The laboratory of Judah Folkman identified the potent endogenous antiangiogenic protein angiostatin in 1994.1 In this issue of Blood, Lee and colleagues propose 2 new mechanisms of action for angiostatin that may represent promising targets for new cancer therapeutics.2
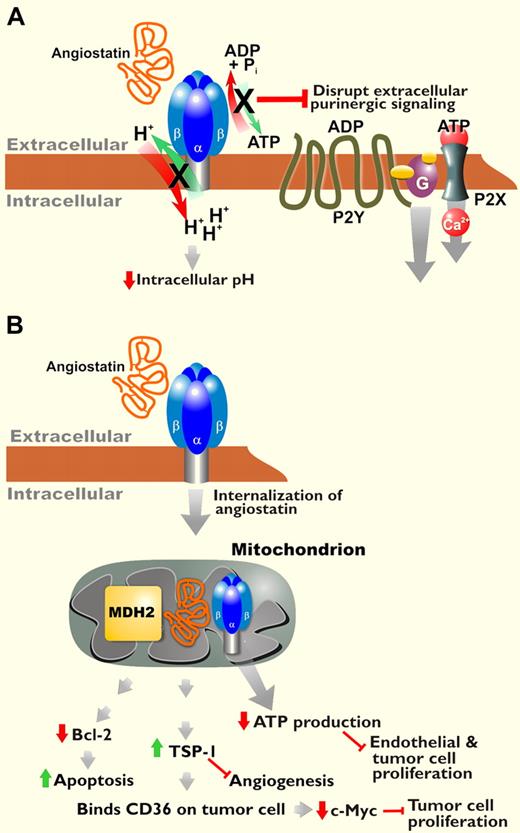
Angiostatin, a proteolytic fragment of plasminogen, blocks angiogenesis and inhibits tumor growth.1,3 Previous studies of angiostatin emphasized its inhibition of angiogenesis via endothelial cell–specific proapoptotic, antimigratory, and antiproliferative effects. F1F0 ATP synthase was identified as a cell-surface receptor for angiostatin.4,5 Angiostatin blocks the enzymatic activity of this receptor, namely transmembrane proton transport coupled to ATP synthesis/hydrolysis. Inhibition of ATP synthase induces intracellular acidification and decreases extracellular ATP production, both of which could contribute to reduced proliferation and cell death via pH dysregulation and/or purinergic signaling effects (see figure, panel A).
Potential mechanisms of action for angiostatin. (A) Angiostatin binds to cell surface ATP synthase, inhibiting proton transport coupled to ATP synthesis/hydrolysis by this enzyme. Interference with intracellular pH and purinergic signaling may account for some effects of angiostatin. (B) Lee et al demonstrate internalization of angiostatin via ATP synthase and binding to mitochondrial MDH2 and ATP synthase. They propose that angiostatin targeting of these enzymes regulates several downstream effects of this antitumorigenic protein.2
Potential mechanisms of action for angiostatin. (A) Angiostatin binds to cell surface ATP synthase, inhibiting proton transport coupled to ATP synthesis/hydrolysis by this enzyme. Interference with intracellular pH and purinergic signaling may account for some effects of angiostatin. (B) Lee et al demonstrate internalization of angiostatin via ATP synthase and binding to mitochondrial MDH2 and ATP synthase. They propose that angiostatin targeting of these enzymes regulates several downstream effects of this antitumorigenic protein.2
Lee et al provide evidence of additional antitumorigenic activities of angiostatin beyond its antiangiogenic properties: increased apoptosis and decreased proliferation of tumor cells, and inhibition of macrophage tumor infiltration. They demonstrate increased TUNEL staining, indicative of DNA fragmentation due to apoptosis, in melanoma cells from angiostatin-treated mice. They also show decreased tumor cell expression of antiapoptotic Bcl-2, but the authors acknowledge that Bcl-2 levels may only correlate with tumor size. However, the decrease in Bcl-2 fits with previous findings that angiostatin increases p53 expression in endothelial cells6 and kills tumor cells expressing cell-surface ATP synthase.7 Lee et al also demonstrate increased expression of thrombospondin-1 (TSP-1) in tumor vascular endothelial cells of mice treated with angiostatin. TSP-1 is an anti-angiogenic protein, but Lee et al present the novel finding that in vitro treatment of melanoma cells with TSP-1 reduces levels of the progrowth protein c-Myc, decreases proliferation, and increases apoptosis.
Angiostatin has known anti-inflammatory effects, including inhibition of macrophage infiltration into atherosclerotic plaques and disruption of monocyte/macrophage motility via effects on the actin cytoskeleton.8 Lee et al here present novel evidence of decreased macrophage influx into tumors in animals treated with angiostatin. Tumor-associated macrophages may promote tumor growth by suppressing adaptive antitumor immune responses, facilitating tumor invasion through extracellular matrix remodeling, and producing progrowth and proangiogenic factors.9 Thus, the interplay between angiostatin and tumor-associated macrophages could figure prominently in the antitumor effects of angiostatin.
Lee et al suggest that after binding to ATP synthase, angiostatin internalizes and binds to malate dehydrogenase (MDH2) in the mitochondrial matrix and/or continues binding to ATP synthase localized in the inner mitochondrial membrane (see figure, panel B). Binding these enzymes in the Krebs cycle and electron transport chain, respectively, is associated with decreased intracellular ATP. The authors suggest that intracellular targeting of angiostatin to mitochondrial proteins plays a key role in its antitumorigenic effects. However, they do not explicitly show that angiostatin blocks the enzymatic activity of MDH2, nor that inhibiting MDH2 and mitochondrial ATP synthase is required for angiostatin-induced increases in TSP-1 expression, tumor cell apoptosis, or the other effects of angiostatin. Follow-up studies are required to clarify the role that angiostatin internalization plays in these downstream effects. The data presented introduce intriguing questions about how angiostatin is internalized and then crosses the outer mitochondrial membrane. Given that data support localization of cell- surface ATP synthase to caveolae, the dynamin-dependent process of caveolar endocytosis is likely responsible for the uptake of angiostatin bound to ATP synthase. However, Lee et al show that some internalization can occur in the absence of ATP synthase, so other receptors and mechanisms of uptake may exist.
Although many important questions remain, the results of Lee et al presented in this issue highlight potential targets for new cancer therapeutics. Given that angiostatin itself has performed poorly in clinical trials, likely due to its short serum half-life, achieving a better understanding of its mechanisms of action can facilitate the development of drugs mimicking the potent antitumor activities of angiostatin without its pharmacokinetic limitations.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal