On recognition of influenza virus (Flu) by TLR7, plasmacytoid dendritic cells (pDCs) produce type I IFN in significant amounts. Synthetic TLR7 ligands induce the maturation of pDCs, as evidenced by the expression of costimulatory molecules and the production of proinflammatory cytokines; however, they induce only low-level production of IFN-α. To dissect the TLR7 signaling in pDCs and how these different profiles are induced, we studied the effects of 2 TLR7 ligands (Flu and CL097) on the activation of blood-isolated pDCs and the human GEN2.2 pDC cell line. Type I IFN production by pDCs correlates with differential interferon regulatory factor 7 (IRF7) translocation into the nucleus induced by the 2 TLR7 ligands. Surprisingly, with both activators we nevertheless observed the rapid expression of the IFN-inducible genes mxa, cxcl10, and trail within 4 hours of stimulation. This expression, controlled by STAT1 phosphorylation, was independent of type I IFN. STAT1 activation was found to be strictly dependent on the PI3K-p38MAPK pathway, showing a new signaling pathway leading to rapid expression of IFN-inducible genes after TLR7 triggering. Thus, pDCs, through this unusual TLR7 signaling, have the capacity to promptly respond to viral infection during the early phases of the innate immune response.
Introduction
Toll-like receptors (TLRs) contain a leucine-rich repeat ectodomain that enables the recognition of pathogen-associated molecular patterns.1 Among the 10 TLRs identified in humans, TLR7 is the least studied. This TLR binds single-stranded viral RNA, localizes to endosomal compartments, and is particularly expressed by plasmacytoid dendritic cells (pDCs).2 Ligand binding to TLR7 activates human pDCs that respond by either producing proinflammatory cytokines or substantial levels of type I IFN and activating specific T cells.3,–5 In addition, we have recently shown that Flu- and TLR7 agonist–activated pDCs express TNF-related apoptosis-inducing ligand (TRAIL). This renders them capable of direct cytotoxic activity toward infected and tumor cells.6 This has been observed in vivo: Stary et al7 describe the presence of TRAIL-expressing and IFN-α–producing pDCs in tumors after topical use of the TLR7 ligand imiquimod in basal cell carcinoma treatment. Taken together, these data suggest that pDCs represent key target cells in the striking antitumor effects observed with synthetic TLR7 agonists, such as imidazoquinolines, in the treatment of cutaneous virus–induced neoplasia (eg, condyloma-HPV),8 and other skin tumors, such as basal cell carcinoma.9
The signaling pathways triggered by TLR7 activation have been partially described. After ligand recognition, endosomal TLR7 interacts with the key adaptor molecule MyD88 (myeloid differentiation primary-response gene 88) that recruits a signal complex comprising IRAK1 (interleukin-1 receptor-associated kinase 1), IRAK4, and TRAF6 (tumor necrosis factor receptor–associated factor 6). TLR7 triggering leads to the activation of NF-κB (nuclear factor-κB) and MAPKs (mitogen-activated protein kinases), inducing the expression of proinflammatory cytokines and costimulatory molecules and the translocation of IRF7 (interferon-regulatory factor 7) into the nucleus, where it can induce the transcription of type I IFN genes.10 Agonistic engagement of TLR7 also leads to the expression of the IFN-inducible genes mxa, cxcl10, and trail.11 A type I IFN autocrine loop is thought to explain the expression of these genes, as has been described in mouse bone marrow–derived DCs.12 However, the precise pathway downstream of TLR7 leading to human pDC activation remains to be determined.
In this study we analyzed the activation profile and signaling events triggered by 2 TLR7 ligands: influenza virus (Flu; natural ligand) and CL097 (synthetic agonist). We studied the effects of these 2 ligands on both blood-isolated pDCs and the pDC model cell line GEN2.2. Results of these studies show the existence of a novel pathway downstream of TLR7 involving early STAT1 phosphorylation and expression of IFN-inducible genes in the absence of type I IFN.
Methods
Antibodies, flow cytometry
Surface or intracellular phenotype was determined by flow cytometry on a FACSCanto II (Becton Dickinson), using specific antibodies, by direct or indirect labeling. The following antibodies were from Immunotech (Beckman Coulter): PE-conjugated anti-CD40 (mAb89), PE-conjugated anti-CD80 (mAb104), and PE-conjugated goat anti–mouse IgG (H+L). PE-conjugated anti-CD123 (9F5) was purchased from PharMingen. PE-conjugated antiphospho-STAT1 (4a/ pY701-stat1) was from BD (Becton Dickinson). FITC-conjugated anti–BDCA-2 (AC144) and FITC-conjugated anti–IFN-α (LT27:295) were from Miltenyi Biotec, and purified anti-TRAIL (2E5) from was from Alexis.
Cells
Normal pDCs were isolated from peripheral blood mononuclear cells (PBMCs) of healthy volunteers with a BDCA-4 cell isolation kit (Miltenyi Biotec). Their purity, as determined with anti–BDCA-2 and anti-CD123 mAbs, was approximately 80% (Figure 1A).
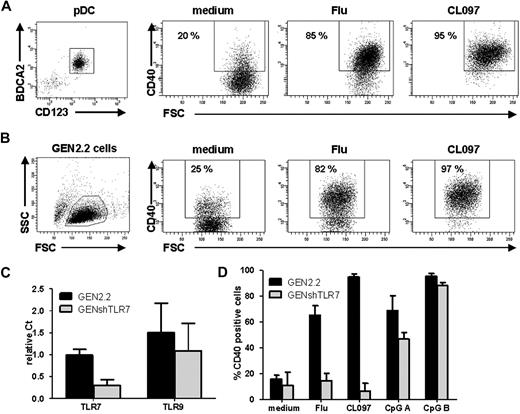
Influenza virus and CL097 induce human pDC activation by triggering TLR7. (A, left) Flow cytometric evaluation of purity of unstimulated pDCs enriched from healthy donor blood labeled with anti-BDCA2 and anti-CD123 antibodies. Cells were untreated (medium) or stimulated with UV-formol–inactivated influenza virus (Flu) or synthetic TLR7 ligand (CL097). Expression of CD40 was measured by flow cytometry on CD123+ cells. Dot plots are shown, the percentage of CD40+ cells is indicated on each plot. Results shown are representative of 4 independent experiments. (B) GEN2.2 cells were untreated or stimulated with Flu or CL097 for 24 hours. CD40 expression was evaluated on forward scatter (FSC)/side scatter (SSC)–gated live cells by flow cytometry. Percentages indicated on dot correspond to the proportion of CD40+ cells. Results shown are representative of at least 5 independent experiments. (C) Expression levels of TLR7 and TLR9 in the GEN2.2 cell line and in lentiviral shRNA TLR7-transfected GEN2.2 cells (GENshTLR7) measured by real-time PCR. Expression levels are normalized to G6PDH. Data are shown as the mean and SD from duplicate values of 2 independent experiments. (D) GEN2.2 and GENshTLR7 cells were untreated or stimulated with Flu, CL097, or 2 different synthetic TLR9 ligands (CpG A and CpG B) for 24 hours. Expression of CD40 was measured by flow cytometry. The mean percentages and SDs from duplicate values of 3 independent experiments are shown.
Influenza virus and CL097 induce human pDC activation by triggering TLR7. (A, left) Flow cytometric evaluation of purity of unstimulated pDCs enriched from healthy donor blood labeled with anti-BDCA2 and anti-CD123 antibodies. Cells were untreated (medium) or stimulated with UV-formol–inactivated influenza virus (Flu) or synthetic TLR7 ligand (CL097). Expression of CD40 was measured by flow cytometry on CD123+ cells. Dot plots are shown, the percentage of CD40+ cells is indicated on each plot. Results shown are representative of 4 independent experiments. (B) GEN2.2 cells were untreated or stimulated with Flu or CL097 for 24 hours. CD40 expression was evaluated on forward scatter (FSC)/side scatter (SSC)–gated live cells by flow cytometry. Percentages indicated on dot correspond to the proportion of CD40+ cells. Results shown are representative of at least 5 independent experiments. (C) Expression levels of TLR7 and TLR9 in the GEN2.2 cell line and in lentiviral shRNA TLR7-transfected GEN2.2 cells (GENshTLR7) measured by real-time PCR. Expression levels are normalized to G6PDH. Data are shown as the mean and SD from duplicate values of 2 independent experiments. (D) GEN2.2 and GENshTLR7 cells were untreated or stimulated with Flu, CL097, or 2 different synthetic TLR9 ligands (CpG A and CpG B) for 24 hours. Expression of CD40 was measured by flow cytometry. The mean percentages and SDs from duplicate values of 3 independent experiments are shown.
The pDC cell line GEN2.2 was grown in complete medium (RPMI 1640 Glutamax; GibcoBRL) supplemented with 1 mM sodium pyruvate, 20 μg/mL gentamicin, nonessential amino acids) to which 10% heat-inactivated fetal calf serum (Gibco) was added.
Generation of lentiviral shRNA TLR7-transfected GEN2.2 cells
GEN2.2 cells were transfected with MISSION Lentiviral transduction particles targeting TLR7 (NM_016562) Clone ID: TRCN0000056975 (Sigma-Aldrich) at MOI 2. Transfected cells were maintained in the presence of 10 μg/mL puromycin for 2 weeks to allow the selection of resistant clones that we called GENshTLR7 cells. The level of TLR7 mRNA expression in GENshTLR7 cells was assessed by real-time polymerase chain reaction (PCR). Silencing was found to diminish TLR7 mRNA expression by 80%.
Activation of GEN2.2 cells
Cells were cultured at 106 cells/mL in complete medium with 10% fetal calf serum. Cells were stimulated with either 640 UHA/mL UV-formol–inactivated influenza virus strain A/H3N2/Wisconsin/67/05 (Sanofi Pasteur) or 1 μg/mL CL097 (TLR7/8 ligand; Invivogen) or 10 μg/mL CpG-A ODN 2336 (TLR9 ligand; Coley Pharmaceuticals) or 10 μg/mL CpG-B ODN 2216 (TLR9 ligand; Invivogen) or 50 000 U/mL human recombinant IFN-α (PeproTech). For some experiments, blocking anti–IFN-α (50 000 U/mL; PBL Medical Laboratories), anti–IFN-β (25 000 U/mL; PBL Medical Laboratories), and anti–IFN-α/βR2 (5 μg/mL; PBL Medical Laboratories) or inhibitors from Calbiochem were added: 5 μM BAY11-7082, 5μM BMS-345541, 10 μM LY-294002, or 50 μM SB203580. After stimulation, phenotypic analyses were performed by flow cytometry. Culture supernatants were cryopreserved for cytokine measurements. These supernatants were tested for IFN-α content by enzyme-linked immunoabsorbent assay (PBL Medical Laboratories) and for IL-6, IL-8, TNF-α, and CXCL10 by Cytometric Bead Array multiplex (CBA; BD Biosciences).
Protein extraction and signaling factor analysis
After stimulation of GEN2.2 cells, cytosolic and nuclear fractions were extracted with the use of the protein extraction kit from Active Motif. Nuclear extracts were probed for NF-κB subunits c-Rel, p50, p52, p65, and RelB content with the use of the TransAM NF-κB family kit (Active Motif). Whole-cell extracts were used for quantification of phospho-STAT1 and phospho-p38MAPK by CBA multiplex (BD Biosciences).
Western blot analysis
After activation, GEN2.2 cells were washed in phosphate-buffered saline (PBS), lysed in 100 mL sample buffer, and heated at 100°C for 5 minutes. Whole-cell extract (20 μg) was loaded onto a 12% SDS–polyacrylamide gel. After electrophoresis, proteins were transferred to a PVDF membrane (Bio-Rad). Nonspecific binding sites were blocked with 5% nonfat milk in PBS Tween20 0.1%. Membranes were then incubated with primary antibodies: antiphospho-STAT1 (4a/pY701), antiphospho-STAT2 (7a/pY; PharMingen), and anti-actin (Sigma-Aldrich). Antibody labeling was shown with horseradish peroxidase–conjugated secondary antibodies (Dako) and was visualized with enhanced chemiluminescence (Amersham Life Science).
Immunofluorescence
Twenty-one–well Teflon-printed slides (Immuno-Cell International) were coated with 1 μg/mL poly-l-lysine (Sigma-Aldrich). GEN2.2 cells (104) were added to each well and allowed to adhere for 1 hour. Cells were then stimulated for 2 and 3 hours with 640 UHA/mL UV-formol–inactivated influenza virus strain A/H3N2/Wisconsin/67/05, 1 μg/mL CL097, or 5000 U/mL human recombinant IFN-α. After activation, medium was removed, slides were fixed in −20°C methanol for 10 minutes and dried at room temperature for 1 hour. Slides were rehydrated in PBS for 10 minutes and then incubated with primary antibody anti-IRF7 (H-246; Santa Cruz Biotechnology). Antibody labeling was revealed with the use of FITC-conjugated goat anti–rabbit secondary antibody (PharMingen). Cells were also colored with 1 mg/mL Evans blue (Sigma-Aldrich) before visualization by fluorescence microscopy. Cells were observed with a Zeiss HBO 50/AC microscope with lens 63×/1.25 oil iris, and images were acquired with a Zeiss MC 80 DX camera by the image-acquisition software Axiovision 4 Version 4.0.1.0.
Quantitative reverse transcription PCR
Total RNA was isolated from stimulated GEN2.2 cells with the use of RNeasy kit (QIAGEN). Reverse transcription to cDNA was carried out by standard methods with reverse transcriptase (Roche Diagnostics) and dNTP (Roche). These cDNAs were amplified with naked primers, LightCycler TaqMan Master mix, and Universal ProbeLibrary probe (Roche). PCRs were conducted in a LightCycler instrument (Roche). Primers were synthesized by Roche; their sequences were as follows (listed 5′-3′): G6PDH (forward, AACAGAGTGAGCCCTTCTTCA; reverse, GGAGGCTGCATCATCGTACT); human TLR7 (forward, GCTAGACTGTCTCAAAAGAACAAAAA; reverse, GCCCACACTCAATCTGCAC); human TLR9 (forward, ATAGCCGTGAGCCGGAAT; reverse, GCAGGCAGAGGTGAGGTG); IFN-α1 (forward, CCCTCTCTTTATCAACAAACTTGC; reverse, TTGTTTTCATGTTGGACCAGA); IFN-α2 (forward, TCCTGCTTGAAGGACAGACA; reverse, TTTCAGCCTTTTGGAACTGG); IFN-β1 (forward, CTTTGCTATTTTCAGACAAGATTCA; reverse, GCCAGGAGGTTCTCAACAAT); IFN-ω1 (forward, ACCAGCTATAGCCCTGTTGG; reverse, AAGTAGGCCATGGTTCTGAGG); MxA (forward, TCCAGCCACCATTCCAAG; reverse, CAACAAGTTAAATGGTATCACAGAGC). Relative threshold cycle values for each gene were normalized to the housekeeping gene G6PDH with the use of the equation 2(−dCp), where Cp is the mean cross point of duplicate runs calculated by Lightcycler software and dCp = gene Cp − G6PDH Cp.
Results
Influenza virus and CL097 induce human pDC activation via TLR7
This study focuses on TLR7 signaling in pDCs. The recently developed human pDC cell line, GEN2.2, was used as a model of pDCs.13,14 We evaluated 2 kinds of TLR ligands: CL097, an imidazoquinoline15,16 and influenza virus (Flu).17 We first verified their activity and their specificity for TLR7. After Flu or CL097 stimulation, both enriched primary pDCs (Figure 1A), and the GEN2.2 cells (Figure 1B) displayed the same activation profile with a high increase of CD40 expression; thus, both ligands were capable of inducing pDC activation. To verify the TLR7 specificity of the effect, TLR7 shRNA lentiviral transfection of GEN2.2 cells was performed, stably transfected cells were selected leading to the establishment of the GENshTLR7 cell line. These cells showed an 80% decrease in TLR7 mRNA expression, whereas levels of TLR9 mRNA were unchanged (Figure 1C). On Flu or CL097 stimulation, GENshTLR7 cells were unable to up-regulate CD40 but maintained their capacity to respond to TLR9 ligands (CpG A and B; Figure 1D). Moreover, GENshTLR7 cells produced neither IFN-α nor inflammatory cytokines after stimulation (data not shown). Control shRNA-transfected cells displayed the same activation profile as untransfected GEN2.2 cells, confirming that the previous results were not attributable to an alteration caused by lentiviral transfection (data not shown). Thus, both Flu and CL097 act specifically on TLR7 to induce CD40 up-regulation and cytokine production. Altogether, these data show that Flu and CL097 activate human pDCs by triggering TLR7.
Influenza virus and CL097 induce differential activation of pDCs
pDCs are known to secrete proinflammatory cytokines or substantial amounts of type I IFN on activation.3,4 The nature of the cytokines produced by GEN2.2 cells after activation by the 2 TLR7 ligands was evaluated. CL097-stimulated GEN2.2 cells produced the proinflammatory cytokines IL-6, IL-8, and TNF-α, whereas Flu-activated cells mainly secreted IFN-α (Figure 2A). To further characterize this secretion, the transcription of 4 type I IFN genes was assessed. Only Flu activation induced the transcription of ifn-α1, ifn-α2, ifn-β1, and ifn-ω1 genes in GEN2.2 cells, whereas CL097 activation did not result in transcription of any of the IFN genes tested (Figure 2B). These results were confirmed with human blood pDCs. A higher number of IFN-α–secreting cells were observed after Flu activation (56%) than after CL097 stimulation (7%; Figure 2C). Thus, the 2 TLR7 ligands chosen induced different activation profiles for pDCs.
Differential pDC maturation is triggered by different TLR7 ligands. GEN2.2 cells were untreated or stimulated with Flu or CL097 for 24 hours. (A) Production of proinflammatory cytokines was measured in culture supernatants by enzyme-linked immunoabsorbent assay and CBA. The mean and SD from duplicate values of 3 independent experiments are shown. (B) After 24 hours of culture, RNA was extracted, and RNA expression levels of type I IFNs: IFN-α1, -α2, -β1, and -ω1 were measured by quantitative PCR. Data shown are normalized to G6PDH and are representative of 2 independent experiments. (C) PBMCs from healthy donors were cultured in the absence or presence of Flu or CL097 for 3 hours. Secretion was blocked by adding brefeldin A for a further 4 hours. Intracellular IFN-α production was measured in HLA-DR+ BDCA4+ pDCs by flow cytometry. Dot plots show the percentage of IFN-α–producing cells among pDCs. Representative results from 3 different donors are shown. (D) GEN2.2 cells were cultured in the absence or presence of Flu or CL097 for 3 hours. Cells were lysed and proteins were extracted. The different NF-κB subunits were quantified in nuclear fractions with the use of the TransAM kit. The mean OD and SD from duplicate values of 3 independent experiments are shown. (E) GEN2.2 cells were cultured in the absence or presence of Flu or CL097 for 3 hours. Cells were immunostained for IRF7 and Evans blue colored. Immunofluorescence was assessed by microscopy. Representative images from 3 independent experiments are shown.
Differential pDC maturation is triggered by different TLR7 ligands. GEN2.2 cells were untreated or stimulated with Flu or CL097 for 24 hours. (A) Production of proinflammatory cytokines was measured in culture supernatants by enzyme-linked immunoabsorbent assay and CBA. The mean and SD from duplicate values of 3 independent experiments are shown. (B) After 24 hours of culture, RNA was extracted, and RNA expression levels of type I IFNs: IFN-α1, -α2, -β1, and -ω1 were measured by quantitative PCR. Data shown are normalized to G6PDH and are representative of 2 independent experiments. (C) PBMCs from healthy donors were cultured in the absence or presence of Flu or CL097 for 3 hours. Secretion was blocked by adding brefeldin A for a further 4 hours. Intracellular IFN-α production was measured in HLA-DR+ BDCA4+ pDCs by flow cytometry. Dot plots show the percentage of IFN-α–producing cells among pDCs. Representative results from 3 different donors are shown. (D) GEN2.2 cells were cultured in the absence or presence of Flu or CL097 for 3 hours. Cells were lysed and proteins were extracted. The different NF-κB subunits were quantified in nuclear fractions with the use of the TransAM kit. The mean OD and SD from duplicate values of 3 independent experiments are shown. (E) GEN2.2 cells were cultured in the absence or presence of Flu or CL097 for 3 hours. Cells were immunostained for IRF7 and Evans blue colored. Immunofluorescence was assessed by microscopy. Representative images from 3 independent experiments are shown.
The transcription factors NF-κB and IRF7 play essential roles in inducing the expression of proinflammatory cytokines and type I IFN, respectively. Therefore, we examined the translocation of these 2 factors into the nucleus after TLR7 triggering. Both Flu and CL097 induced similar nuclear translocation of p50 and p65 subunits of the canonical NF-κB dimer (Figure 2D). The quantities of p52 and RelB subunits of the noncanonical NF-κB pathway were not modified during activation. Interestingly, the NF-κB c-Rel subunit was detectable only in the nucleus of the CL097-stimulated GEN2.2 cells (Figure 2D). In contrast, IRF7 nuclear translocation was observed only in Flu-activated GEN2.2 cells (Figure 2E). This result corroborates the type I IFN production profile described in the second paragraph. These data suggest that, although both ligands induce inflammatory cytokine secretion via the NF-κB pathway, Flu but not CL097 induces IFN-α secretion after IRF7 translocation.
IFN-inducible genes are expressed after TLR7 triggering in a type I IFN–independent manner
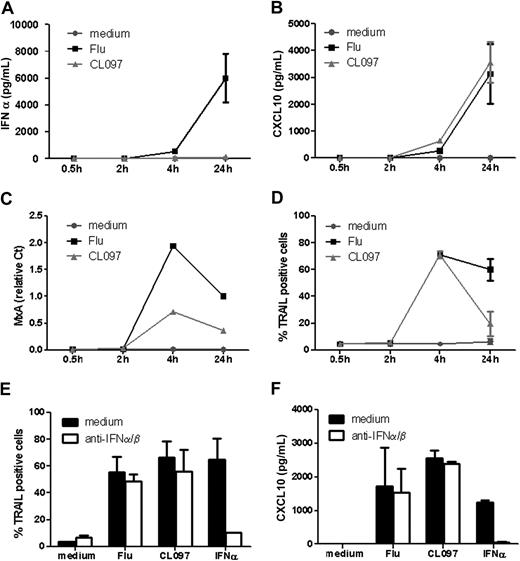
Secretion of type I IFNs by pDCs leads to the expression of a group of IFN-inducible genes, including mxa, cxcl10, and trail.18,–20 We next examined whether the differential type I IFN secretion observed after TLR7 triggering could induce differential expression of these IFN-inducible genes. IFN-α secretion was observed within 4 hours after TLR7 stimulation by Flu, whereas no detectable IFN-α was measured at any time point (up to 24 hours) after CL097 activation (Figure 3A). Surprisingly, the IFN-inducible gene products were nevertheless detected within 4 hours after activation by both TLR7 ligands (Figure 3B-D). The expression profiles were identical whatever the ligand applied: expression peaked at 4 hours and decreased at 24 hours for MxA and TRAIL gene products (Figure 3C-D), whereas CXCL10 production continuously increased up to and beyond 24 hours (Figure 3B). This suggests that, in our model, the induction of MxA, CXCL10, and TRAIL could be independent of IFN-α.
TLR7 triggers expression of IFN-inducible genes in a type I IFN–independent manner. GEN2.2 cells were untreated or stimulated with Flu or CL097 for different times. Supernatants were collected for evaluation of (A) IFN-α and (B) CXCL10 production. (C) RNA was extracted for measurement of MxA transcription levels by real-time PCR. (D) TRAIL expression was evaluated by flow cytometry. GEN2.2 cells were untreated or stimulated with Flu or CL097 in the absence or presence of anti–IFN-α/β and anti–IFN-α/βR neutralizing antibodies for 4 hours. (E) TRAIL expression was evaluated by flow cytometry, and (F) CXCL10 production was measured in culture supernatants by CBA. Data shown are the means and SDs from duplicate values from 2 independent experiments except data in panel C, which are representative of 2 independent experiments.
TLR7 triggers expression of IFN-inducible genes in a type I IFN–independent manner. GEN2.2 cells were untreated or stimulated with Flu or CL097 for different times. Supernatants were collected for evaluation of (A) IFN-α and (B) CXCL10 production. (C) RNA was extracted for measurement of MxA transcription levels by real-time PCR. (D) TRAIL expression was evaluated by flow cytometry. GEN2.2 cells were untreated or stimulated with Flu or CL097 in the absence or presence of anti–IFN-α/β and anti–IFN-α/βR neutralizing antibodies for 4 hours. (E) TRAIL expression was evaluated by flow cytometry, and (F) CXCL10 production was measured in culture supernatants by CBA. Data shown are the means and SDs from duplicate values from 2 independent experiments except data in panel C, which are representative of 2 independent experiments.
Very low levels of type I IFN could be responsible for inducing the expression of IFN-inducible genes after CL097 stimulation of GEN2.2 cells. To investigate this possibility, GEN2.2 cells were stimulated by the 2 TLR7 ligands in the presence or absence of neutralizing anti–IFN-α/β and anti–IFN-α/βR antibodies. In accordance with the absence of any detectable IFN-α production, the blocking of type I IFN signaling had no effect on TRAIL and CXCL10 expression induced by TLR7 ligands (Figure 3E-F). The preincubation of cells with the neutralizing antibodies for 30 or 60 minutes before ligand exposure to exclude the involvement of any autocrine intracellular type I IFN pathway had no effect on these expressions (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, the neutralizing antibodies could block both TRAIL and CXCL10 expression induced by exogenous IFNα/β. Altogether, these data show that expression of IFN-inducible genes could be triggered independently of type I IFN within 4 hours after TLR7 triggering.
Expression of IFN-inducible genes is independent of the NF-κB pathway and extracellular factors
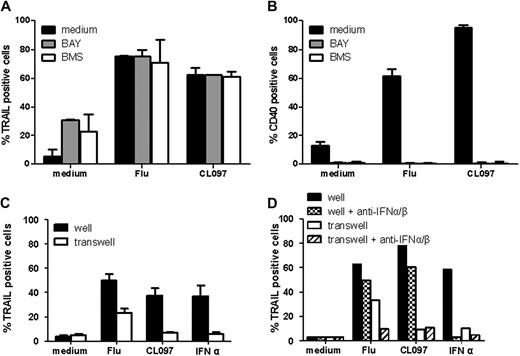
To analyze whether NF-κB was involved in the observed expression of IFN-inducible genes, we measured TRAIL expression in GEN2.2 cells 4 hours after activation by Flu and CL097 in the presence of the I-κB kinase inhibitors BAY11-708221 and BMS-345541.22 Neither of these inhibitors altered the TRAIL expression profile (Figure 4A), whereas they both abrogated NF-κB–dependent CD40 up-regulation (Figure 4B). These results indicate that the expression of IFN-inducible genes after TLR7 triggering is independent of NF-κB activation.
Expression of IFN-inducible genes is independent of the NF-κB pathway and of extracellular factors. GEN2.2 cells were untreated or stimulated with Flu or CL097 in the absence or presence of the NF-κB inhibitors BAY and BMS. TRAIL (A) and CD40 (B) expressions were evaluated by flow cytometry after 4 hours and 24 hours of culture, respectively. The mean percentages and SDs from duplicate values of 4 independent experiments are shown. (C) GEN2.2 cells were untreated or stimulated for 2 hours. After washing, cells were placed in 24-well plates; untreated cells were added in transwells. After 4 hours of culture, TRAIL expression was evaluated by flow cytometry on cells in wells and transwells. Data shown are the means and SDs from duplicate values of 3 independent experiments. (D) GEN2.2 cells were untreated or stimulated for 2 hours in the absence or presence of anti–IFN-α/β and anti–IFN-α/βR neutralizing antibodies. After washing, cells were placed in 24-well plates; untreated cells were added in transwells. After 4 hours of culture, TRAIL expression was evaluated by flow cytometry on cells in wells and transwells. Data from 1 representative experiment are shown.
Expression of IFN-inducible genes is independent of the NF-κB pathway and of extracellular factors. GEN2.2 cells were untreated or stimulated with Flu or CL097 in the absence or presence of the NF-κB inhibitors BAY and BMS. TRAIL (A) and CD40 (B) expressions were evaluated by flow cytometry after 4 hours and 24 hours of culture, respectively. The mean percentages and SDs from duplicate values of 4 independent experiments are shown. (C) GEN2.2 cells were untreated or stimulated for 2 hours. After washing, cells were placed in 24-well plates; untreated cells were added in transwells. After 4 hours of culture, TRAIL expression was evaluated by flow cytometry on cells in wells and transwells. Data shown are the means and SDs from duplicate values of 3 independent experiments. (D) GEN2.2 cells were untreated or stimulated for 2 hours in the absence or presence of anti–IFN-α/β and anti–IFN-α/βR neutralizing antibodies. After washing, cells were placed in 24-well plates; untreated cells were added in transwells. After 4 hours of culture, TRAIL expression was evaluated by flow cytometry on cells in wells and transwells. Data from 1 representative experiment are shown.
Extracellular factors could also contribute to the induction of expression of IFN-inducible genes. To rule out their involvement, TRAIL expression was measured on untreated GEN2.2 cells to which supernatants from TLR7-activated GEN2.2 cells were applied. No induction of TRAIL expression was observed in cells exposed to supernatants from CL097-activated cells (Figure 4C). The expression of TRAIL induced by the supernatant from Flu-activated cells was the result of the presence of secreted IFN-α in this supernatant. This expression could be blocked with the use of neutralizing anti–IFN-α/β antibodies (Figure 4D). These latter results show that expression of IFN-inducible genes after TLR7 triggering is caused by a previously undescribed intracellular signaling pathway.
Phosphorylation of STAT1 in response to TLR7 triggering depends on the PI3K-p38MAPK pathway
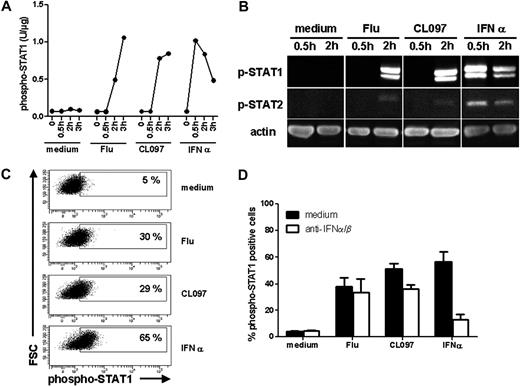
Expression of IFN-inducible genes is regulated by the transcription factor IFN-stimulated gene factor 3.23 IFN-stimulated gene factor 3 is in fact a complex of proteins, including STAT1, STAT2, and IRF9. We next examined whether this complex was activated in response to TLR7 triggering. Activation of GEN2.2 cells by both Flu and CL097 induced phosphorylation of STAT1 within 2 hours (Figure 5A-B) at levels comparable to the classic type I IFN activation (Figure 5B). Low-level phosphorylation of STAT2 was also observed (Figure 5B). In these analyses, no variation in total STAT protein quantity was observed (data not shown). These results were further confirmed by flow cytometric analysis. Results showing that STAT1 was phosphorylated in one-third of the GEN2.2 cells within 2 hours after TLR7 activation are presented in Figure 5C. The presence of neutralizing anti–IFN-α/β and anti–IFN-α/βR antibodies, which prevented IFN-α–induced STAT1 activation, had no effect on the level of STAT1 phosphorylation induced subsequent to TLR7 triggering (Figure 5D). Thus, STAT1 activation could also be induced via TLR7 signaling in a type I IFN–independent manner.
STAT1 is phosphorylated independently of type I IFN after TLR7 triggering with either ligand. GEN2.2 cells were untreated or stimulated with Flu, CL097, or IFN-α for 30 minutes, 2 hours, and 3 hours. Whole-cell protein extracts were prepared. (A) Phospho-STAT1 (pY701) was quantified in the protein extracts by CBA. Data shown are representative of 2 independent experiments. (B) Western blot analysis of phospho-STAT1 (pY701) and phospho-STAT2 (pY690) after activation of GEN2.2 cells. Data shown are representative of 2 independent experiments (C) After 2 hours of stimulation, cells were fixed and permeabilized for phospho-STAT1 (pY701) analysis by flow cytometry. Representative dot plots of 4 independent experiments are shown. (D) Activation of GEN2.2 cells in the absence or presence of anti–IFN-α/β and anti–IFN-α/βR neutralizing antibodies for 2 hours. Phospho-STAT1 (pY701) was analyzed by flow cytometry. Data shown are the means and SDs from duplicate values of 2 independent experiments.
STAT1 is phosphorylated independently of type I IFN after TLR7 triggering with either ligand. GEN2.2 cells were untreated or stimulated with Flu, CL097, or IFN-α for 30 minutes, 2 hours, and 3 hours. Whole-cell protein extracts were prepared. (A) Phospho-STAT1 (pY701) was quantified in the protein extracts by CBA. Data shown are representative of 2 independent experiments. (B) Western blot analysis of phospho-STAT1 (pY701) and phospho-STAT2 (pY690) after activation of GEN2.2 cells. Data shown are representative of 2 independent experiments (C) After 2 hours of stimulation, cells were fixed and permeabilized for phospho-STAT1 (pY701) analysis by flow cytometry. Representative dot plots of 4 independent experiments are shown. (D) Activation of GEN2.2 cells in the absence or presence of anti–IFN-α/β and anti–IFN-α/βR neutralizing antibodies for 2 hours. Phospho-STAT1 (pY701) was analyzed by flow cytometry. Data shown are the means and SDs from duplicate values of 2 independent experiments.
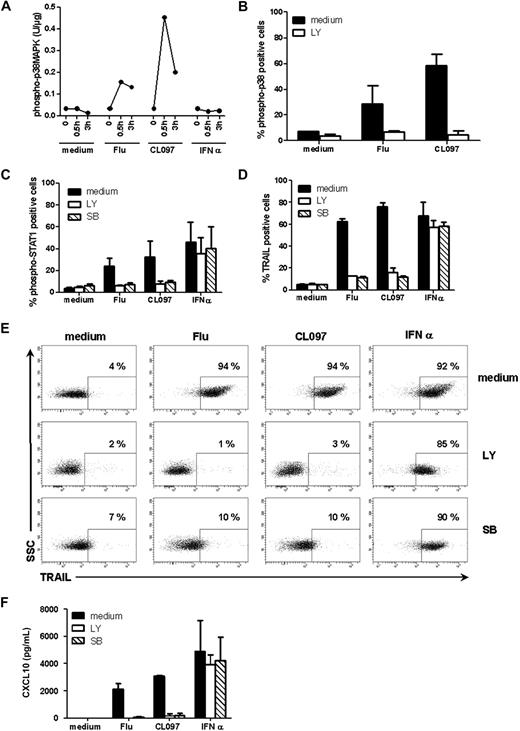
Other groups have suggested that STAT1 phosphorylation in response to TLR9 triggering depends on p38MAPK activation.24 To test this hypothesis in the TLR7 model, we analyzed the activation of p38MAPK in stimulated GEN2.2 cells. Phosphorylation of p38MAPK was observed within 30 minutes after stimulation of GEN2.2 cells by either Flu or CL097, but not after activation by type I IFN, as was expected (Figure 6A). Thus, phosphorylation of p38MAPK is induced by TLR7 stimulation. We next investigated the possible pathway linking TLR7 via p38MAPK to STAT1. We found PI3K, which has previously been linked to the TLR7 pathway,25 to have a role in this pathway. The PI3K inhibitor LY294002, in addition to altering the p38MAPK phosphorylation profile in response to TLR7 triggering (Figure 6B), also impaired STAT1 phosphorylation and TRAIL expression by GEN2.2 cells. A similar effect was observed in the presence of the specific26 p38MAPK inhibitor SB203580 (Figure 6C-D). These results were confirmed on blood-isolated pDCs after stimulation of cells with Flu or CL097 whereby the presence of LY or SB inhibited TRAIL expression (Figure 6E) and CXCL10 production (Figure 6F). Taken together, these data show that both STAT1 activation and the expression of IFN-inducible genes after TLR7 triggering depended on the PI3K-p38MAPK pathway.
STAT1 phosphorylation and expression of IFN-inducible genes after TLR7 triggering depends on the PI3K-p38MAPK pathway. GEN2.2 cells were untreated or stimulated with Flu, CL097, or IFN-α for 30 minutes or 3 hours. (A) Phospho-p38MAPK (pT180/pY182) was quantified on whole-cell lysates by CBA. Data shown are representative of 2 independent experiments. (B) GEN2.2 cells were untreated or stimulated with Flu, CL097, or IFN-α for 30 minutes in the absence or presence of the PI3K inhibitor LY. Cells were fixed and permeabilized for phospho-p38MAPK (pT180/pY182) analysis by flow cytometry. The mean percentages and SDs from duplicate values of 2 independent experiments are shown. (C) Phospho-STAT1 (pY701) and (D) TRAIL expression were analyzed by flow cytometry after 2 hours or 4 hours of stimulation of GEN2.2 cells with Flu, CL097, or IFN-α in the absence or presence of LY and the specific p38MAPK inhibitor SB203580 (SB). The mean percentages and SDs from duplicate values of 3 independent experiments are shown. Blood-isolated pDCs were unstimulated or stimulated with Flu or CL097 for 4 hours in the absence or presence of LY and SB. (E) Expression of TRAIL was evaluated by flow cytometry. Dot plots representative of 2 experiments are shown. The percentage of TRAIL-positive cells is indicated on each plot. (F) CXCL10 production was measured in cell culture supernatants by CBA. The mean and SD from duplicate values of 2 experiments performed with 2 different donors are shown.
STAT1 phosphorylation and expression of IFN-inducible genes after TLR7 triggering depends on the PI3K-p38MAPK pathway. GEN2.2 cells were untreated or stimulated with Flu, CL097, or IFN-α for 30 minutes or 3 hours. (A) Phospho-p38MAPK (pT180/pY182) was quantified on whole-cell lysates by CBA. Data shown are representative of 2 independent experiments. (B) GEN2.2 cells were untreated or stimulated with Flu, CL097, or IFN-α for 30 minutes in the absence or presence of the PI3K inhibitor LY. Cells were fixed and permeabilized for phospho-p38MAPK (pT180/pY182) analysis by flow cytometry. The mean percentages and SDs from duplicate values of 2 independent experiments are shown. (C) Phospho-STAT1 (pY701) and (D) TRAIL expression were analyzed by flow cytometry after 2 hours or 4 hours of stimulation of GEN2.2 cells with Flu, CL097, or IFN-α in the absence or presence of LY and the specific p38MAPK inhibitor SB203580 (SB). The mean percentages and SDs from duplicate values of 3 independent experiments are shown. Blood-isolated pDCs were unstimulated or stimulated with Flu or CL097 for 4 hours in the absence or presence of LY and SB. (E) Expression of TRAIL was evaluated by flow cytometry. Dot plots representative of 2 experiments are shown. The percentage of TRAIL-positive cells is indicated on each plot. (F) CXCL10 production was measured in cell culture supernatants by CBA. The mean and SD from duplicate values of 2 experiments performed with 2 different donors are shown.
Discussion
Many studies have suggested that the effects triggered by TLR7 ligands as adjuvants in cancer immunotherapy could be attributed to the activation of pDCs and their subsequent type I IFN production.7,27,28 To understand how agonists for the same receptor lead to different activated pDC phenotypes, we initiated a study of the signaling pathways induced in pDCs by 2 different TLR7 ligands. In this study we show that, in contrast to Flu activation, CL097 stimulation of pDCs does not induce production of high levels of IFN-α. However, we were able to show that both TLR7 ligands led to rapid expression of IFN-inducible genes via a type I IFN–independent pathway.
The data presented here show that Flu and CL097 both trigger human TLR7 but induce differential cytokine production. With the use of stably shRNA-transfected cells, GENshTLR7, we could show that human pDC activation by inactivated influenza virus required TLR7, as had been previously described in a mouse model.17 Type I IFN production after CL097 activation was found to be null or extremely low compared with the massive amounts of IFN-α produced in the presence of Flu. This result corroborated those concerning IRF7 translocation into the nucleus of pDCs after stimulation with the different ligands. This differential type I IFN production is reminiscent of results obtained with TLR9 ligands.29 Kerkmann et al29 have shown that TLR9 triggering with CpG A ODNs leads to a high production of type I IFN, whereas CpG B ODNs, although they bind TLR9, are unable to induce IFN secretion. The mechanism responsible for the differential behavior of pDCs after TLR9 triggering has been suggested to be the result of the spatiotemporal location of the ligand within the cell and its higher order structure.30 Indeed, multimeric CpG A ODNs are retained within endosomes, whereas monomeric CpG B ODNs traffic to lysosomal compartments where they trigger the activation of different signaling factors.31,32 Similarly, Heil et al33 observed that synthetic TLR7 ligands induce higher IFN-α production by PBMCs when complexed to the cationic lipid DOTAP.33 Thus, Flu's RNAs may follow the same cell trafficking as multimeric CpG A ODNs, whereas CL097 may rather stay in a monomeric structure such as CpG B, leading to a lysosomal location and absence of type I IFN production.
MxA, CXCL10, and TRAIL, through their role in the inhibition of virus replication, in the chemotaxis of immune cells, and in the apoptosis of transformed and infected cells, are key players in countering infection and cancers.34 Here, we have shown that these genes, which have been described to be tightly regulated by IFNs in various hematopoietic cells,23,35 are rapidly expressed in TLR7-activated pDCs in the absence of type I IFN production, even when type I IFN–receptor signaling was blocked with neutralizing anti–IFN-α/β and anti–IFN-α/βR antibodies. Although expression of the “IFN-inducible” genes was independent of IFN, it remained dependent on STAT1 phosphorylation on Tyr-701. This phosphorylation was found to be p38MAPK dependent in our model, a similar signaling pathway was also described in CpG A–activated pDCs.24 However, because p38MAPK is a serine kinase, known to phosphorylate STAT1 on Ser-727,36 this tyrosine phosphorylation of STAT1 must be mediated by an intermediate tyrosine kinase whose activation depends on p38MAPK. Both the Janus kinases 1 and 2 and c-Src are able to phosphorylate STAT1 on Tyr-701,23,37 but their activation is generally associated with cytokine receptors.38 In our model, we have shown that no soluble factors were involved. Therefore, the intermediate kinase responsible for STAT1 phosphorylation in response to TLR7 stimulation remains to be identified.
Here, we have shown that PI3K is involved in the activation of p38MAPK, whereas other groups have shown its implication in IRF7 translocation.25,39 Moreover, the NF-κB–dependent expression of CD80 and cytokine production were also impaired in the presence of LY after TLR7 triggering (data not shown), suggesting that PI3K is a key early signaling factor in the TLR7 pathway.
Altogether, exhaustive characterization of the signaling pathway downstream of TLR7 in pDCs led us to describe the expression of IFN-inducible genes in the absence of cytokine receptor engagement. This originality may confer on pDCs the capacity to respond more rapidly to viral infection, reinforcing their role in the early phases of the immune response. Finally, the work described here may help to define new therapeutic targets that could favor pDC activation in tumor and viral immunotherapy or to dampen it in pDC-driven autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by 2 grants from the Institut National du Cancer and the Cancéropôle Lyon- Auvergne Rhône-Alpes (ACI-63-04 and Cancéropôle 2004-5). J.D.D.'s PhD was financed by the Ministère de l'Education Nationale de la Recherche et de la Technologie. A.B. was the recipient of a grant from EFS. M.G.-G. received a Young Researcher's grant from Inserm.
Authorship
Contribution: J.D.D. performed the experiments, analyzed the results, made the figures, and wrote the paper; A.B. and J.-P.M. performed part of the experiments; M.G.-G., L.C., and J.P. proofread the manuscript; and J.D.D., L.C., and J.P. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurence Chaperot, R&D Laboratory, EFS Rhônes-Alpes, 29 Av Maquis du Gresivaudan, BP35 La Tronche, 38701 France; e-mail: laurence.chaperot@efs.sante.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal