Abstract
Immune deficiency viruses such as SIV in macaques or HIV-1 in human beings have evolved mechanisms to defeat host immunity that also impact the efficacy of vaccines. A key factor for vaccine protection is whether immune responses elicited by prior immunization remain at levels sufficient to limit disease progression once a host is exposed to the pathogen. One potential mechanism for escaping pre-existing immunity is to trigger death among antigen-activated cells. We tested whether FasL/CD178 is involved in destroying preexisting immunity. Rhesus macaques were immunized with recombinant vesicular stomatitis virus vaccine expressing SIV Gag to elicit cellular immune responses, then treated with antibody that neutralizes FasL and challenged with intravenous SIVmac251. Compared with animals injected with control antibody, anti-FasL–treated macaques had superior preservation of central memory CD4+ and CD8+ cells and decreased regulatory T cells in the blood. The CD4+ and CD8+ lymphocytes from treated animals responded better to SIV Gag compared with controls, evidenced by higher cell-mediated immune responses to viral antigens for at least 17 weeks after SIV challenge. Anti-FasL treatment during the initial stages of acute SIV infection preserved the T-cell compartment and sustained cell-mediated immunity to SIV.
Introduction
Pathogenic HIV infection in humans or SIV infection in macaques is characterized by an acute viremia linked to immune cell activation. Studies on SIV infection in macaques revealed profound cell depletion in gut lamina propria1-3 and thymus.4 Direct virus infection and cytopathic effects promoted the loss of CD4+ T cells at these sites. Other cell types including B cells and CD8+ T cells that are not susceptible to virus infection are depleted by indirect mechanisms. Cell killing by apoptosis, even among uninfected lymphocytes, has been recognized as an important contributor to disease progression and is one of the indirect depletion mechanisms.5-8 Virus-mediated changes in the local microenvironment4 or a generalized IL-7 response to lymphopenia9 likely increases the expression of FasL/CD178 and, when combined with increased Fas/CD95 expression on activated immune cells, triggers cell death and promotes the loss of several cell types. Other mechanisms have been proposed for indirect cell death during SIV infection, including mitochondrial membrane depolarization independent of Fas/FasL interactions.10-12 Intervention studies performed in a relevant animal model for AIDS are needed to assess the FasL-mediated killing mechanism and its importance in disease progression
In addition to the generalized immune destruction during HIV infection, there is evidence that vaccine-induced immunity is depleted after HIV or SIV infection.13-16 Loss of preexisting immunity would limit vaccine effectiveness irrespective of levels reached during the immunization period. Mechanisms for the destruction of preexisting immune cells likely involve both direct viral infection and indirect mechanisms such as the contact between uninfected immune cells bearing Fas receptors and cells bearing FasL. Even soluble FasL in serum from HIV-infected patients caused higher cell death of uninfected cells such as natural killer cells.15 Elevated levels of immunosuppressive moieties in plasma such as TRAIL, FasL, and plasma microparticles can reduce or delay HIV-protective immune responses early after HIV infection14 and also control responses to the vaccine itself. Plasmid DNA immunization of mice triggered a response by class II–restricted CD4+ T cells that expressed FasL and killed the transfected antigen-presenting cells.17 When plasmid DNA immunizations were performed in Fas−/− mice17 or class II−/− mice,18 the damping of CD8+ T-cell responses was not observed. The damping effect was independent of perforin expression and depended only on Fas/FasL interactions. Thus, even in a noninfectious situation with normal Fas/FasL expression, killing through this pathway affects the response to vaccine.
Our previous study demonstrated that blocking FasL during acute SIV infection slowed disease progression in a small group of rhesus macaques.19 In the present study, we tested the role for FasL in macaques that were immunized with recombinant vesicular stomatitis virus (VSV)–expressing SIV proteins and then challenged with pathogenic SIVmac251. We show that the Fas/FasL pathway has an effect on SIV-specific cellular immunity in vaccinated macaques that are challenged with SIV.
Methods
Animals and treatments
Rhesus macaques were screened for MHC-unrestricted cytolysis as described earlier19 to identify those with higher cytolysis levels that were most suitable for this study; 16 animals with high cytolytic activity were selected. We showed previously that approximately 50% of rhesus macaques naturally have high MHC-unrestricted cytolysis20 and this activity is FasL dependent.19 By selecting for high unrestricted cytolysis, we reduced variation within the groups. The 16 animals were divided randomly into 2 groups and treated with the following antibodies: 8 animals received intravenous injections of a humanized anti-FasL monoclonal antibody, RNOK20321 at 4 mg/kg body weight. The antibody was injected 5 times at weekly intervals starting 2 weeks before infection with SIV. Eight control animals received the same concentrations of isotype-matched human IgG (catalog no. I-5029; Sigma). After the animal study was completed, we obtained genetic typing of our animals to identify MHC class I and class II genotypes. The study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
The MHC typing results for anti-FasL–treated and control groups are listed in Table 1. All macaques were negative for Mamu B08 and Mamu B17 alleles that are considered protective or slow-progressing. One macaque in the anti-FasL group and 2 in the control group were Mamu A01–positive, the first allele ever linked to slow progression.22
MHC genotyping results
| Monkey no. . | Anti-FasL . | MHC haplotype . |
|---|---|---|
| 1: 5A5 | + | DRB1*03† |
| 2: 20V | + | A08‡ |
| 3: 4B3 | + | A02, DRB1*03, DRB1*10 |
| 4: AK42 | + | DRB1*03 |
| 5: DL6P | + | A02, DRB1*03, DRB1*10 |
| 6: RQ4432 | + | AG01 but A01-negative |
| 7: 6A2 | + | A01 |
| 8: CP2E | + | DRB1*03 |
| 9: 7A7 | − | A08, DRB1*03, DRB1*10 |
| 10: 5A6 | − | A01, DRB1*03 |
| 11: 8A6 | − | A01, A08, DRB1*03, DRB1*10 |
| 12: AM24 | − | A08 |
| 13: 95Z | − | A08, DRB1*03, DRB1*10 |
| 14: DL6M | − | A08, DRB1*03, DRB1*10 |
| 15: DM4E | − | A08, B01 |
| 16: DM4D | − | A08, B01 |
| Monkey no. . | Anti-FasL . | MHC haplotype . |
|---|---|---|
| 1: 5A5 | + | DRB1*03† |
| 2: 20V | + | A08‡ |
| 3: 4B3 | + | A02, DRB1*03, DRB1*10 |
| 4: AK42 | + | DRB1*03 |
| 5: DL6P | + | A02, DRB1*03, DRB1*10 |
| 6: RQ4432 | + | AG01 but A01-negative |
| 7: 6A2 | + | A01 |
| 8: CP2E | + | DRB1*03 |
| 9: 7A7 | − | A08, DRB1*03, DRB1*10 |
| 10: 5A6 | − | A01, DRB1*03 |
| 11: 8A6 | − | A01, A08, DRB1*03, DRB1*10 |
| 12: AM24 | − | A08 |
| 13: 95Z | − | A08, DRB1*03, DRB1*10 |
| 14: DL6M | − | A08, DRB1*03, DRB1*10 |
| 15: DM4E | − | A08, B01 |
| 16: DM4D | − | A08, B01 |
Data are from a MHC-typing service at the University of Wisconsin, http://ink.primate.wisc.edu/∼watkins/services.html. Testing was performed for a sample of the most common MHC class I and class II alleles in captive rhesus macaques. + indicates treated; and −, untreated.
Major histocompatibility complex class II allele.
Major histocompatibility complex class I allele.
Immunization and virus infection
Attenuated, recombinant VSV expressing the SIV Gag protein was kindly provided by Dr John Rose (Yale University).23 The rVSV Gag was complemented first with the Indiana serotype of envelope G protein and all macaques received an intranasal instillation of 1.5 × 109 plaque-forming units 12 weeks before virus challenge. A second virus batch was prepared using complementation to express the Chandipura serotype G protein that avoids vector immunity. All macaques received a booster immunization via intranasal instillation with 1.5 × 109 plaque forming units of rVSV at 8 weeks before challenging them with SIV. In addition, all macaques received a single booster of SIVp27 Gag protein,16 which was 1 mL of a 60-μg/mL emulsion with incomplete Freund adjuvant (Sigma), given by intramuscular injection at 4 weeks before challenge.
Macaques were also immunized with the standard human pediatric dose (0.5 μg, intramuscular injection) of hepatitis B vaccine (Recombivax HB; Merck) at −7, 0, and 13 weeks relative to virus challenge to measure the effect of SIV infection on immune responses to a reference vaccine.
At week 0 (day of challenge), animals were inoculated with SIVmac251 (provided by Drs R. Desrosier, Harvard University, and Nancy Miller, National Institute of Allergy and Infectious Diseases, National Institutes of Health). All animals received 10 TCID50 of the virus by intravenous route (saphenous vein) and were followed for 45 weeks.
vRNA assays
SIV viral RNA levels were assessed in plasma samples. The number of viral copies per milliliter of plasma was measured with a real-time NASBA assay (Advanced Biosciences Laboratory).
Antibodies and staining
Antibodies were purchased from BD Biosciences (unless stated otherwise). Whole blood collected in EDTA was used to stain for surface and intracellular markers using the following antibodies: CD3 FITC (clone SP34), CD3 PE (clone SP34), CD4 (clone L200), CD8 (clone SK1), CD95 (clone DX2), CD28 (clone 28.2), CCR5 (clone 3A9), Ki-67 (cone B56), IL-2 (clone MQ1-17H12), and IFNγ (clone 4S.B3). Regulatory T cells were analyzed by staining peripheral blood mononuclear cells using a FoxP3 staining kit (clone PCH101; eBiosciences).
Cytokine responses were measured by intracellular cytokine staining. Whole blood cultures were stimulated with a pool of overlapping 15-mer peptides from the SIV Gag p27 region (5 μg/mL for each peptide obtained from the AIDS Research and Reference Program). Alternately, polyclonal stimulations used PHA (10 μg/mL) or PMA (20 ng/mL) plus ionomycin (1 μg/mL) and cells were cultured for 16 hours at 37°C, 5% CO2. All cultures contained monensin (Golgi stop; BD Biosciences) and 1 μg/mL anti-CD49d (BD Biosciences). Cells were stained for surface antigens CD4 and CD8 and then for intracellular antigens IL-2 and IFNγ using the BD cytofix cytoperm kit and the manufacturer's protocol. Samples were analyzed on a FACSCalibur instrument with 100 000 events in the lymphocyte gate and results were analyzed using FlowJo software (TreeStar). Samples with a percentage of cytokine-positive cells at least twice the control values were considered positive.
IFN-γ enzyme-linked immunosorbent spot assay
Primary responses to SIV antigens were analyzed by measuring IFN-γ production in peripheral blood mononuclear cells (PBMCs) collected before and after infection, using the monkey IFN-γ enzyme-linked immunosorbent spot (ELISPOT) kit (U-Cytech Biosciences) and the manufacturer's protocol. PBMCs were thawed and rested for 6 hours, then suspended at 2 × 105 cells/well with triplicates for each assay. Cells were stimulated with a pool of SIV Gag peptides (15-mers) at 2 μg/mL each, to assess antigen-specific IFN-γ production; ConA (10 μg/mL) was used as a positive control and medium alone was the negative control. After an 18-hour incubation, plates were washed and the assay products were visualized with a colorimetric assay that was measured with an ELISPOT reader (Immunospot Acquisition V 5.0.1; Cellular Technology Ltd); Gag-specific IFN-γ production was reported as spot-forming units (SFU)/million cells after subtracting background values.
Anti-SIV antibodies
Anti-SIV antibody titers were assayed in plasma samples from 6 and 25 weeks after infection by enzyme-linked immunosorbent assay (ELISA) using plates coated with p27 Gag antigen as described previously19 and limiting dilution of plasma to determine the binding titer. Western blotting for p27-specific antibodies was done using diluted plasma samples (ZeptoMetrix Corporation).
Antibodies against the hepatitis B surface antigen were measured 4 weeks after the third hepatitis B vaccination (17 weeks after SIV challenge) using a commercial ELISA Kit (BioChain).
Statistical analysis
Differences between animal groups treated with anti-FasL or control antibody were analyzed by Student t test. P value less than .05 was considered significant.
Results
SIV viral RNA levels in blood
Levels of viral RNA in plasma were measured in specimens from before challenge to 29 weeks after SIV inoculation. The peak viral RNA levels occurred by 2 weeks after infection in both groups (Figure 1). The average viral loads were higher in the untreated group (Figure 1B), but differences were not statistically significant for peak or set point levels (P = .94).
Plasma viral loads after SIVmac251 infection. (A) Viral RNA from individual animals is shown with anti-FasL animals in bold lines. (B) The differences between average log10 for viral loads of the groups were not statistically significant at any time point. Error bars indicate SEM.
Plasma viral loads after SIVmac251 infection. (A) Viral RNA from individual animals is shown with anti-FasL animals in bold lines. (B) The differences between average log10 for viral loads of the groups were not statistically significant at any time point. Error bars indicate SEM.
Central memory lymphocytes are preserved by anti-FasL treatment
Specific lymphocyte subsets were examined to assess the impact of anti-FasL antibody on SIVmac251. The first objective was to measure central memory CD4 lymphocytes, which are increasingly accepted as a reliable predictor of SIV disease progression.24-26 Central memory CD4+ or CD8+ lymphocytes were assessed among control or anti-FasL–treated monkeys. The percentages of CD95+CD28+ central memory CD4 lymphocytes were significantly higher in the anti-FasL–treated group starting as early as 2 weeks after infection. The absolute numbers of CD4 central memory lymphocytes were also higher in the treated group, demonstrating this subset was preserved throughout at least 21 weeks after infection in the treated group (Figure 2A-C) compared with controls (P < .05). Animals treated with anti-FasL also had significantly lower percentages of naive (CD95−CD28+) CD4 lymphocytes at all time points after challenge (data not shown). Similar patterns were observed for CD8+ T cells. The CD8 central memory subset was well preserved among macaques treated with anti-FasL and the naive subset was proportionally lower (Figure 2D). Overall, anti-FasL treatment preserved the central memory CD4+ and CD8+ T-cell population in peripheral blood.
Central memory lymphocytes are preserved in blood after anti-FasL treatment. (A) Central memory (CM), effector memory (EM), and naive lymphocyte populations were defined on the basis of CD95 and CD28 expression. Significantly higher percentages (B) as well as absolute numbers (C) of CD95+CD28+ CM CD4 lymphocytes were observed at various time points after SIV infection in anti-FasL–treated animals. (D) The anti-FasL–treated animals had significantly higher percentages of CM CD8 lymphocytes at most time points after SIV infection. The percentages of naive (CD95−CD28+) CD8 lymphocytes were significantly lower at these times. *P < .05 (significant differences by paired testing). Error bars indicate SEM.
Central memory lymphocytes are preserved in blood after anti-FasL treatment. (A) Central memory (CM), effector memory (EM), and naive lymphocyte populations were defined on the basis of CD95 and CD28 expression. Significantly higher percentages (B) as well as absolute numbers (C) of CD95+CD28+ CM CD4 lymphocytes were observed at various time points after SIV infection in anti-FasL–treated animals. (D) The anti-FasL–treated animals had significantly higher percentages of CM CD8 lymphocytes at most time points after SIV infection. The percentages of naive (CD95−CD28+) CD8 lymphocytes were significantly lower at these times. *P < .05 (significant differences by paired testing). Error bars indicate SEM.
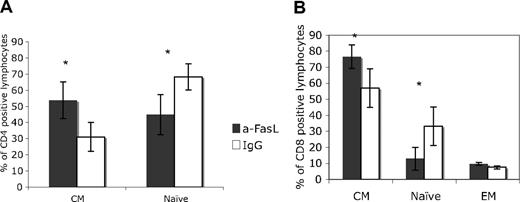
The proportions of central memory and naive T cells were similar for blood and lymph nodes. During the acute infection (2 to 3 weeks after SIV inoculation), we collected lymph node biopsies from 4 animals in each group. Again, anti-FasL treatment was associated with significantly higher central memory CD4+ or CD8+ T cells and with significantly decreased naive T-cell subsets in lymph nodes (Figure 3A-B).
Higher percentages of central memory lymphocytes in lymph nodes from animals treated with anti-FasL. Memory and naive lymphocytes were examined in lymph node biopsies collected at the time of peak virus replication (2 weeks after infection). Consistent with data for blood samples, the CM CD4 (A) and CD8 (B) lymphocyte percentages were significantly higher in the anti-FasL–treated group. *P < .05. Error bars indicate SEM.
Higher percentages of central memory lymphocytes in lymph nodes from animals treated with anti-FasL. Memory and naive lymphocytes were examined in lymph node biopsies collected at the time of peak virus replication (2 weeks after infection). Consistent with data for blood samples, the CM CD4 (A) and CD8 (B) lymphocyte percentages were significantly higher in the anti-FasL–treated group. *P < .05. Error bars indicate SEM.
The relative level of total memory CD4 lymphocytes is another biomarker for measuring vaccine protection against SIV infection.2 When we looked at the total memory lymphocyte pools, treated macaques had significant preservation of both CD4 and CD8 memory lymphocyte subsets throughout the 17-week study period (data not shown). The proportions of effector memory (CD95+CD28−) CD4 lymphocytes were higher in anti-FasL group at several times after infection (data not shown), however the differences from controls did not reach significance (eg, effector memory CD4 lymphocytes at 17 weeks after SIV infection were 3.12% in anti-FasL group versus 1.26% in control group; P = .065).
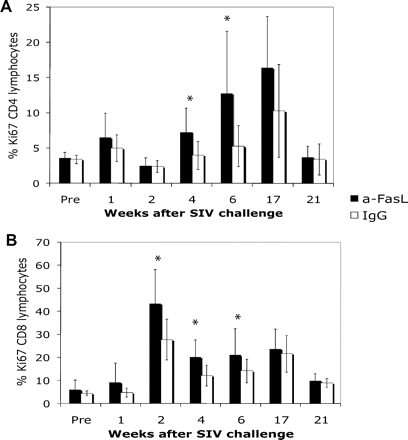
Treatment with anti-FasL increases the levels of lymphocytes expressing Ki-67 antigen
A high turnover of lymphocytes indicates an active cellular response to infection and the body's effort to replenish lost cells. The Ki-67 antigen is a convenient marker for actively dividing cells and has been used to characterize lymphocyte responses in SIV-infected animals.27 The percentage of Ki-67–expressing CD4 lymphocytes was significantly higher in treated animals compared with controls from weeks 4 to 17 after infection (Figure 4A). The Ki-67+ CD8 lymphocytes rose rapidly at the time of peak virus replication and remained higher among treated animals throughout the acute phase of infection (Figure 4B). After 21 weeks for CD4+ cells and 17 weeks for CD8+ cells, Ki-67+ cells were similar among anti-FasL and control groups.
The fraction of proliferating lymphocytes was higher among anti-FasL–treated animals. (A) The CD4 Ki-67+ lymphocytes showed an increase in both groups after the peak virus replication phase, with significantly more cells in anti-FasL group at 4 and 6 weeks after infection. (B) The CD8 Ki-67+ lymphocytes were highest at the time of peak virus replication for both groups; anti-FasL–treated animals had significantly higher percentages of this subset at 2, 4, and 6 weeks after infection. *P < .05. Error bars indicate SEM.
The fraction of proliferating lymphocytes was higher among anti-FasL–treated animals. (A) The CD4 Ki-67+ lymphocytes showed an increase in both groups after the peak virus replication phase, with significantly more cells in anti-FasL group at 4 and 6 weeks after infection. (B) The CD8 Ki-67+ lymphocytes were highest at the time of peak virus replication for both groups; anti-FasL–treated animals had significantly higher percentages of this subset at 2, 4, and 6 weeks after infection. *P < .05. Error bars indicate SEM.
Regulatory T cells are decreased after anti-FasL treatment
Declining immune capacity during SIV disease reflects lymphocyte destruction and dysregulation, including the generation of T regulatory cells (Tregs) that suppress cellular immunity.28-30 In our study, we measured Tregs with the phenotype CD4+CD25highFoxP3+ at 6 and 17 weeks after SIV challenge (Figure 5A-B). Control animals had significantly higher Treg levels compared with animals treated with anti-FasL monoclonal antibody. The role for Tregs in HIV infection is still not well understood, however recent investigations suggested a lower Treg response might be beneficial for the generation of effective antiviral immunity.31
Regulatory T cells are lower after FasL blocking. (A) Regulatory T cells (Tregs) were defined as the CD4+CD25high subset of CD3 lymphocytes that also express FoxP3. (B) The anti-FasL–treated group had significantly lower percentages of Tregs at 6 and 17 weeks after infection. *P < .05. Error bars indicate SEM.
Regulatory T cells are lower after FasL blocking. (A) Regulatory T cells (Tregs) were defined as the CD4+CD25high subset of CD3 lymphocytes that also express FoxP3. (B) The anti-FasL–treated group had significantly lower percentages of Tregs at 6 and 17 weeks after infection. *P < .05. Error bars indicate SEM.
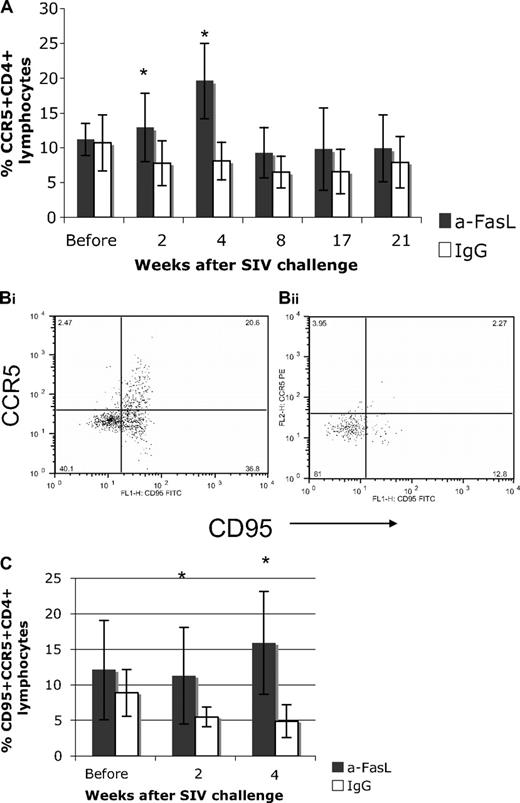
Increased CCR5 expression was observed in anti-FasL–treated animals
CCR5-expressing CD4 lymphocytes are targeted preferentially by M-tropic viruses such as SIVmac251, and their loss is a marker for disease progression.32 We found a significantly higher percentage of CCR5+CD4+ lymphocytes in anti-FasL–treated animals compared with controls at 2 and 4 weeks after SIV infection (Figure 6A), with the 4-week values showing a substantial increase over baseline for the treated group. The proportion of memory CD95+CCR5+CD4+ T cells declined after infection for the control group, but were significantly increased early and then preserved near prechallenge levels for the treated group (Figure 6B-C). Because plasma viremia levels were comparable in control and treated animals, our data suggest that these cells were preserved in macaques by the action of anti-FasL antibody. Further, this result argues that FasL is not required for CCR5+CD4+ T-cell expansion and may not act as a stimulatory ligand under these conditions.
CCR5-expressing CD4 lymphocytes were higher in treated compared with control macaques at early times after SIV infection. (A) A significantly higher percentage of CCR5+CD4+ lymphocytes was observed in the anti-FasL–treated group at 2 and 4 weeks after SIV infection. In this group, CCR5+CD4+ T cells were nearly twice the baseline values from 2 to 4 weeks after infection (an effect not seen in the control group). (B) The CD95+CCR5+ subset of CD4 lymphocytes was compared at 2 and 4 weeks to study the effect of infection on the memory CCR5+CD4+ subset. Representative dot plots from anti-FasL (Bi) and IgG (Bii) animals showed a decline in this subset for control animals. (C) The average values from the groups show the memory population declined in control animals but not in the anti-FasL–treated group. Error bars (panels A and C) indicate SEM. Asterisks indicate significant differences (P <.05).
CCR5-expressing CD4 lymphocytes were higher in treated compared with control macaques at early times after SIV infection. (A) A significantly higher percentage of CCR5+CD4+ lymphocytes was observed in the anti-FasL–treated group at 2 and 4 weeks after SIV infection. In this group, CCR5+CD4+ T cells were nearly twice the baseline values from 2 to 4 weeks after infection (an effect not seen in the control group). (B) The CD95+CCR5+ subset of CD4 lymphocytes was compared at 2 and 4 weeks to study the effect of infection on the memory CCR5+CD4+ subset. Representative dot plots from anti-FasL (Bi) and IgG (Bii) animals showed a decline in this subset for control animals. (C) The average values from the groups show the memory population declined in control animals but not in the anti-FasL–treated group. Error bars (panels A and C) indicate SEM. Asterisks indicate significant differences (P <.05).
Blocking FasL results in higher Gag-specific cellular immune responses
Gag-specific IFN-γ production was measured with ELISPOT and intracellular staining. The anti-FasL–treated group showed a significant increase in cytokine spot-forming units (SFU) at 4, 6, and 17 weeks after virus challenge compared with controls. Untreated macaques had an average of 158 and 279 SFU/million lymphocytes at 4 and 6 weeks after infection. In contrast, the anti-FasL–treated group had significantly higher ELISPOT responses of 528 and 790 SFU/106 lymphocytes at these same time points (Figure 7A). Intracellular cytokine staining also showed a higher percentage of IFNγ+ CD4 lymphocytes in this group at 4 weeks after infection (Figure 7B); differences were not significant for CD8+ T cells. Also at 4 weeks after infection, the average frequencies of IFNγ+IL-2+ double-positive CD4 lymphocytes were 1.01% and 0.06%, respectively, for anti-FasL and control groups; this difference was not statistically significant (P = .09). We also looked at cytokine production in response to polyclonal stimulation. Again, the anti-FasL–treated group had significantly greater numbers of IFNγ-positive CD4+ and CD8+ lymphocytes during both acute and chronic phases of infection (Figure 7C), showing that treatment preserved antigen-specific and bulk cytokine responses in CD4 cells and bulk responses in CD8 cells.
Antigen-specific and polyclonal cellular immune responses were higher in anti-FasL–treated animals compared with controls. (A) Gag-specific responses were measured by IFNγ ELISPOT. There were higher antigen-specific responses in the anti-FasL group at weeks 4 and 6 after infection compared with controls. (B) The SIV Gag-specific responses, measured as the percentage of IFNγ-producing CD4+ T cells detectable by intracellular flow cytometry, were also higher in anti-FasL–treated animals at 4 weeks after infection. (C) Significantly higher percentages of IFNγ+ CD4 and CD8 lymphocytes were detected in response to polyclonal stimulation in anti-FasL–treated animals at 4 weeks after SIV challenge. *P < .05. Error bars indicate SEM.
Antigen-specific and polyclonal cellular immune responses were higher in anti-FasL–treated animals compared with controls. (A) Gag-specific responses were measured by IFNγ ELISPOT. There were higher antigen-specific responses in the anti-FasL group at weeks 4 and 6 after infection compared with controls. (B) The SIV Gag-specific responses, measured as the percentage of IFNγ-producing CD4+ T cells detectable by intracellular flow cytometry, were also higher in anti-FasL–treated animals at 4 weeks after infection. (C) Significantly higher percentages of IFNγ+ CD4 and CD8 lymphocytes were detected in response to polyclonal stimulation in anti-FasL–treated animals at 4 weeks after SIV challenge. *P < .05. Error bars indicate SEM.
Humoral immune responses to SIV Gag or reference antigen were not altered by anti-FasL treatment
Specific viral antibodies were measured by ELISA and Western blot assays and were present at similar levels in anti-FasL–treated or control groups. All animals developed antibody against Gag antigens, however, there were no significant differences between the titers from anti-FasL– or control antibody–treated animals (average ELISA titers at 25 weeks were between 1000 and 10 000 for both anti-FasL and control groups; data not shown) unlike our previous report that showed higher virus-binding antibodies in unvaccinated, anti-FasL–treated animals compared with controls.19
The antibody responses to hepatitis B vaccine were similar among the 2 groups (data not shown) showing that reference immune responses were not damaged by anti-FasL treatment.
Disease progression
We followed the animals for 45 weeks after infection with SIV. Three of 8 control animals succumbed to disease between weeks 32 and 34 weeks after infection, whereas all anti-FasL–treated animals were alive at week 45. This difference in survival was not statistically significant (P = .06, Kaplan-Meier log-rank test) but confirms a trend toward slower disease progression in this group that is similar to our earlier findings.19
Discussion
We tested the role for FasL in the SIV mechanisms for evading vaccine-induced immunity. Rhesus macaques were immunized with recombinant VSV expressing SIV Gag, a vaccine known to produce partial protection against disease.23 Half of the immunized animals were treated briefly with intravenous injection of a monoclonal antibody against FasL. Treated animals preserved higher levels of central memory T cells including both CD4+ and CD8+ subsets, showed accelerated T-cell regeneration with increased proportions of CCR5+CD4+ cells, and had 3-fold higher antigen-specific cytokine responses and lower levels of Tregs despite viremia that was similar in treated and control groups. There were trends toward slower disease in the treatment group, with no deaths by 45 weeks compared with 3 deaths from 8 starting animals in the control group. Overall, the addition of intravenous anti-FasL antibody injected last at 2 weeks after SIV inoculation altered the immunologic status of treated animals, giving them characteristics consistent with greater protection against disease.
The impact of anti-FasL treatment was seen in CD4 and CD8 T cells even though absolute T-cell counts were similar in treatment and control groups. Using the Ki-67 marker for cell proliferation, we noted significantly increased CD4 and CD8 T-cell proliferation in PBMCs from the anti-FasL–treated group compared with controls during early stages of infection. Increased levels of Ki-67+ T cells during the asymptomatic stage of SIV infection were linked previously to immune depletion and disease progression,27 consistent with the view that chronic immune activation promotes SIV disease progression and is detrimental to the host.33,34 Similarly, elevated frequencies of Ki-67+ T cells and increased proportions of CCR5+CD8+ T cells were implicated in SIV disease progression, because they were higher in SIVmac251 compared with SIVdeltaNef infection.35 Our data show that Ki-67 and CCR5 increases can occur among animals, even when central memory T-cell subsets are preserved and viremia is indistinguishable from controls. Pathogenic SIV infection in rhesus macaque and nonpathogenic SIVsmm infection in sooty mangabey are both associated with comparable levels of immune activation during early infection; sustained immune activation observed in rhesus macaques determines the outcome of infection in this model.31 We also know that SIV-infected resting CD4 cells, more than activated CD4 cells, contribute the majority of peak virus production during acute SIV infection.1,36 The role for immune activation in HIV disease is to generate virus-susceptible CD4+ target cells, leading to higher replication of the virus as well as severe depletion of CD4 T lymphocytes.33,34 However, we saw no differences in peak, set point virus replication or CD4 depletion among treated or control groups. Interestingly, Ki67 expression during the acute stage was lowest among 3 control animals that died during this study. Thus, it appears that the Ki-67 increase observed during acute infection in the anti-FasL treatment group likely contributes to preserving critical T-cell subsets, suggesting it is a component of the protective immune response in early infection.
The phenotype differences between treated and control animals were reflected in the strength of cellular immunity. Antigen-specific stimulation of IFN-γ secretion was significantly higher for animals that received anti-FasL treatment compared with controls. These differences were significant through 6 weeks after virus challenge, with the treated group having 3- to 4-fold higher spot-forming units in ELISPOT assays. These differences were also significant for virus-specific IFN-γ+ CD4+ responses. The virus-specific IFN-γ+ CD8+ responses tended to be higher for the treated group but differences were not significant. It has been reported that CCR5 is expressed preferentially on IFN-γ Th1 cells37 and that Fas/FasL drives activation-induced cell death in this subset. In our study, anti-FasL treatment preserved CCR5+CD4+ cells and virus-specific CD4+ IFN-γ− production. We also know that Fas/FasL interactions reduce CD8+ T-cell responses to plasmid-encoded antigens by destroying the transfected antigen-presenting cell.17,18 The superior ELISPOT responses in our study suggest that interfering with the Fas/FasL interaction protected these antigen-specific cells from destruction. Preservation of CCR5+ CD4 T cells upon anti-FasL treatment did not result in any increase in viral replication. Similar observations of vaccine-induced preservation of CCR5+CD4+ T cells in mucosal sites of macaques infected with SIV have been reported,38,39 and such vaccine-generated CD4+CCR5+ T effector memory cells were also not associated with enhanced replication in “breakthrough” infections.38 The anti-FasL treatment did not influence humoral responses (to SIV or a reference antigen). Previously, we showed an effect of such treatment in preserving SIV-binding antibodies.19 The different result observed in this study might be due to vaccination with VSVgag, which promoted cellular responses and may have reduced the effect of anti-FasL on antibody responses.
We also observed significant differences in the levels of Tregs. Recent studies suggest Tregs contribute to HIV/SIV pathogenesis by suppressing HIV-specific immune responses in vivo, as they do in vitro.40,41 In SIV infection of rhesus macaques, premature induction of Tregs during the acute phase facilitated the establishment of chronic infection by blunting SIV-specific T-cell responses before the virus could be cleared.31 Supporting evidence from human studies showed that circulating or lymph node Treg levels were correlated with viral load,28,42,43 although some HIV-infected individuals were reported to have strong Treg function in vitro with significantly lower viral loads and higher CD4+ T-cell counts.30 Tregs also have been shown to suppress other immune responses, including effector T-cell proliferation.44 Our results showed reductions in Treg percentages in anti-FasL–treated animals and increased Ki-67 expression among CD4 and CD8 lymphocytes, arguing that Treg levels in vivo are suppressing proliferation and blocking immune responses. FasL is important for some Treg-suppression mechanisms,29 and the therapeutic antibody treatment might have reduced the numbers and activity of Tregs.
Intervention strategies targeting another immune regulator, PD-1, were reported to enhance viral immunity in SIV-infected rhesus macaques.45,46 The effects of treating with antibody to PD-1 on viral RNA levels were inconsistent, with some observations of transient viremia changes but no durable impact despite increased CD8+ T-cell responses. In our study, blocking FasL boosted the central memory CD4 lymphocyte subsets and resulted in higher SIV-specific cellular immunity, both of which are predictors of slower progression in SIV-infected macaques, although we did not observe changes in viral loads. Our demonstration that blocking FasL during early stages of infection has a measurable impact on SIV immunity underscores the importance of developing strategies that modulate host immune activation and immune regulatory pathways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Harry Davis, Joseph Bryant, Nicolas Epie, and Cheryl Armstrong for expert assistance with animal and laboratory studies. We are especially grateful to Dr John Rose for providing the recombinant VSV constructs and advice on their use. We thank Dr Nancy Miller and Dr Ranagit Pal for helpful advice and assistance with vRNA assays.
This work was funded by a grant from the National Institutes of Health (AI068508) to C.D.P.
National Institutes of Health
Authorship
Contribution: B.P. performed experiments, analyzed data, and wrote the paper; M.S.S. designed the study, interpreted data, and wrote the paper; H.Y., T.M., and K.O. provided critical reagents and designed the study; and C.D.P. designed the study, performed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. David Pauza, Institute of Human Virology, University of Maryland School of Medicine, 725 W Lombard St, Baltimore, MD 21201; e-mail: cdpauza@ihv.umaryland.edu.