Abstract
Methotrexate and 6-mercaptopurine, important components of acute lymphoblastic leukemia treatment, are substrates for multidrug resistance-associated protein MRP4. Eight single nucleotide polymorphisms were analyzed in MRP4 gene, and 4 variants were identified as tagSNPs with frequency more than or equal to 5%. They were investigated for association with treatment responses in 275 children with acute lymphoblastic leukemia. The TC genotype of the regulatory T-1393C polymorphism was associated with better event-free survival (P = .02) and lower methotrexate plasma levels (P = .01). The CA genotype of A934C (Lys304Asn) substitution correlated in contrast with lower event-free survival (P = .02) and higher frequency of high-grade thrombocytopenia (P = .01). Gene reporter assay showed that the promoter haplotype uniquely tagged by the C-1393 allele conferred higher promoter activity compared with remaining haplotypes (P < .001). Further analyses are needed to replicate this pilot study and get closer insight into the functional effect of these polymorphisms.
Introduction
The treatment of pediatric acute lymphoblastic leukemia (ALL) has greatly improved since the introduction of effective combination risk-adapted therapies.1,2 Nevertheless, therapy resistance in a significant number of children is still a major obstacle to successful treatment, whereas intensive treatment has also important side effects. Pharmacogenetic studies identified certain genetic variations that may contribute to variability in ALL treatment responses.1,2 The drug effects depend, among other factors, on the activity and expression of multidrug resistance-related proteins (MRPs).3 MRP4 has a remarkable ability to transport a range of drugs and physiologic substrates.4 MRP4 is ubiquitously expressed, including high expression in the hematopoietic cells, thus possibly affecting both drug intended and drug side effects in these cells.5 MRP4 affects disposition of physiologic folates, but also of methotrexate (MTX) and 6-mercaptopurine (6-MP), which are key components of ALL treatment.4,6-8 MRP4 protects cells against thiopurine-induced toxicity by actively exporting thioguanine nucleotides (TGNs),6 whereas MRP4-transfected cells displayed increased resistance to MTX.4,7 Wide variation in MRP4 expression has been reported in pediatric leukemia lymphoblasts.9 This can be in part the result of functional genetic polymorphisms. Here we report the analysis of MRP4 gene variations.
Methods
The children enrolled in the study are 275 whites diagnosed with ALL at Hospital Sainte-Justine, between January 1989 and December 2003. The patients underwent treatment with Dana-Farber Cancer Institute ALL Consortium protocols DFCI 87-01, 91-01, 95-01, or 2000-01.10 Patient samples were obtained after informed consent was given, in accordance with the Declaration of Helsinki, and the Ethics Committee of Centre Hospitalier Universitaire Sainte-Justine approved the study protocol. Eight MRP4 polymorphisms in regulatory and coding gene regions were selected from the National Center for Biotechnology Information database.11 All selected polymorphisms were genotyped in 49 controls to estimate allele and haplotype frequency (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Selected tagSNPs (single nucleotide polymorphism sufficient to define common haplotypes) with frequency more than or equal to 5% (T-1393C, C-1015T, C934A, and A4131C) were subsequently genotyped in 275 ALL patients. Genotyping details are given in supplemental Table 1. Survival differences for patients with different genotypes were estimated by Kaplan-Meier analysis. The hazard ratio (with a 95% confidence interval [CI]) for MRP4 variants was estimated by the Cox regression analysis, with and without inclusion of the common prognostic factors or other genetic variants (dihydrofolate reductase, [DHFR], thymidylate synthase [TS], reduced folate carrier 1, methylene-tetrahydrofolate reductase, cyclin D1, and glutathione S transferase M1) shown by us and others to influence the risk of relapse10,12-19 (supplemental Table 2). Toxicity on bone marrow and liver function (the appearance of toxicity and toxicity rates) was based, as previously described,20 on the results of weekly laboratory tests collected in 174 patients during consolidation and maintenance treatment. MTX plasma levels at both 36 hours and 48 hours after high-dose MTX were available for 197 patients; 267 patients had measured or conclusive MTX levels at 72 hours after high-dose MTX. All analyses were performed by SPSS statistical package, Version 13.0.
For the gene reporter experiments, haplotype-specific fragments corresponding to proximal promoter of MRP4 were amplified from genomic DNA of persons with known genotype and subcloned into the promoterless pGL3-basic firefly luciferase reporter vector (Promega) as previously described.10 Expression data for lymphoblastoid cells lines were taken from Gene Expression Omnibus at the National Center for Biotechnology Information.21
Results and discussion
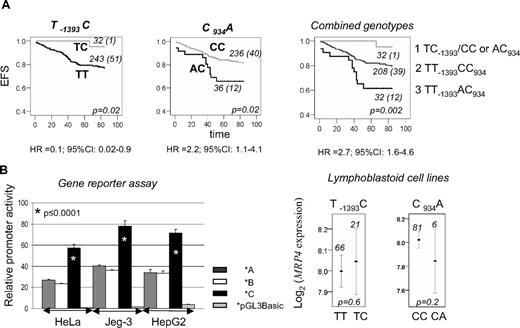
Association of MRP4 genotypes with event-free survival (EFS) showed that persons with the TC-1393 genotype had better EFS and those with the CA934 genotype had reduced EFS compared with remaining genotypes (P = .02, Figure 1A). When 2 polymorphisms were combined, 3 genotype groups could be distinguished with EFS, decreasing from 95% to 80% and 62% (Figure 1A). Significant associations with EFS were retained in the Cox regression, when other prognostic factors or genotypes previously shown to affect EFS10,12-19 were included in the models (supplemental Table 2). Among event-predisposing genotypes, MRP4 CA934, DFHR haplotype *1, and TS 3R homozygosity retained significance (P ≤ .05). Dihydrofolate reductase and TS, in accordance with our previous observation,10 result in more important EFS reduction when acting together; MRP4 leads to equally important reduction individually and combined with other genotypes (supplemental Figure 1).
The impact of MRP polymorphisms on ALL outcome and transcription. (A) EFS curves for TC-1393, CA934, and combined MRP genotypes. Three groups are distinguished: group 1, with TC-1393 and CC or AC934 genotypes; group 2, with TT-1393 and CC934 genotypes; and group 3, with TT-1393 and AC934 genotypes. The genotype and the number of patients in each curve, numbers of persons with an event (in parentheses), as well as the P value, estimated by log-rank test for the survival differences between the patients groups, are indicated on each plot. Times to event were measured as the time between diagnosis and the event of interest; for censored cases, it corresponded to time between diagnosis and the study endpoint (5 years after treatment, ie, 84 months after diagnosis) or to the last observational period. Risk of event associated with the given genotype, expressed as univariable hazard ratio (HR) with 95% CI estimated by Cox regression analysis, is indicated below the plots. Similar results as those shown for EFS were obtained for disease-free survival (P ≤ .02). A similar, but not significant, trend was observed for overall survival for persons with the TC-1393 genotype (P = .07), whereas there was no association for AC934 genotype (P = .4). (B) Relative promoter activity and mRNA levels in lymphoblastoid cell lines in relation to MRP promoter haplotypes. Relative promoter activity (mean ± SD) obtained by luciferase reporter assay for MRP haplotypes *A, *B, and *C is represented by  , □, and ■, respectively. Empty vector is represented by a light gray bar. The values are given for 3 different cell lines, Human placental Jeg-3, cervical cancer HeLa, and hepatoma HepG2 (ATCC). The difference in promoter activity obtained by Student t test (*p) between haplotype *C and remaining haplotypes (*A or *B) is indicated on the plot. MRP expression derived from wide-genome expression dataset GSE172621 is presented. The mean value of expression with 95% CI, the number of persons represented by each line, and P value obtained by analysis of variance for the difference of expression between indicated genotypes, taken from HapMap data24 are given on the plots.
, □, and ■, respectively. Empty vector is represented by a light gray bar. The values are given for 3 different cell lines, Human placental Jeg-3, cervical cancer HeLa, and hepatoma HepG2 (ATCC). The difference in promoter activity obtained by Student t test (*p) between haplotype *C and remaining haplotypes (*A or *B) is indicated on the plot. MRP expression derived from wide-genome expression dataset GSE172621 is presented. The mean value of expression with 95% CI, the number of persons represented by each line, and P value obtained by analysis of variance for the difference of expression between indicated genotypes, taken from HapMap data24 are given on the plots.
The impact of MRP polymorphisms on ALL outcome and transcription. (A) EFS curves for TC-1393, CA934, and combined MRP genotypes. Three groups are distinguished: group 1, with TC-1393 and CC or AC934 genotypes; group 2, with TT-1393 and CC934 genotypes; and group 3, with TT-1393 and AC934 genotypes. The genotype and the number of patients in each curve, numbers of persons with an event (in parentheses), as well as the P value, estimated by log-rank test for the survival differences between the patients groups, are indicated on each plot. Times to event were measured as the time between diagnosis and the event of interest; for censored cases, it corresponded to time between diagnosis and the study endpoint (5 years after treatment, ie, 84 months after diagnosis) or to the last observational period. Risk of event associated with the given genotype, expressed as univariable hazard ratio (HR) with 95% CI estimated by Cox regression analysis, is indicated below the plots. Similar results as those shown for EFS were obtained for disease-free survival (P ≤ .02). A similar, but not significant, trend was observed for overall survival for persons with the TC-1393 genotype (P = .07), whereas there was no association for AC934 genotype (P = .4). (B) Relative promoter activity and mRNA levels in lymphoblastoid cell lines in relation to MRP promoter haplotypes. Relative promoter activity (mean ± SD) obtained by luciferase reporter assay for MRP haplotypes *A, *B, and *C is represented by  , □, and ■, respectively. Empty vector is represented by a light gray bar. The values are given for 3 different cell lines, Human placental Jeg-3, cervical cancer HeLa, and hepatoma HepG2 (ATCC). The difference in promoter activity obtained by Student t test (*p) between haplotype *C and remaining haplotypes (*A or *B) is indicated on the plot. MRP expression derived from wide-genome expression dataset GSE172621 is presented. The mean value of expression with 95% CI, the number of persons represented by each line, and P value obtained by analysis of variance for the difference of expression between indicated genotypes, taken from HapMap data24 are given on the plots.
, □, and ■, respectively. Empty vector is represented by a light gray bar. The values are given for 3 different cell lines, Human placental Jeg-3, cervical cancer HeLa, and hepatoma HepG2 (ATCC). The difference in promoter activity obtained by Student t test (*p) between haplotype *C and remaining haplotypes (*A or *B) is indicated on the plot. MRP expression derived from wide-genome expression dataset GSE172621 is presented. The mean value of expression with 95% CI, the number of persons represented by each line, and P value obtained by analysis of variance for the difference of expression between indicated genotypes, taken from HapMap data24 are given on the plots.
An increase in the frequency of thrombocytopenia grade 3 or 4 was seen for persons with MRP4 CA934 genotype (P = .01, Figure 2A),20 whereas differences in MTX plasma levels were observed for MRP4-1393 variation, being lowest in patients with the TC genotype (P = .01 for MTX retention at 72 hours after high-dose MTX, Figure 2B; and P = .006 for 36 hours and 48 hours after high-dose MTX levels, Figure 2C).
Hematologic toxicity and MTX levels according to MRP4 genotypes. (A) Relationship between MRP polymorphisms and frequency of high-grade thrombocytopenia. The presence (■) and absence (□) of at least one episode of thrombocytopenia grade 3 and/or 4 in persons with different MRP genotype groups (top panel) or genotypes of CA934 polymorphism (bottom panel). P values for the difference between genotypes are obtained by χ2 (p1) and by Kruskal-Wallis or Mann-Whitney test (p2) for the difference in toxicity rates. Toxicity was graded using the common criteria for adverse events of the National Cancer Institute.20 The mean number of weeks assessed per patient was 80. (B) Relationship between MRP polymorphisms and retention of MTX in plasma at concentration higher than 1 μM. Concentration more than 1 μM 72 hours after high-dose MTX are indicated by ■, and concentrations less than or equal to 1 μM are indicated by □ in persons with different MRP genotype groups (top panel) or genotypes of TC-1393 polymorphism (bottom panel). MTX plasma levels were measured by fluorescence polarization immunoassay (TDx; Abbott Laboratories) following the manufacturer's instructions. P value for the difference between genotypes is obtained by χ2. (C) Relationship between MRP polymorphisms and MTX plasma levels. Lines represent log values of MTX levels (μM, measured at 2 time points after high-dose MTX) in persons with different MRP genotype (top panel) or genotypes of TC-1393 polymorphism (bottom panel). P values are obtained by repeated measures analysis of variance. In panels A and B, genotypes are indicated on the x-axis. The number of persons represented by white and black bars is indicated above and below each plot, respectively. In panel C, genotype groups, genotypes, and number of persons represented by each line are indicated on the plots. Given that the data on toxicity were available for 174 patients only, we analyzed an association between MRP4 polymorphisms and EFS in this subgroup. The same trend was observed (P = .1 for TC-1393 and AC934 genotype, and P = .02 for genotype groups).
Hematologic toxicity and MTX levels according to MRP4 genotypes. (A) Relationship between MRP polymorphisms and frequency of high-grade thrombocytopenia. The presence (■) and absence (□) of at least one episode of thrombocytopenia grade 3 and/or 4 in persons with different MRP genotype groups (top panel) or genotypes of CA934 polymorphism (bottom panel). P values for the difference between genotypes are obtained by χ2 (p1) and by Kruskal-Wallis or Mann-Whitney test (p2) for the difference in toxicity rates. Toxicity was graded using the common criteria for adverse events of the National Cancer Institute.20 The mean number of weeks assessed per patient was 80. (B) Relationship between MRP polymorphisms and retention of MTX in plasma at concentration higher than 1 μM. Concentration more than 1 μM 72 hours after high-dose MTX are indicated by ■, and concentrations less than or equal to 1 μM are indicated by □ in persons with different MRP genotype groups (top panel) or genotypes of TC-1393 polymorphism (bottom panel). MTX plasma levels were measured by fluorescence polarization immunoassay (TDx; Abbott Laboratories) following the manufacturer's instructions. P value for the difference between genotypes is obtained by χ2. (C) Relationship between MRP polymorphisms and MTX plasma levels. Lines represent log values of MTX levels (μM, measured at 2 time points after high-dose MTX) in persons with different MRP genotype (top panel) or genotypes of TC-1393 polymorphism (bottom panel). P values are obtained by repeated measures analysis of variance. In panels A and B, genotypes are indicated on the x-axis. The number of persons represented by white and black bars is indicated above and below each plot, respectively. In panel C, genotype groups, genotypes, and number of persons represented by each line are indicated on the plots. Given that the data on toxicity were available for 174 patients only, we analyzed an association between MRP4 polymorphisms and EFS in this subgroup. The same trend was observed (P = .1 for TC-1393 and AC934 genotype, and P = .02 for genotype groups).
In this study, the A allele of C934A (Lys304Asn) correlated with reduced EFS and higher incidence of high-grade thrombocytopenia. The functional impact of this polymorphism has not been clearly shown. There was no difference in protein expression in liver or in accumulation of 6-MP and antiviral agents in kidney cells in relation to the MRP4 genotypes.8,22 Gradhand and Kim,23 in contrast, showed reduction in MRP4 expression in liver samples associated with the A934 allele. Because of the small number of patients, the significance of this finding was not conclusive. We also noticed nonsignificant reduction in mRNA expression in lymphoblastoid cell lines (P = .2, Figure 1B). One of possible explanations for our finding is that the higher frequency of toxicity presumably associated with lower MRP4 expression would lead to more frequent drug withdrawal or dose reduction, which might cause higher frequency of relapse. Indeed, relapsed ALL patients do not have higher MRP4 expression, contrasting other MRPs whose higher levels correlated with a relapse.25 MRP4 was also expressed at a lower level in T-cell ALL, having higher resistance to treatment than B-cell ALL.25 The other mechanisms are probably involved as well; down-regulation of MRPs might result in decreased folate efflux, thereby leading to expansion of the intracellular folate pool and antifolate resistance.3
Carriers of the C-1393 allele, which uniquely tags promoter haplotype *C, were associated with better EFS and with lower MTX plasma levels after high drug dose. Gene reporter assay showed 2-fold higher promoter activity for haplotype *C, compared with the remaining haplotypes (P ≤ .001, Figure 1B). No association with basal mRNA expression in lymphoblastoid cell lines was found (Figure 1B). Several reasons might account for this discrepancy. The difference seen in vitro does not necessarily reflect an expression in vivo, which is further influenced by a variety of genetic and nongenetic factors. The difference in expression might exist between different cell types. MRP4 is expressed in various tissues, including kidney, and has affinity for many substrates.4 MTX levels assessed in this study probably reflect renal MRP4 transport and not the efflux from lymphocytes given that the renal excretion is the main MTX elimination route.4 Higher MRP4 activity in kidney associated with the C-1393 allele could then explain lower MTX levels and the absence of toxicity associated with this allele. MRP4 was also shown to protect cells against 6-MP hematopoietic toxicity.6 It would be thus important to correlate these polymorphisms with 6-MP/TGN levels, which was not possible in this study because of the lack of 6-MP/TGN data.
Several additional nonsynonymous polymorphisms were identified in the MRP gene. Few of them were found to influence protein expression or drug transport.6,8,22 Given their low frequency (1%–2%), they were not analyzed here.
In conclusion, we provided additional insight into the possible genetic modulation of treatment responses in childhood ALL. Further functional analysis and replication in independent cohort are needed to support the validity of this pilot study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and their parents who consented to participate in genetics study related to leukemia.
This work was supported by grants from the Leukemia-Lymphoma Society of Canada, Canadian Institute of Health Research, Research Center of Centre Hospitalier Universitaire Sainte-Justine, Charles Bruneau Foundation, and Center d'excellence en Oncologie pédiatrique et en soins palliatifs. M.K. and D.S. are scholars of the Fonds de la Recherche en Santé du Québec. M.A. is scholar of the Swiss National Fund, Charles Bruneau and Telemaque foundation. Gene reporter assay was carried out within the projects of Genome Quebec/Genome Canada: Gene regulators in disease.
Authorship
Contribution: M.A., G.S., M.L., and V.G. performed experiments; M.A., C.L., and A.M. contributed to the retrieval of clinical data; M.L. retrieved the public data on MRP4 expression; M.A., V.G., and M.K. performed the analysis; M.K. designed the research; D.S. designed gene reporter experiments; and M.A. and M.K. drafted the article. All authors contributed to the interpretation of data and revised the manuscript critically.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maja Krajinovic, Centre de recherche, CHU Sainte-Justine, 3175 chemin de la Côte-Ste-Catherine, Montréal, QC, H3T 1C5 Canada; e-mail: maja.krajinovic@umontreal.ca.