Abstract
Pretargeted radioimmunotherapy (PRIT) is designed to enhance the directed delivery of radionuclides to malignant cells. Through a series of studies in 19 nonhuman primates (Macaca fascicularis), the potential therapeutic advantage of anti-CD45 PRIT was evaluated. Anti-CD45 PRIT demonstrated a significant improvement in target-to-normal organ ratios of absorbed radiation compared with directly radiolabeled bivalent antibody (conventional radioimmunotherapy [RIT]). Radio-DOTA-biotin administered 48 hours after anti-CD45 streptavidin fusion protein (FP) [BC8 (scFv)4SA] produced markedly lower concentrations of radiation in nontarget tissues compared with conventional RIT. PRIT generated superior target:normal organ ratios in the blood, lung, and liver (10.3:1, 18.9:1, and 9.9:1, respectively) compared with the conventional RIT controls (2.6:1, 6.4:1, and 2.9:1, respectively). The FP demonstrated superior retention in target tissues relative to comparable directly radiolabeled bivalent anti-CD45 RIT. The time point of administration of the second step radiolabeled ligand (radio-DOTA-biotin) significantly impacted the biodistribution of radioactivity in target tissues. Rapid clearance of the FP from the circulation rendered unnecessary the addition of a synthetic clearing agent in this model. These results support proceeding to anti-CD45 PRIT clinical trials for patients with both leukemia and lymphoma.
Introduction
Indolent B-cell lymphomas are incurable with standard doses of chemotherapy, monoclonal antibody (Ab) therapy, and radiation. Despite high initial response rates to combinations of these treatments, patients invariably relapse. Recurrent disease is frequently responsive to further therapy, but a pattern of relapse and remission ensues, characterized by progressively shorter durations of response and a shrinking pool of responders.1 Myeloid leukemias demonstrate a similarly high initial sensitivity to both chemotherapy and radiation. Yet, acute myeloid leukemia (AML) patients with high-risk cytogenetic or gene mutation abnormalities frequently relapse without human leukocyte antigen-matched allogeneic stem cell transplantation; and irrespective of prior risk status, recurrence portends a poor prognosis for all patients
Myeloablative doses of anti-CD20 radioimmunotherapy (RIT) followed by stem cell rescue results in dramatically improved rates of response for patients with relapsed B-cell lymphomas. Objective remissions are seen in 85% to 90% of such patients, with 45% to 80% experiencing durable complete remissions lasting 3 years or more.2-5 Although this represents a promising advance, most groups still report a relapse rate of 50%.3 The improved response rate seen with myeloablative regimens suggests that the high disease recurrence rates after nonmyeloablative RIT are a function of suboptimal levels of radiation absorbed by tumor. Similarly, in patients with AML, clinical trials have demonstrated excellent response rates when either anti-CD33 or anti-CD45 RIT is combined with high-dose chemotherapy before hematopoietic stem cell transplantation, but a significant proportion still relapse.6,7
Multistep pretargeting is designed to optimize delivery of radioimmunoconjugates to tumor targets while limiting normal organ radiation exposure. Several approaches to pretargeting have been described.8-11 The method used in these studies involves a tetrameric scFv antibody (SA) fusion protein (FP) followed by administration of a small molecule, radio-DOTA-biotin. Disassociating the slow Ab distribution phase from the radionuclide delivery phase generates more favorable target-to-normal organ ratios.11-16
Anti-CD45 FP retains the full antigen-binding capacity of intact anti-CD45 Ab. CD45 possesses several potentially advantageous characteristics for RIT targeting of both leukemias and lymphomas. It is expressed on the surface of virtually all cells of hematopoietic origin, except mature erythrocytes and platelets,17 and is found on the surface of 85% to 95% of both B-cell lymphoma and leukemic cells with a relatively high copy number (100-300 000 antigenic sites per leukemic cell).18 The CD45 antigen remains stably fixed on the cell surface with minimal internalization after ligand binding.19 Radiolabeled anti-CD45 Abs have been previously demonstrated to preferentially localize in the spleen, lymph nodes (LNs), and bone marrow (BM) in both mouse and macaque models.20-22 Our group has reported on the efficacy of incorporating high-dose radiolabeled Ab therapy targeting CD45 into hematopoietic stem cell transplantation conditioning regimens for patients with relapsed or refractory myeloid leukemia.7,23,24 We have demonstrated this antigen to be a promising target in B-cell lymphoma as well. In mice bearing human (Ramos) lymphoma xenografts, we have compared anti-CD20 (1F5) and anti-CD45 (BC8) Abs using both conventional and pretargeted RIT. Whereas 1F5 reagents delivered significant doses of radiation to tumor, equimolar concentrations of BC8 reagents consistently delivered 2- to 4-fold more radiation.12 CD45 exhibits superior cell surface retention compared with other anti-lymphoma antibodies tested and is unaffected by the presence of circulating rituximab,25 a theoretical limitation to anti-CD20–directed therapies. Patients with CD20-negative lymphomas, such as T-cell non-Hodgkin lymphoma (NHL), do not benefit from targeted therapy directed at the CD20 antigen, but the majority exhibit robust surface expression of CD45.26,27
In the current report, we describe a series of experiments characterizing BC8-FP pharmacokinetics and biodistribution in 19 fascicularis macaques. We show, for the first time, that multistep anti-CD45 pretargeting is feasible and safe in a nonhuman primate model. Further, we document the efficacy of this approach by demonstrating superior target-to-normal organ ratios of measured radiation.
Methods
Animals
Nineteen macaques (Macaca fascicularis) were studied at the Washington National Primate Research Center at the University of Washington (15 male and 4 female). The animals weighed between 2.8 and 9.0 kg (median, 5.6 kg) and varied in age from 3.5 to 13.8 years (median, 11.0 years). Unless otherwise noted, each experiment involved 2 animals, one experimental and one control. For each study, a dedicated veterinary anesthetist and surgical staff were required. Concurrent general anesthesia, gamma camera imaging, serial blood draws, and tissue biopsy procedures made study groups of larger than 2 animals impractical. Major findings were confirmed through repeat experiments as described in “Results.” All animal care and procedures were performed in accordance with guidelines set forth by the Institute of Laboratory Animal Resources of the National Research Council, National Academy of Sciences, and the Association for Assessment and Accreditation of Laboratory Animal Care. Studies received direct approval from the Institutional Animal Care and Use Committee at the University of Washington.
Monoclonal antibodies
Hybridoma cells secreting the murine IgG1a monoclonal Ab BC8 were a gift of Claudio Anasetti (Fred Hutchinson Cancer Research Center). BC8 recognizes all isoforms of the human CD45 antigen (hCD45) and binds approximately 2 × 105 molecules per macaque peripheral blood lymphocyte. BC8-Ab was produced in hollow fiber bioreactors in the Biologics Production Facility at the Fred Hutchinson Cancer Research Center. Purification was by protein A immunoabsorption column chromatography.7,24
FPs
Expression, purification, and characterization of the BC8 (scFv)4SA FP (BC8-FP) construct has been previously described.28 The CC49 (scFv)4SA FP (CC49-FP), a nonbinding negative control that recognizes the TAG-72 antigen on human adenocarcinomas, and the E121-3-10 plasmid for producing BC8-FP were gifts from NeoRx. Construction of CC49-FP has also been reported previously.29-31
CA
Two synthetic biotinylated thiogalactoside-containing clearing agents (CAs) were studied. One (C405H728O113N113N50S17; MW: 8652 Da), with an outer dendrimeric shell functionalized by 16 N-acetyl-galactosamine residues per dendrimer (16-mer), was a gift from NeoRx. The second (biotin-N-methylcaproate-C105H188N14O29S5; MW: 2271 Da), with an outer dendrimeric shell functionalized by 4 N-acetyl-galactosamine residues per dendrimer (4-mer), was synthesized by our group using procedures similar to those previously reported.32 The CAs were designed to reduce excess BC8-FP from the circulation before infusion of radiolabeled biotin. The 16-mer construct has been previously shown to facilitate blood clearance through hepatocyte endocytosis mediated by hepatic asialoglycoprotein receptors with a high affinity for the N-acetyl-galactosamine residues on the CA.11 The 4-mer CA is synthesized with a backbone identical to the 16-mer construct and demonstrates equivalent blood clearance of BC8-FP in mice (data not shown). The dose of 16-mer CA administered (45 mg/m2) was based on previous human clinical studies10 and through extrapolation from murine experience.13 The dose of 4-mer CA (4 mg/m2) was based on the initial 16-mer study (corrected for molar equivalence).
Radiolabeling
BC8-FP and CC49-FP were trace radioiodinated with 1 to 2 mCi of either Na125I or Na131I (PerkinElmer Life and Analytical Sciences) by the chloramine T method.33 DOTA-biotin and intact DOTA-BC8-Ab were conjugated with trace (2-3 mCi) 111In (PerkinElmer Life and Analytical Sciences) under metal-free conditions using a process of radiometal chelation previously described.33,34 Labeling efficiencies were more than 90% as determined by binding to avidin-agarose beads.
Immunoreactivity/avidity for BC8-FP and intact BC8-Ab
The immunoreactivities of BC8-FP and intact BC8-Ab were assessed by a competitive flow cytometric binding assay in a CD45+ lymphoma cell line (Ramos). Compared with BC8-Ab on a molar basis, the IC50 values for the tetravalent BC8-FP demonstrated 2-fold greater avidity. Lineweaver-Burke and Scatchard cell binding assays confirmed that BC8-FP retains the full immunoreactivity and avidity of the parent BC8-Ab. In the Ramos lymphoma cell line, the immunoreactivities for BC8-FP and BC8-Ab were 83% and 80%, respectively. In the Raji line, these values were 59% and 58%. The calculated avidity constants for BC8-FP were 2.76 plus or minus 0.18 × 108 L/mol (Ramos) and 2.42 plus or minus 0.12 × 108 L/mol (Raji); whereas for BC8-Ab, the values were 2.28 plus or minus 0.14 × 108 L/mol (Ramos) and 1.93 plus or minus 0.15 × 108 L/mol (Raji). The slightly higher avidity is presumably a consequence of the tetravalent structure of FP.28 Biotin-binding capacity was determined through incubation of the FP (100 μL, 1-2 nmol/mL) with freshly diluted biotin-cyanocobalamin (10 μL, 2.16 mg/mL; Quanta Biodesign), followed by quantification of the unbound biotin-cyanocobalamin by high-performance liquid chromatography using an unbound serial titration standard curve. This revealed an average of 3.9 of 4 biotin binding sites available on each FP molecule.28
Dose identification for BC8 (scFv)4 SA
Single-cell fresh lymphocyte suspensions from LN biopsy specimens obtained 24 to 72 hours after infusion were cryopreserved. The percentage saturation of antigenic sites was determined through flow cytometric analysis comparing the mean fluorescence intensity of target cells with and without incubation of the cells with supersaturating concentrations of exogenous BC8-FP before staining with phycoerythrin-labeled-F(ab′)2 goat anti–mouse Ig (Jackson ImmunoResearch Laboratories). The percentage Ab saturation was computed as previously published.35
Radiometal conjugated DOTA-biotin has been previously described.36 In the current studies, 1.2 mg/m2 of 111In labeled DOTA-biotin was used. This dose was selected based on previous clinical trial data studying pretargeting in patients with B-cell lymphoma.10 The dose is based on body surface area and is therefore proportional to doses used previously in patient clinical trials.
Pharmacokinetic and biodistribution studies
Animals received a single intravenous injection of either 125I-BC8-FP or 131I-BC8-Ab over 10 minutes while under general anesthesia. Vital signs were monitored throughout the infusion and then daily for the duration of each study. All animals had blood samples collected before the Ab/FP infusion, 5 minutes after the infusion, and 0.5, 1, 2, 24, 48, 72, and 96 hours after infusion (except when necropsy was at 72 hours). Animals receiving pretargeted radioimmunotherapy (PRIT) had additional samples collected 5 minutes, 30 minutes, 1 hour, and 2 hours after administration of 111In-DOTA-biotin reagent (which occurred either 24 or 48 hours after FP administration). At each time point, complete blood counts, serum chemistries, and radioactivity were measured. In addition, liver function and coagulation studies were obtained before Ab or FP infusion and daily during the study. BM (femur or humeral) and excisional LN biopsies (femoral or axillary) were obtained 2, 24, 48, and 96 hours after receiving intact Ab or FP. These time intervals correlated with tissue sampling 24 or 48 hours before DOTA-biotin, as well as 2, 24, and, in some studies, 48 hours after infusion for animals receiving 2-step pretargeting. BM core biopsy specimens were obtained with a Jamshidi needle (Baxter). Necropsies were performed after death resulting from an overdose of sodium pentobarbital under ketamine sedation, and spleen, LN, liver, lung, kidney, skeletal muscle, and BM core specimens were obtained in triplicate (∼ 100 mg each). Colon and small intestine samples were also collected in selected studies. BM core samples were collected from the humerus or femur in initial studies; however, in later studies, larger red marrow specimens were obtained from the lumbar vertebral bodies (L2 or L3).
Blood and tissue samples were collected at each time point, weighed, and analyzed by γ-counting (Cobra II; Packard Instruments). The percentage injected dose per gram of tissue (%ID/g) was calculated after correction for radioactive decay using an aliquot of the injectate. An adjustment was also made for crossover from the 131I to the 125I channel. Target-to-normal tissue ratios were calculated. BM core biopsy samples were assumed to consist of 50% hematopoietic tissue and 50% trabecular bone or fat based on previous studies,37 and estimates of radiation absorbed to marrow were increased by a factor of 2.
In the CA studies, 16-mer synthetic CA was administered 24 hours after BC8-FP. Blood samples were collected before CA infusion and at 5 minutes, 30 minutes, 1 hour, and 2 hours after infusion. 111In-DOTA-biotin was administered after 2 hours and thereafter at time points identical to those described for 2-step pretargeting in this section. BM and LN samples were obtained before CA and 4 hours after CA infusion (2 hours after DOTA-biotin). In a second study, the 4-mer synthetic CA was administered 24 hours after BC8-FP and specimens were collected at identical time points as outlined for the 16-mer CA, except that necropsy was performed after 120 hours.
Results
BC8 (scFv)4SA dose determination
The dose of BC8-FP required to optimize biodistributions in the macaque was identified through a series of dose escalation experiments. In a pilot experiment, a dose of 0.6 mg/kg (3.4 nmol/kg) of 131I-BC8-FP was administered, and no target tissue specific uptake was evident compared with a coinjected equimolar concentration of nonbinding control FP (data not shown). At this low dose, CD45 antigen expressed by circulating leukocytes appeared to act as a sink for the BC8-FP, preventing delivery to less accessible compartments (BM and LN). A 5-fold dose escalation to 3.0 mg/kg (16.9 nmol/kg) demonstrated specific target organ uptake as measured by dual-channel gamma counting of LN, spleen, and BM. FP was calculated to saturate 34% to 70% of CD45 binding sites on LN cells at this dose35 (Figure 1). Further dose escalation to 15.0 mg/kg (84.8 nmol/kg) was evaluated. FP saturation was not increased at this dose level, and the %ID/g of target organ (LN, spleen, BM) was not significantly different at the higher dose level compared with the 3.0 mg/kg dose level in target LN specimens assessed at serial time points.
Cell-binding saturation studies. (A) Flow cytometry analysis of fresh single-cell lymphocyte suspensions from the LN of an untreated macaque. The histograms compare the mean florescence intensity of lymphocytes after incubation with a saturating concentration of exogenous BC8-FP followed by PE-F(ab′)2 (shaded) versus background staining without the second-step reagent (nonshaded). (B) Flow cytometry analysis of fresh single-cell lymphocyte suspensions from the LN of a macaque obtained 96 hours after intravenous administration of BC8-FP (16.9 nmol/kg). The histograms demonstrate the mean florescence intensity after staining with PE-F(ab′)2 (nonshaded) compared with cells from the same specimen incubated with a saturating concentration of exogenous BC8-FP followed by PE-F(ab′)2 (shaded). The maximum calculated saturation of binding sites was 70%.
Cell-binding saturation studies. (A) Flow cytometry analysis of fresh single-cell lymphocyte suspensions from the LN of an untreated macaque. The histograms compare the mean florescence intensity of lymphocytes after incubation with a saturating concentration of exogenous BC8-FP followed by PE-F(ab′)2 (shaded) versus background staining without the second-step reagent (nonshaded). (B) Flow cytometry analysis of fresh single-cell lymphocyte suspensions from the LN of a macaque obtained 96 hours after intravenous administration of BC8-FP (16.9 nmol/kg). The histograms demonstrate the mean florescence intensity after staining with PE-F(ab′)2 (nonshaded) compared with cells from the same specimen incubated with a saturating concentration of exogenous BC8-FP followed by PE-F(ab′)2 (shaded). The maximum calculated saturation of binding sites was 70%.
Blood clearance and biodistribution
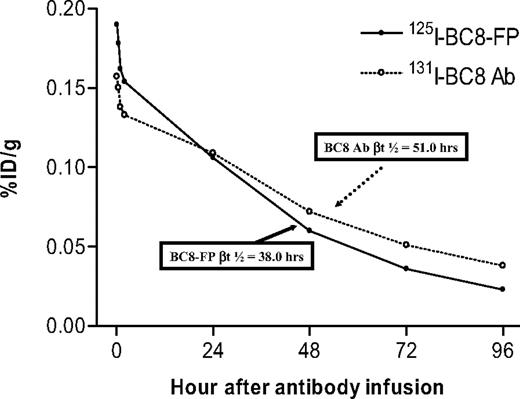
A pilot pharmacokinetic study (experiment 1) was performed to compare the blood clearance of 131I-labeled BC8-Ab and 125I-labeled BC8-FP in fascicularis macaques using the double-label method of Pressman et al.38 Blood clearance was biphasic, and clearance half-lives were determined to be 4.18 hours and 4.30 hours in the α-phase and 38.0 hours and 51.0 hours in the β-phase, respectively, after coinjection of equimolar (8.45 nmol/kg) concentrations of BC8-FP and BC8-Ab into the same animal (Figure 2). The clearance half-times for BC8-FP were reproducibly analyzed in 3 subsequent animals demonstrating an α-phase t1/2 of 3.83 plus or minus 0.43 hours and a βt1/2 of 39.15 plus or minus 4.2 hours.
Blood clearance of 131I-BC8-Ab and 125I-BC8-FP. Blood clearance of 131I labeled BC8 antibody (○) and 125I labeled BC8-FP (●) in a fascicularis macaque after coinjection of equimolar concentrations (8.45 nmol/kg) of BC8-Ab and BC8-FP into the same animal. Results are expressed as percentage of injected dose per gram (%ID/g) and were analyzed by dual-channel gamma counting.
Blood clearance of 131I-BC8-Ab and 125I-BC8-FP. Blood clearance of 131I labeled BC8 antibody (○) and 125I labeled BC8-FP (●) in a fascicularis macaque after coinjection of equimolar concentrations (8.45 nmol/kg) of BC8-Ab and BC8-FP into the same animal. Results are expressed as percentage of injected dose per gram (%ID/g) and were analyzed by dual-channel gamma counting.
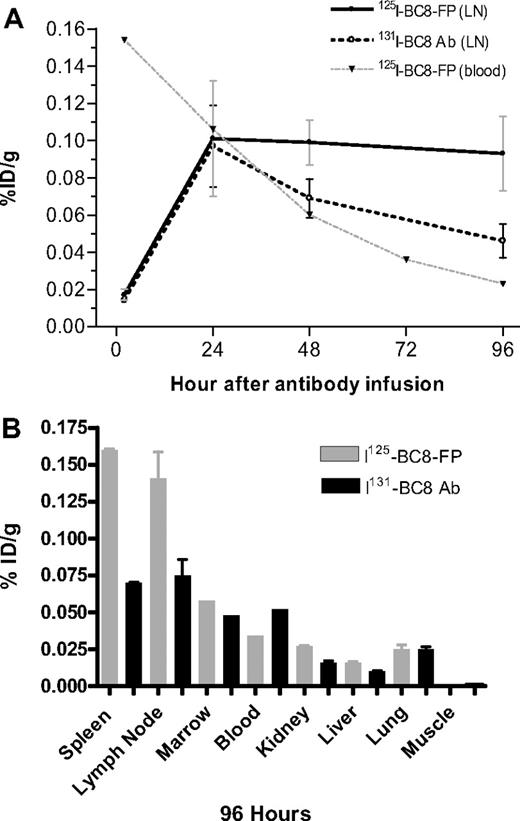
To compare the biodistribution of BC8-FP with BC8-Ab (experiment 2), equimolar doses (8.45 nmol/kg) of 125I-BC8-Ab and 131I-BC8-FP were coinjected and blood, LN, and BM were serially sampled. Ten organ tissues were harvested at necropsy, performed 96 hours after injection of the reagents. Special attention was focused on the targeting of lymphoid organs because of the desire to apply CD45 PRIT to patients with malignant lymphoma. The contents of the trace-labeled reagents in serial LN specimens were measured in a dual-channel gamma counter and expressed as the %ID/g. The uptake 2 hours after infusion was 0.017% plus or minus 0.003%ID/g for BC8-FP and 0.014% plus or minus 0.002% ID/g for intact BC8-Ab (Figure 3A). The measured contents of Ab and FP were also similar 24 hours after infusion (0.097% ± 0.038% for BC8-FP and 0.101% ± 0.031% for BC8-Ab). However, a difference in the amounts of the reagents retained in lymphoid tissue became evident by 48 hours (Figure 3A) and further pronounced by the 96-hour necropsy time point (0.093% ± 0.020% for BC8-FP and 0.046% ± 0.013% for BC8-Ab). The differences in biodistribution, reflecting the altered retention kinetics for BC8-FP compared with intact BC8-Ab, were largely limited to LN and spleen (considered surrogate target sites because of high levels of CD45 receptor expression). In the nontarget tissues, which historically experience the highest levels of nonspecific radiation (kidney, liver, lung),21,22 the BC8-FP demonstrated more favorable target organ to blood ratios compared with intact BC8-Ab after 96 hours (Table 1).
Target specific LN uptake of 125I-BC8-FP and 131I-BC8-Ab. With 96-hour target (A) and nontarget tissue biodistribution (B). (A) Concentrations of coinjected 125I-BC8-FP (●) and 125I-BC8-Ab (○) in LN and blood (▼) specimens after coinjection of equimolar concentrations (8.45 nmol/kg) of BC8-Ab and BC8-FP. Results are expressed as %ID/g as assessed by dual-channel gamma counting 2 to 96 hours after coinjection. (B) Shown are %ID/g for both 125I-BC8-FP and 125I-BC8-Ab coinjected at equimolar concentrations (96-hour necropsy).
Target specific LN uptake of 125I-BC8-FP and 131I-BC8-Ab. With 96-hour target (A) and nontarget tissue biodistribution (B). (A) Concentrations of coinjected 125I-BC8-FP (●) and 125I-BC8-Ab (○) in LN and blood (▼) specimens after coinjection of equimolar concentrations (8.45 nmol/kg) of BC8-Ab and BC8-FP. Results are expressed as %ID/g as assessed by dual-channel gamma counting 2 to 96 hours after coinjection. (B) Shown are %ID/g for both 125I-BC8-FP and 125I-BC8-Ab coinjected at equimolar concentrations (96-hour necropsy).
Target-to-blood ratios and measured activities of 131I-BC8-FP and 125I-BC8-Ab coinjected 96 hours before tissue harvest
| Experiment/organ . | BC8-FP . | SD . | BC8-Ab . | SD . |
|---|---|---|---|---|
| Experiment 2 | ||||
| LN | 0.093% | ± 0.020% | 0.046% | ± 0.013% |
| Spleen | 0.158% | ± 0.00% | 0.057% | ± 0.00% |
| Kidney | 0.016% | ± 0.001% | 0.003% | ± 0.000% |
| Liver | 0.015% | ± 0.000% | 0.005% | ± 0.001% |
| Lung | 0.008% | ± 0.002% | 0.007% | ± 0.001% |
| LN:blood | 4:1 | — | 1.3:1 | — |
| Spleen:blood | 6.9:1 | — | 1.5:1 | — |
| BM:blood | 0.8:1 | — | 0.4:1 | — |
| Experiment 3 | ||||
| LN | 0.141% | ± 0.031% | 0.075% | ± 0.019% |
| Spleen | 0.160% | ± 0.001% | 0.070% | ± 0.001% |
| Kidney | 0.027% | ± 0.001% | 0.016% | ± 0.002% |
| Liver | 0.016% | ± 0.001% | 0.010% | ± 0.001% |
| Lung | 0.025% | ± 0.005% | 0.025% | ± 0.003% |
| LN:blood | 4:1 | — | 1.4:1 | — |
| Spleen:blood | 4.7:1 | — | 1.3:1 | — |
| BM:blood | 1.7:1 | — | 0.92:1 | — |
| Experiment/organ . | BC8-FP . | SD . | BC8-Ab . | SD . |
|---|---|---|---|---|
| Experiment 2 | ||||
| LN | 0.093% | ± 0.020% | 0.046% | ± 0.013% |
| Spleen | 0.158% | ± 0.00% | 0.057% | ± 0.00% |
| Kidney | 0.016% | ± 0.001% | 0.003% | ± 0.000% |
| Liver | 0.015% | ± 0.000% | 0.005% | ± 0.001% |
| Lung | 0.008% | ± 0.002% | 0.007% | ± 0.001% |
| LN:blood | 4:1 | — | 1.3:1 | — |
| Spleen:blood | 6.9:1 | — | 1.5:1 | — |
| BM:blood | 0.8:1 | — | 0.4:1 | — |
| Experiment 3 | ||||
| LN | 0.141% | ± 0.031% | 0.075% | ± 0.019% |
| Spleen | 0.160% | ± 0.001% | 0.070% | ± 0.001% |
| Kidney | 0.027% | ± 0.001% | 0.016% | ± 0.002% |
| Liver | 0.016% | ± 0.001% | 0.010% | ± 0.001% |
| Lung | 0.025% | ± 0.005% | 0.025% | ± 0.003% |
| LN:blood | 4:1 | — | 1.4:1 | — |
| Spleen:blood | 4.7:1 | — | 1.3:1 | — |
| BM:blood | 1.7:1 | — | 0.92:1 | — |
— indicates not applicable.
In experiments 2 and 3, equimolar doses (8.45 nmol/kg) of 125I-BC8-Ab and 131I-BC8-FP were coinjected. Organ tissues were harvested at necropsy and performed 96 hours after injection of the reagents. The contents of the trace-labeled reagents in tissue specimens were measured in a dual-channel gamma counter and are expressed as the percentage injected dose per gram of tissue (%ID/g). SDs reflect 3 biopsy specimens from each tissue.
A follow-up study, conducted under the same conditions, reproduced the major findings of experiment 2. The differences in biodistribution, reflecting the altered retention kinetics for BC8-FP compared with intact BC8-Ab, were again largely limited to LN and spleen (experiment 3, Figure 3B). In the nontarget tissues, the uptake of BC8-FP and intact BC8-Ab again demonstrated minimal variation and target organ to blood ratios again favored BC8-FP in LN, BM, and spleen tissue after 96 hours (Table 1).
To further evaluate the retention kinetics of FP in target tissues, we injected 125I-labeled BC8-FP and performed serial target tissue biopsies. Necropsy, with a detailed biodistribution analysis, was performed after 168 hours (experiment 4). In the LN, the BC8-FP %ID/g was 0.115% plus or minus 0.03%, 0.080% plus or minus 0.01%, 0.082% plus or minus 0.03%, and 0.050% plus or minus 0.02% at 98, 120, 144, and 168 hours, respectively. The LN:blood ratios were 4.8:1; 5.7:1; 10.2:1, and 10:1 at 98, 120, 144, and 168 hours, respectively. The spleen to blood ratio was 25:1 and the BM to blood ratio was 13.4:1 after 168 hours. The organ with the highest nonspecific uptake at 168 hours was the kidney (0.026% ± 0.003%).
BC8 (scFv)4SA and radio-DOTA-biotin kinetics
BC8-FP antigen binding kinetics were initially evaluated in experiments comparing the biodistribution of 3.0 mg/kg (16.9 nmol/kg) of 131I-labeled BC8-FP coinjected with 3.0 mg/kg (16.9 nmol/kg) of 125I-labeled nonbinding control FP, CC49-FP (experiment 5). Blood clearance for both FPs was identical (data not shown). The ratio of BC8-FP to CC49-FP in LN was almost 3:1 at 24 hours. After 96 hours, the LN:blood ratio for CC49-FP was 1:1, whereas for BC8-FP it was more than 2:1. The animals were not killed after this experiment so detailed radioactivity contents for other organs are not available.
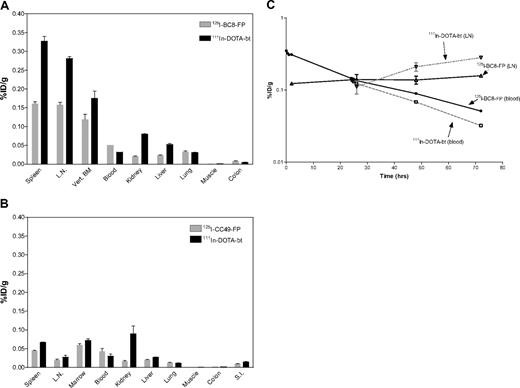
To assess the antigen specificity of this 2-step pretargeting method animals were given 16.9 nmol/kg of either 125I-BC8-FP or 125I-CC49-FP followed 24 hours later by 1.2 mg/m2 of 111In-DOTA-biotin (experiment 6). BC8-FP uptake in target tissues (LN and spleen) again demonstrated antigen specificity. The %ID/g in LN was 0.158% plus or minus 0.01% after 72 hours for BC8-FP compared with 0.02% plus or minus 0.004% for CC49-FP, whereas the values in the spleen were 0.161% plus or minus 0.01% and 0.045% plus or minus 0.001%, respectively (Figure 4A-B). The measured activities for the second-step reagent, 111In-DOTA-biotin, reflected the relative uptakes of the FPs in the target tissues (Table 2). The biodistributions for 125I-BC8-FP (first-step) and 111In-DOTA-biotin (second-step) demonstrate an amplification of adsorbed radiation with the second step (Figure 4A).
111In-DOTA-biotin biodistribution in target and nontarget tissues. After 125I-BC8-FP pretargeting (A), 125I-CC49-FP pretargeting (B), and serial target site activity with BC8 PRIT (C). (A) Uptake of 125I-BC8-FP and 111In-DOTA-biotin in tissues (%ID/g) after pretargeted RIT. 125I-BC8-FP (16.9 nmol/kg) was administered 24 hours before the 111In-DOTA-biotin (1.2 mg/m2). Tissues were collected 72 hours after 125I-BC8-FP and 48 hours after 111In-DOTA-biotin. (B) Comparative uptake of control fusion protein (125I-CC49-FP and 111In-DOTA-biotin after pretargeted RIT). 125I-CC49-FP (16.9 nmol/kg) was administered 24 hours before the 111In-DOTA-biotin (1.2 mg/m2). Specimens shown were collected 72 hours after 125I-CC49-FP and 48 hours after 111In-DOTA-biotin. (C) Time-activity curves of 125I-BC8-FP in the blood (●) and LNs (Δ) and 111In-DOTA-biotin in the blood (□) and LNs (▽) of macaques treated with pretargeted RIT 125I-BC8-FP (16.9 nmol/kg) administered 24 hours before the 111In-DOTA-biotin (1.2 mg/m2). Serial blood and LN specimens were obtained at the time points indicated.
111In-DOTA-biotin biodistribution in target and nontarget tissues. After 125I-BC8-FP pretargeting (A), 125I-CC49-FP pretargeting (B), and serial target site activity with BC8 PRIT (C). (A) Uptake of 125I-BC8-FP and 111In-DOTA-biotin in tissues (%ID/g) after pretargeted RIT. 125I-BC8-FP (16.9 nmol/kg) was administered 24 hours before the 111In-DOTA-biotin (1.2 mg/m2). Tissues were collected 72 hours after 125I-BC8-FP and 48 hours after 111In-DOTA-biotin. (B) Comparative uptake of control fusion protein (125I-CC49-FP and 111In-DOTA-biotin after pretargeted RIT). 125I-CC49-FP (16.9 nmol/kg) was administered 24 hours before the 111In-DOTA-biotin (1.2 mg/m2). Specimens shown were collected 72 hours after 125I-CC49-FP and 48 hours after 111In-DOTA-biotin. (C) Time-activity curves of 125I-BC8-FP in the blood (●) and LNs (Δ) and 111In-DOTA-biotin in the blood (□) and LNs (▽) of macaques treated with pretargeted RIT 125I-BC8-FP (16.9 nmol/kg) administered 24 hours before the 111In-DOTA-biotin (1.2 mg/m2). Serial blood and LN specimens were obtained at the time points indicated.
Measured activities for first step reagents (125I-BC8-FP or 125I-CC49-FP) and second-step reagent (111In-DOTA-biotin) from target tissues obtained 72 hours after FP administration
| . | BC8-FP . | SD . | CC49-FP . | SD . | DOTA-biotin (BC8-FP) . | SD . | DOTA-Biotin (CC49-FP) . | SD . |
|---|---|---|---|---|---|---|---|---|
| LN | 0.158% | ± 0.01% | 0.020% | ± 0.004% | 0.280% | ± 0.01% | 0.027% | ± 0.01% |
| Spleen | 0.161% | ± 0.01% | 0.045% | ± 0.001% | 0.330% | ± 0.022% | 0.070% | ± 0.002% |
| BM | 0.119% | ± 0.024% | 0.060% | ± 0.013% | 0.175% | ± 0.033% | 0.071% | ± 0.016% |
| . | BC8-FP . | SD . | CC49-FP . | SD . | DOTA-biotin (BC8-FP) . | SD . | DOTA-Biotin (CC49-FP) . | SD . |
|---|---|---|---|---|---|---|---|---|
| LN | 0.158% | ± 0.01% | 0.020% | ± 0.004% | 0.280% | ± 0.01% | 0.027% | ± 0.01% |
| Spleen | 0.161% | ± 0.01% | 0.045% | ± 0.001% | 0.330% | ± 0.022% | 0.070% | ± 0.002% |
| BM | 0.119% | ± 0.024% | 0.060% | ± 0.013% | 0.175% | ± 0.033% | 0.071% | ± 0.016% |
Animals received 16.9 nmol/kg of either 125I-BC8-FP or 125I-CC49-FP followed 24 hours later by 1.2 mg/m2 of 111In-DOTA-biotin. The contents of the trace-labeled reagents in tissue specimens were measured in a dual-channel gamma counter and are expressed as the percentage injected dose per gram of tissue (%ID/g). SDs reflect 3 biopsy specimens from each tissue.
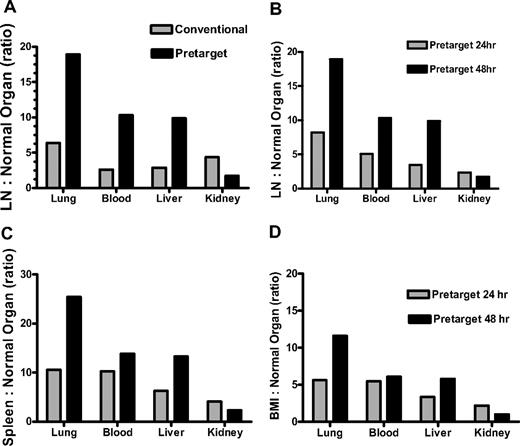
The initial time point selected for infusion of DOTA-biotin was based on prior murine BC8-Ab studies and macaque studies with the nonhuman primate anti-CD45 Ab AC8.12-14,22 These models predicted the optimal time point for 111In-DOTA-biotin (second-step) infusion to be 24 to 26 hours after FP. In experiment 6, accumulation of BC8-FP in LN was identical at 26 and 48 hours after infusion; the measured activity of BC8-FP in LN 26 hours after infusion was 0.14% plus or minus 0.042% and after 48 hours measured activity was 0.14% plus or minus 0.031% (Figure 4C). However, the LN:blood ratio was superior at the later time point. The measured activity of the second-step 111In-DOTA-biotin was 1.6:1 at 48 hours after BC8-FP administration; whereas after 26 hours, it was 0.8:1. Experiment 7 confirmed this finding. BC8-FP was again followed 24 hours later with 111In-DOTA-biotin at identical doses to experiment 6. The LN:blood ratio, based on the measured activity of the second-step 111In-DOTA-biotin, was 3:1 at 48 hours after BC8-FP administration; whereas after 26 hours, it was less than 1:1. The superiority of radio-DOTA-biotin given 48 hours after FP, compared with the 24-hour radio-DOTA-biotin administration in the initial studies, is confirmed by comparison of target-to-normal organ ratios from both time points (Figure 5B-D). The amounts of FP measured serially in the initial biodistribution studies (experiments 2, 3, and 5) further demonstrate 48 hours to be a superior time point for DOTA-biotin administration (Table 3).
Target-to-normal organ ratios. Comparing conventional RIT vs PRIT (A) and 24-hour vs 48-hour 111In-DOTA-biotin administration (B-D). (A) LN-to-normal organ ratios for macaques receiving either pretargeted (111In-DOTA-biotin) or directly labeled Ab (111In-DOTA-BC8) measured at 48-hour necropsy. 111In-DOTA-biotin was administered 48 hours after 125I-BC8-FP. (B-D) Target-to-normal organ ratios comparing 111In-DOTA-biotin administration at 24 hours versus 48 hours after 125I-BC8-FP.
Target-to-normal organ ratios. Comparing conventional RIT vs PRIT (A) and 24-hour vs 48-hour 111In-DOTA-biotin administration (B-D). (A) LN-to-normal organ ratios for macaques receiving either pretargeted (111In-DOTA-biotin) or directly labeled Ab (111In-DOTA-BC8) measured at 48-hour necropsy. 111In-DOTA-biotin was administered 48 hours after 125I-BC8-FP. (B-D) Target-to-normal organ ratios comparing 111In-DOTA-biotin administration at 24 hours versus 48 hours after 125I-BC8-FP.
Target-to-blood ratios of 125I-BC8-FP activity measured at 24 and 48 hours after infusion
| . | BC8-FP . | 24 h . | 48 h . |
|---|---|---|---|
| Experiment 2 | LN:blood ratio | 1:1 | 1.5:1 |
| Experiment 3 | LN:blood ratio | 0.8:1 | 1.3:1 |
| Experiment 5 | LN:blood ratio | 0.83:1 | 2:1 |
| . | BC8-FP . | 24 h . | 48 h . |
|---|---|---|---|
| Experiment 2 | LN:blood ratio | 1:1 | 1.5:1 |
| Experiment 3 | LN:blood ratio | 0.8:1 | 1.3:1 |
| Experiment 5 | LN:blood ratio | 0.83:1 | 2:1 |
Lymph node (LN) and blood samples were collected at serial time points, and specimen activity was measured in a dual-channel gamma counter. Calculated ratios of measured activity were superior at 48 versus 24 hours after 125I-BC8-FP infusion.
Conventional versus pretargeted RIT
After identification of 48 hours as the best time point for radio-DOTA-biotin administration, a biodistribution study was conducted to compare directly radiolabeled intact BC8-Ab with BC8-FP head-to-head (experiment 8). One animal received directly labeled 111In-DOTA-BC8 (16.9 nmol/kg), and a second animal received BC8-FP (16.9 nmol/kg) followed 48 hours later by 111In-DOTA-biotin (1.2 mg/m2). At necropsy, the measured activities in target organs for both animals were comparable. The spleen uptake was 0.230% plus or minus 0.002% and LN uptake 0.205% plus or minus 0.088% in the animal receiving intact Ab, whereas comparative values in the pretargeted animal were 0.305% plus or minus 0.011% for spleen and 0.227% plus or minus 0.057% for LN. Although the advantages of pretargeting compared with directly labeled RIT seem modest in target tissues, it must be emphasized that PRIT had markedly lower concentrations of radiation in normal organs/tissues (blood 0.0220% ± 0.00%[FP] vs 0.0780% ± 0.00%[Ab]; lung 0.0120% ± 0.0010%[FP] vs 0.0320% ± 0.0040%[Ab]; and liver 0.0230% ± 0.003%[FP] vs 0.0710% ± 0.003%[Ab]). The decreased measured radiation in the blood and normal organs of the animal receiving the FP is reflected by target:normal organ ratios favoring pretargeting for all organs of interest except kidney (Figure 5A). In the blood, lung, and liver, these ratios were 10.3:1, 18.9:1, and 9.9:1, respectively, in the pretargeted animal; and 2.6:1, 6.4:1, and 2.9:1 in the animal given directly labeled Ab. The kidney was the only nontarget organ that experienced higher levels of measured radiation in the pretargeted animal (LN:kidney ratio of 1.7:1 compared with 4.4:1 in the animal treated with directly labeled Ab). The concentrations of radiation in all other nontarget organs assessed were minimal in both the directly labeled Ab and pretargeted animals (< 0.01%).
Clearing agent
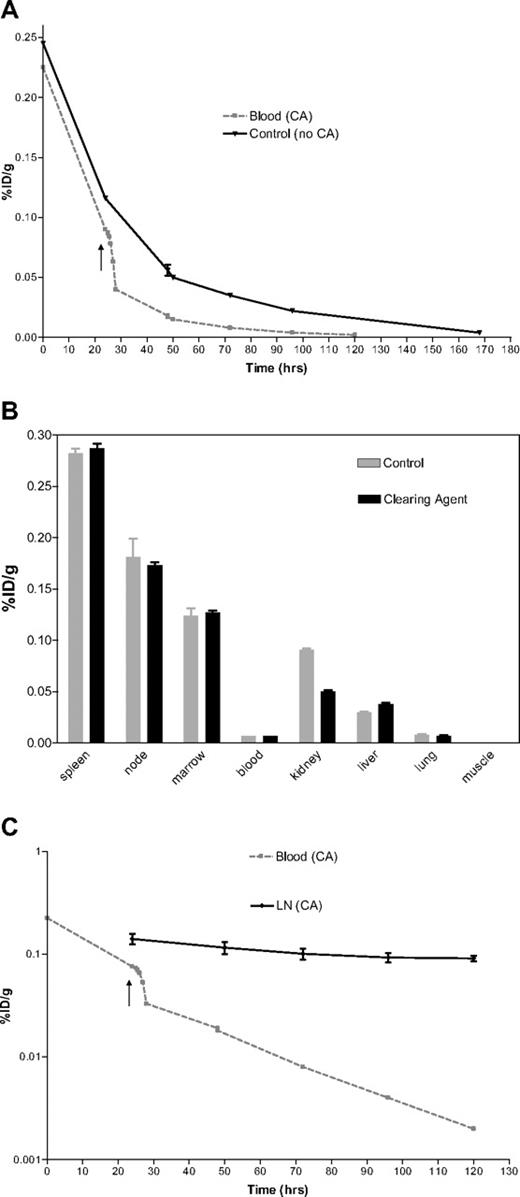
In an attempt to improve the target to nontarget ratios of radioactivity further, we investigated the efficacy of 2 CAs designed to accelerate clearance of excess FP from the bloodstream after optimal localization of the FP in target tissues (LN, spleen, BM). In one experiment, we administered 45 mg/m2 of a synthetic 16-mer CA intravenously 24 hours after 125I-BC8-FP and 2 hours before 111In-DOTA-biotin to evaluate its impact on blood clearance and organ biodistribution. A control animal received 125I-BC8-FP and 111In-DOTA-biotin at identical time points, but no CA (experiment 9; Figure 6B). CA reduced the measured activity in the blood by 30% 2 hours after infusion. In the control animal, the measured activity decreased by 10% over the same period. The CA-mediated blood clearance had no effect on subsequent amounts of 125I-BC8-FP measured in nontarget organs at 96-hour biodistribution (Table 4), nor on 111In-DOTA-biotin uptake in target organs at 96 hours (Figure 6B). The CA did appear to offer a mild salutary effect on nonspecific 111In-DOTA-biotin exposure to the kidney; however, this was not evident in other nontarget organs (Figure 6B).
The effect of CA administration. (A) Biodistribution. (B) Blood clearance. (C) Target organ retention. (A) Time-activity curves demonstrating the impact of a CA on the radioactivity present in the blood after administration of 16.9 nmol/kg of 125I-BC8-FP. One animal received a 4-mer CA (■) 24 hours after the BC8-FP (arrow), and an 83% decrease in activity was measured in the blood at t = 48 hours. No CA was administered to the control animal (▼). (B) Uptake of 111In-DOTA-biotin in tissues (%ID/g) after pretargeted RIT. 125I-BC8-FP (16.9 nmol/kg) was administered 26 hours before the 111In-DOTA-biotin (1.2 mg/m2). One animal received a 16-mer CA 24 hours after the BC8-FP. Tissues were collected 96 hours after 125I-BC8-FP and 72 hours after 111In-DOTA-biotin. (C) Time-activity curves demonstrating the impact of a CA on the radioactivity present in the blood (■) and LN (♦) after administration of 16.9 nmol/kg of 125I-BC8-FP. 4-mer CA (↑) was administered 24 hours after the BC8-FP.
The effect of CA administration. (A) Biodistribution. (B) Blood clearance. (C) Target organ retention. (A) Time-activity curves demonstrating the impact of a CA on the radioactivity present in the blood after administration of 16.9 nmol/kg of 125I-BC8-FP. One animal received a 4-mer CA (■) 24 hours after the BC8-FP (arrow), and an 83% decrease in activity was measured in the blood at t = 48 hours. No CA was administered to the control animal (▼). (B) Uptake of 111In-DOTA-biotin in tissues (%ID/g) after pretargeted RIT. 125I-BC8-FP (16.9 nmol/kg) was administered 26 hours before the 111In-DOTA-biotin (1.2 mg/m2). One animal received a 16-mer CA 24 hours after the BC8-FP. Tissues were collected 96 hours after 125I-BC8-FP and 72 hours after 111In-DOTA-biotin. (C) Time-activity curves demonstrating the impact of a CA on the radioactivity present in the blood (■) and LN (♦) after administration of 16.9 nmol/kg of 125I-BC8-FP. 4-mer CA (↑) was administered 24 hours after the BC8-FP.
Measured activities for 125I-BC8-FP in target organs at 96 hours in animals treated with and without 16-mer synthetic clearing agent at 24 hours
| . | CA . | SD . | Control . | SD . |
|---|---|---|---|---|
| LN | 0.173% | ± 0.005% | 0.181% | ± 0.031% |
| Spleen | 0.287% | ± 0.008% | 0.282% | ± 0.008% |
| BM | 0.127% | ± 0.003% | 0.124% | ± 0.012% |
| . | CA . | SD . | Control . | SD . |
|---|---|---|---|---|
| LN | 0.173% | ± 0.005% | 0.181% | ± 0.031% |
| Spleen | 0.287% | ± 0.008% | 0.282% | ± 0.008% |
| BM | 0.127% | ± 0.003% | 0.124% | ± 0.012% |
A total of 45 mg/m2 of a synthetic 16-mer CA was administered intravenously 24 hours after 125I-BC8-FP (16.9 nmol/kg) and 2 hours before 111In-DOTA-biotin (1.2 mg/m2) to evaluate its impact on blood clearance and organ biodistribution. A control animal received 125I-BC8-FP and 111In-DOTA-biotin at identical time points, but no CA. The contents of the trace-labeled reagents in tissue specimens were measured in a dual-channel gamma counter and are expressed as the percentage injected dose per gram of tissue (%ID/g). SDs reflect 3 biopsy specimens from each tissue.
Because of expense and limited availability of the 16-mer CA, we next tested the utility of a less expensive and more abundant 4-mer CA, which was administered at a dose of 4.0 mg/m2 24 hours after injection of 125I-BC8-FP. Twenty-four hours after CA infusion, the measured 125I-BC8-FP activity was 83% lower than preinfusion. In a control animal, 125I-BC8-FP activity decreased by 57% over the same time interval (Figure 6A). CA did not reduce 125I-BC8-FP retention in target tissue based on the measured activity at serial time points (Figure 6C).
Safety of BC8-FP and Ab infusions
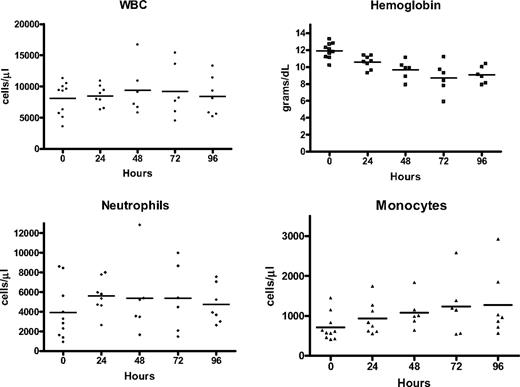
Infusions of BC8-Ab, FP, and radio-DOTA-biotin were performed with animals under general anesthesia. Frequent monitoring of basic vital signs revealed no hemodynamic consequence of BC8-Ab, FP, radio-DOTA-biotin, or CA infusions. No abnormalities in serum electrolytes, platelet count, liver enzymes, or creatinine were observed. A transient increase in absolute neutrophil count (t0 = 3908 cells/μL ± 2798; t24 = 5618 cells/μL ± 1742; t96 = 4733 cells/μL ± 1951) was noted 24 hours after FP infusion; however, measured levels returned to baseline by 96 hours (Figure 7). A mild monocytosis was also observed (t0 = 712 cells/μL ± 341;t96 = 1272 cells/μL ± 837; Figure 7). Daily phlebotomy resulted in a progressive daily decline in measured hemoglobin values (t0 = 11.9 g/dL ± 0.9; t96 = 8.7 g/dL ± 1.4; Figure 7).
The effect of BC8-FP on serial blood counts. White blood cell (WBC), hemoglobin, neutrophil, and monocyte measurements collected from the peripheral blood before (0 hours) and at serial time points (24, 48, 72, and 96 hours) after BC8-FP administration (n = 10). Horizontal line represents the average value at each time point.
The effect of BC8-FP on serial blood counts. White blood cell (WBC), hemoglobin, neutrophil, and monocyte measurements collected from the peripheral blood before (0 hours) and at serial time points (24, 48, 72, and 96 hours) after BC8-FP administration (n = 10). Horizontal line represents the average value at each time point.
Discussion
In 2007, an estimated 8990 people died of recurrent AML and an additional 18 660 died of relapsed NHL.39 Many of these deaths were presumably caused by malignant cells that remained radiosensitive but evaded prior therapies. The dose of radiation therapy that can be administered to such patients is limited by toxicity to normal organs. Promising results have been reported by many groups targeting the CD20 antigen with either 131I-tositumab or 90Y-ibritumomab tiuxetan for patients with relapsed NHL. Although successful in achieving improved response rates, in most patients the duration of remission is short, with an average progression-free survival of less than 12 months. Incorporating conventional RIT into hematopoietic cell transplantation conditioning regimens has improved outcomes, but at least 50% of these patients still relapse.2,4,5
The efficacy of RIT targeting CD45 is well established. In the context of B-cell malignancies, we think that targeting CD45 is also a promising avenue for future therapy. We selected CD45 as our target antigen based on 2 factors considered crucial for obtaining a favorable biodistribution of radiolabeled Ab: antigen density and binding site accessibility. A high density of expression directly correlates with accumulation of Ab at tumor sites40 and is characteristic of both CD45 and CD20 in B-cell malignancies. Recently, our group has demonstrated that the presence of circulating anti-CD20 Ab blocks the binding of anti-CD20 125I-tositumomab but does not block anti-CD45 BC8 uptake in murine lymphoma tumor xenografts.25 Currently, a large majority of patients with B-cell lymphomas receive regimens that include anti-CD20 immunotherapy (rituximab) before being considered for RIT-based treatments. As a consequence, CD45 may offer superior binding site accessibility, and we consider this alternate RIT antigen target to be promising for treatment of B-cell lymphoma.
Studies in murine models, nonhuman primates, and human subjects have documented the importance of appropriate Ab dosing to achieve optimal radiolabeled Ab biodistributions.22,41,42 The broad expression of CD45 antigen raises some concerns about its capacity to selectively target hematolymphoid tissues in lymphoma at doses realistically achievable in patients. These concerns have not been fully addressed by murine xenografts studies because murine CD45 does not bind the BC8-Ab. However, our group has demonstrated the effectiveness of murine CD45 (mCD45) pretargeting in a syngeneic murine leukemia model.43
Multistep pretargeting has demonstrated dramatic improvements in target-to-normal ratios of absorbed radiation in models of both human and murine leukemia and human lymphoma.8,10-13,43-45 Our studies document that anti-CD45 PRIT is capable of significantly improving the target-to-normal organ ratio of absorbed radiation compared with conventional RIT in macaques. We report 6 major findings that contribute to the overall efficacy of PRIT in this animal model. First, we demonstrate the safety and feasibility of pretargeting. Second, we show that a tetrameric FP construct exhibits superior retention in target tissues compared with the comparable, directly radiolabeled bivalent Ab. Third, we demonstrate that the time point at which the second-step radiolabeled ligand (radio-DOTA-biotin) is delivered has significant impact on biodistribution. Fourth, we show that anti-CD45 FP pretargeting is capable of selectively delivering high doses of radiation to target organs, with relative sparing of nontarget tissue. Fifth, we validate previous findings in smaller animals, documenting that our FP construct and small molecule DOTA-biotin both retain the full binding capacity of their parent molecules. Sixth, we demonstrate that the rapid α-phase blood clearance of BC8-FP in primates obviates the need for synthetic CA.
We report that BC8-FP exhibited superior retention in serial LN samples compared with simultaneously coadministered anti-CD45 Ab by a factor of almost 2-fold at 96 hours after infusion. Similar findings were observed in another target organ, the spleen, with 2.3-fold greater retention of FP than Ab 96 hours after infusion. BM, a third potential target, demonstrated a less impressive 1.2-fold greater retention of the BC8-FP compared with intact BC8-Ab. However, the BM figures may underestimate the superiority of the pretargeted approach because one limitation of our studies was the variable quality of BM core samples collected. In initial studies, we found these specimens were difficult to obtain in macaques and demonstrated the largest SEs for measured reagent uptake of any tissue evaluated. In later experiments, more reliable BM core specimens were obtained at necropsy from lumbar vertebral sites and demonstrated significantly less variability. This underscores a limitation of the macaque model, as there are no reliable leukemia or lymphoma models available in nonhuman primates. Nonetheless, retention of the FP construct was specific to target sites. Nontarget organs, which lack CD45-antigen–expressing cells, revealed no difference in FP and intact Ab activity in necropsy specimens collected after 96 hours.
In patients with disease, we anticipate that anti-CD45 PRIT will enable delivery of higher doses of radiation to BM and extramedullary target sites than are achievable through conventional RIT. We do not think that higher doses of radiation delivered to the BM compartment will impact subsequent marrow function. Dose augmentation through PRIT in our murine models has demonstrated no evidence of BM stromal damage or difficulties with hematopoietic reconstitution.12,14,43,46 Over the past 22 years, 209 patients have been treated with conventional anti-CD45 RIT as hematopoietic cell transplantation conditioning at our center, and none has failed to engraft.7,47 Nonetheless, initial anti-CD45 PRIT dose escalation trials must be designed to closely monitor patients for BM toxicity, including delayed engraftment at each dose level.
Our studies demonstrate that 2-step PRIT yields target-to-normal organ ratios that are significantly superior to the conventional, directly labeled Ab approach. Studies were also conducted to evaluate the potential for synthetic CA to further enhance the therapeutic index. Streptavidin-Ab conjugates have been used to target 90Y-DOTA-biotin to lung cancer xenografts in nude mice and have demonstrated striking tumor-to-blood ratios in this model (> 20:1 at 2 hours and > 1000:1 at 144 hours), as a result of rapid removal of more than 90% of circulating SA-Ab by the CA.11 Our group has reported similarly favorable results in murine B-cell lymphoma (using anti-CD20 and anti-CD45 directed PRIT) and leukemia models.12,13,43 In the nonhuman primate, however, we demonstrate that the rapid decrease in circulating BC8-FP during the α-phase of blood clearance minimizes the requirement for a CA (Figure 6A). We therefore plan to proceed to initial clinical studies using a 2-step pretargeting approach without a CA.
Our findings suggest that nonspecific radiation exposure to the kidney may define the dose-limiting toxicity with 2-step pretargeted RIT. When formal kidney dosimetry was performed in experiment 8, the kidneys received an estimated radiation dose of 19.2cGy per mCi with pretargeting using BC8-FP followed by 111In-DOTA-biotin, whereas the kidneys received a somewhat lower dose of 12.4 cGy/mCi with conventional RIT using 111In-BC8. Whether this moderate increase in renal radiation exposure will translate to significant nephrotoxicity is unknown, but close and frequent monitoring of renal function will be integral to our patient studies. Long-term biochemical monitoring has demonstrated no clinical evidence of renal impairment in our previously published murine PRIT studies.12,13,15,16,43,46 Lymphoma-bearing athymic mice, cured with high doses of 90Y-DOTA-biotin, lived for more than one year after therapy in good health. Mice killed after one year occasionally had evidence of mild to moderate membranous glomerulonephropathy at necropsy. The significance of subclinical membranous glomerulonephritis should be viewed in the context of a 100% mortality rate within 40 days for the control mice and conventional RIT-treated mice in these experiments compared with a cure rate of 90% to 100% for experimental animals receiving optimal doses of PRIT. We think the dramatic differences in survival seen in PRIT mouse models and the limited potential risk for nephrotoxicity seen in both mice and macaques warrant proceeding to clinical trials for patients with incurable disease who have failed standard therapies.
In conclusion, our studies demonstrate that 2-step pretargeting offers a significant advantage over conventional RIT. The relatively rapid blood clearance of the BC8-FP in nonhuman primates suggests that a CA may not be necessary to achieve excellent target-to-normal organ ratios of radioactivity in human clinical trials. Such trials will need to monitor renal function carefully but hold great promise for improved therapy of CD45-expressing leukemias and lymphomas.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the veterinary and research staff of the Washington National Primate Research Center for their technical support.
This work was supported by grants from the Lymphoma Research Foundation (D.J.G., O.W.P., J.M.P., A.K.G.), the National Institutes of Health (grants PO1 CA44991 and RO1 CA109663, O.W.P.; grant K23 CA100394, E.R.N), the American Society of Clinical Oncology Young Investigator Award Program (D.J.G.), and the Damon Runyan Cancer Research Foundation (J.M.P.) and by gifts from David and Patricia Giuliani, Mary and Geary Britton-Simmons, James and Sherry Raisbeck, the Wyner-Stokes Foundation, and the Hext Family Foundation (O.W.P.)
National Institutes of Health
Authorship
Contribution: D.J.G., E.R.N., and O.W.P. designed the experiments; D.J.G., A.P., Y.L., A.K., and D.K.H. performed experiments; D.J.G., J.M.P., D.R.F., D.S.W., J.G.R., A.K.G., S.I.P., and O.W.P. analyzed the results; D.J.G. wrote the manuscript and produced the figures; and O.W.P., J.M.P., E.R.N., and D.S.W. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Damian J. Green, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, MS D3-190, Seattle, WA 98109; e-mail: dgreen@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal