18 (8) | 55 (12) |
| Other | 3 (1) | 5 (2) | 8 (2) |
| Subject choice | 33 (14) | 35 (15) | 68 (15) |
| Investigator decision | 16 (7) | 11 (5) | 27 (6) |
| Adverse event | 4 (2) | 26 (11) | 30 (7) |
| Complete remission | 0 | 9 (4) | 9 (2) |
| Lost to follow-up | 1 (< 1) | 0 | 1 (< 1) |
| Ongoing | 8 (3) | 1 (< 1) | 9 (2) |
AML indicates acute myeloid leukemia; and BSC, best supportive care, including hydroxyurea.
Concomitant therapy
A total of 125 (55%) patients on the BSC arm received treatment with hydroxyurea. There were 41 patients in the BSC arm whose WBC exceeded 50 000 during the treatment period. Among those 41 patients, 35 (85%) received hydroxyurea.
Nearly all patients in the study had at least one blood product transfusion. There were slightly more blood product transfusions with tipifarnib treatment (93% vs 86%). Most of the increased transfusion need was evident within the first 30 days; from 31 days onward (up to 90 days), there were no differences in the number of transfusions for the 2 treatment groups. The proportion of patients receiving anti-infectives or cytokines was similar on both treatment arms (84% tipifarnib vs 79% BSC) and among sites across geographic regions (total patients: Asia, 82%; Eastern Europe, 77%; Western Europe, 83%; North America, 87%; South America, 81%).
Subsequent therapy
Eighty-nine (39%) patients in the BSC group and 93 (41%) patients in the tipifarnib group received subsequent therapy. The most frequent therapy given was hydroxyurea (12% BSC group, 15% tipifarnib group) or some form of single-agent chemotherapy, such as low-dose cytarabine or etoposide (11% BSC group, 15% tipifarnib group). Subsequent induction chemotherapy was received by 15 (7%) patients in the BSC group and 9 (4%) patients in the tipifarnib group. The main reason for receiving subsequent induction chemotherapy was that a change in the disease status required aggressive therapy (18 patients) or that the patient now accepted intravenous chemotherapy (6 patients). Only one patient (BSC group) had a response (CR). Five of the 18 patients who had CR on tipifarnib were retreated with tipifarnib on relapse, as allowed per protocol. One patient had a subsequent partial response (PR) lasting 6 months, and 2 had stable disease lasting 4 months each.
Discussion
This phase 3, multicenter, open-label study evaluated the effect on survival of tipifarnib compared with BSC, including hydroxyurea, as first-line therapy in elderly patients with newly diagnosed AML and considered to be unfit for or unwilling to be treated with induction chemotherapy. There was no survival benefit with tipifarnib treatment for the overall population.
The concept of this study was based on an earlier phase 2 study, CTEP-20, demonstrating that tipifarnib treatment resulted in CR among elderly patients with AML.19 In that study of 154 patients, the median age was 74 years (range, 34-85 years) with a high proportion of the patients (75%) having prior MDS. Twenty-two (14%) patients had a CR, with a further 15 (9%) patients having a PR or hematologic improvement. The median duration of CR was 7.3 months, and the median survival of complete responders was 18 months. In the current study, which had a minimum age of 70 years, the median age of the study population was 76 years, with 24% being older than 80 years. The 2 study arms were well balanced for baseline demographic and disease characteristics, specifically for the presence/absence of unfavorable karyotype, the World Health Organization classification of AML, and the time since initial diagnosis of AML. The best response to treatment in this patient population was a CR in 18 (8%) patients treated with tipifarnib (there were no CRs in the BSC group), with a further 20 (9%) patients having a PR or hematologic improvement. The CR rate was lower than previously observed in the phase 2 study, CTEP-20, but the median CR duration of 8 months was similar and the median overall survival of CR patients of 21.9 months was longer. CRs were observed in patients with all types of baseline characteristics, including 3 patients with complex karyotypes and several patients with baseline bone marrow blast counts more than 90%. The reason for the lower CR rate in this study compared with the phase 2 study is not clear but could be related to the generally older population and higher proportion of patients with ECOG performance status 2. In addition, this study was conducted at 115 sites around the world compared with 4 sites in one country for the phase 2 study, so variation in treatment practice and experience with tipifarnib could also play a role. Although the numbers of patients recruited were not sufficient to analyze outcome by country, an analysis of supportive care treatments by geographic region revealed no obvious differences.
In the current study, the CR rate was probably too low to have a positive effect on overall survival. Survival was not negatively affected by the rate of early deaths (17% for BSC and 21% for tipifarnib) or total drug-related deaths (4 patients, 2%). Nor is it probable that survival was influenced by subsequent therapy. The 2 arms were similarly matched for numbers of patients receiving subsequent induction (7% on BSC and 4% on tipifarnib) or other chemotherapy, or treatment with other agents. It is probable that a higher CR rate is needed to positively affect survival. The challenge is to identify the patients who probably respond to tipifarnib. Review of the baseline disease characteristics of responding patients in this and other studies has not identified any particular predictors of response. However, recently, a 2-gene classifier for predicting response to tipifarnib has been identified and validated, which could have application in future studies.20
AML represents a heterogeneous disease associated with poor outcomes. Some of the factors complicating the poor outcomes include older age, presence of specific karyotypes, and properties of multidrug resistance.21-24 The current study represents the largest study of elderly (> 70 years) patients ever conducted. Because there was no confounding effect of treatment, the prognostic factors of survival are particularly interesting. Consistent with previous reports, older age (≥ 75 years), higher ECOG performance score, unfavorable cytogenetics, and high BM blast count were strong predictors of poor survival. Interestingly, AML with myelodysplasia (including prior MDS), baseline LDH more than 1500 U/L, and baseline WBC more than 25 giga/L did not significantly affect survival.
A large number of older patients with AML do not receive specific treatment, and those who receive standard regimens have a median survival time of less than 1 year.1 Recently, 2 large retrospective analyses have been conducted in older patients with AML, particularly those 75 years of age or older, by the Southwest Oncology Group and the M. D. Anderson Cancer Center, demonstrating poor median overall survival durations, particularly in the 75 years and older age group.25,26 The poor outcomes observed in this group of elderly patients are most probably a reflection of low initial remission rates, higher treatment-related toxicity, high likelihood of relapse, and increased mortality.1,4,11 A more recent study (AML-14 trial) conducted in elderly patients by the National Cancer Research Institute (United Kingdom) demonstrated that low-dose cytarabine therapy was associated with a higher CR rate (18% vs 1%) and longer overall survival compared with hydroxyurea (P < .001).27 In that study, the 1-year survival of the entire population was reported as 13%, and it is clear from the survival curve that the majority of patients who were alive were in the low-dose cytarabine arm, with all patients in the hydroxyurea arm having died shortly after 1 year. In our study, the 1-year survival rate of patients on tipifarnib was 14.9%, with a median survival of 15.3 weeks, which was in line with the assumed median (16 weeks) for the sample size calculation. However, the median survival of the BSC patients of 15.6 weeks was higher than anticipated and, with a 1-year survival rate of 17.7%, suggests that this group included several patients with smoldering leukemia. A review of patient characteristics for those surviving more than 300 days did not reveal major differences between the 2 arms (data not shown).
This study was predicated on the CR rate observed in previous single-arm studies. The assumption was that CR is a good surrogate for survival in AML. This study confirmed that assumption in responding patients. However, the results of this study indicate that treatment with tipifarnib does not result in a rate of CR sufficient to increase the survival in this patient population (elderly [median age, 76 years] and not suitable for induction chemotherapy) compared with BSC.
The online version of this article contains a data supplement.
Presented in part at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2007 [Abstract 952392].
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who took part in this study as well as Dr Ali Nourbakhsh for assistance with medical review, Angelique Langlois for trial management, Dr Xinyu Wei for statistical analysis, and Dr Namit Ghildyal for writing contributions. This study, R115777-AML-301, was sponsored by Johnson & Johnson Pharmaceutical Research & Development LLC.
This work was supported by Johnson & Johnson Pharmaceutical Research & Development LLC.
A complete list of the investigators of the FIGHT-AML-301 study appears in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Authorship
Contribution: J.-L.H., Y.C.P., P.D.P., and A.J.H. were responsible for concept and design; A.B. and A.J.H. provided administrative support; J.-L.H., G.M., W.W.J., J.M.B., D.B., T.M., G.J.O., J.A.A., G.B., J.M., M.-B.V., H.D., X.T., A.K.B., T.R., N.K.K., A.K.G., E.T., and L.M. provided study materials or patients; Y.C.P., A.B., and A.J.H. collected and assembled the data; J.-L.H., Y.C.P., P.D.P., and A.J.H. analyzed and interpreted the data and wrote the manuscript; and J.-L.H., G.M., W.W.J., J.M.B., D.B., T.M., G.J.O., J.A.A., G.B., J.M., M.-B.V., H.D., X.T., A.K.B., T.R., N.K.K., A.K.G., E.T., and L.M. gave final approval of the manuscript.
Conflict-of-interest disclosure: J.-L.H. has received honoraria from OrthoBiotech Janssen Cilag. G.M. has been a consultant to Novartis and Bristol-Myers Squibb and has received research funding from Novartis. Y.C.P. and A.B. are employed by Johnson & Johnson Pharmaceutical Research & Development. P.D.P. and A.J.H. are employed by Ortho-Biotech Oncology Research & Development. Y.C.P., A.B., P.D.P., and A.J.H. own stock in Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Jean-Luc Harousseau, Service d'Hématologie, Hôtel Dieu, Place Alexis Ricordeau, 44093, Nantes Cedex 01, France; e-mail: jl-harousseau@nantes.fnclcc.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal