Abstract
During platelet activation, phosphoinositide 3-kinases (PI3Ks) produce lipid second messengers participating in the regulation of functional responses. Here, we generated a megakaryocyte-restricted p110β null mouse model and demonstrated a critical role of PI3Kβ in platelet activation via an immunoreceptor tyrosine-based activation motif, the glyco-protein VI-Fc receptor γ-chain complex, and its contribution in response to G-protein–coupled receptors. Interestingly, the production of phosphatidylinositol 3,4,5-trisphosphate and the activation of protein kinase B/Akt were strongly inhibited in p110β null platelets stimulated either via immunoreceptor tyrosine-based activation motif or G-protein–coupled receptors. Functional studies showed an important delay in fibrin clot retraction and an almost complete inability of these platelets to adhere onto fibrinogen under flow condition, suggesting that PI3Kβ is also acting downstream of αIIbβ3. In vivo studies showed that these mice have a normal bleeding time and are not protected from acute pulmonary thromboembolism but are resistant to thrombosis after FeCl3 injury of the carotid, suggesting that PI3Kβ is a potential target for antithrombotic drugs.
Introduction
Platelet activation is a highly regulated process involving various signaling pathways initiated by specific receptors coupled to heterotrimeric G proteins (GPCRs), integrins, or immunoreceptor tyrosine-based activation motif (ITAM)–containing proteins. In all cases, key signaling enzymes, such as phospholipases C (PLC) and phosphoinositide 3-kinase (PI3K) isoforms, are activated. If the situation is clear for PLC (ie, the PLCβ isoforms are activated by heterotrimeric Gq proteins, whereas the γ isoforms are stimulated via tyrosine phosphorylation and ITAM signaling), the implication of the different PI3K isoforms downstream of the major platelet receptors is still poorly known. Class Ia PI3Ks (α,β,δ), composed of a catalytic subunit (p110) and a regulatory subunit, are classically activated by their association with phosphotyrosine residues containing sequences via the SH2 domains of their regulatory subunit.1 However, the p110β isoform may not follow this rule because its activation has been proposed to involve both Gβγ and phosphotyrosyl peptides.2-5 Using a selective inhibitor of p110β, Jackson et al6 have proposed a role of p110β in the regulation of αIIbβ3 integrin in a shear-dependent manner. Interestingly, this inhibitor prevented the formation of an occlusive thrombus generated in vivo. To firmly establish the role of the PI3Kβ in platelet activation and evaluate its impact on hemostasis in vivo, we created a mouse line in which this isoform has been inactivated by gene targeting selectively in the megakaryocyte lineage.
Methods
Materials
Collagen was from Nycomed, U46619 from QBiogen Inc; integrilin from Glaxo Group Ltd; p110α, β, γ, and δ antibodies from Santa Cruz Biotechnology; p85 antibody from Upstate Biotechnology; TGX-221 from Cayman Chemical; and other reagents from Sigma-Aldrich.
Animals
Generation of the mice model is described in the supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article). All mice experiments were approved by the IFR150-Toulouse Purpan institutional review board.
Preparation of murine platelets
Whole blood was drawn from the inferior vena cava of anesthetized mice into a syringe containing acid citrate dextrose (1 vol anticoagulant/9 vol blood). Platelets were then prepared as previously described7 and stimulated in the presence or absence of integrilin (40 μg/mL). Aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900 rpm.
In vitro PI3K assay
Lipid extraction and analysis
Platelets were labeled with 0.4 mCi/mL [32P]orthophosphate, stimulated, and their phosphoinositide content was analyzed as described previously.10
Clot retraction experiments
The platelet-rich plasma was obtained from pooled blood samples from several mice by centrifugation for 4 minutes at 250g at 37°C. Clot retraction studies were performed as described.11
Platelet interaction on immobilized fibrinogen under flow conditions
This technique is described in the supplemental Methods.
Ferric chloride carotid artery injury model of thrombosis
This technique is described in the supplemental Methods.
Statistical analysis
Statistical significance was analyzed using the unpaired Student test using Microsoft Excel 2007.
Results and discussion
Conditional genetic inactivation of p110β in the megakaryocytic lineage
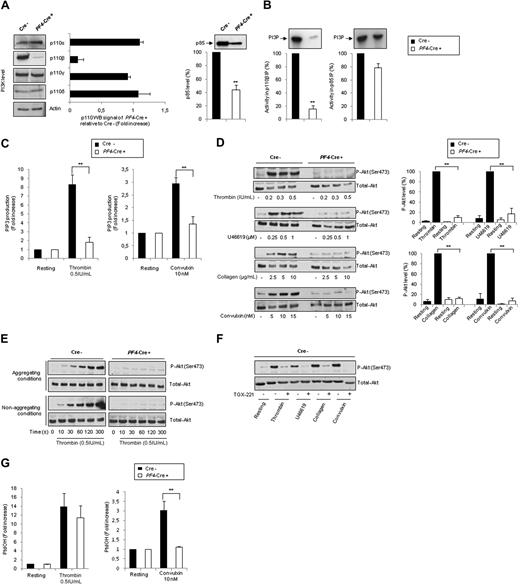
To determine the stability in platelets of p110β protein, which lacks the coding sequence of exons 21 and 22 of the kinase domain after Cre excision (p110Δ21,22 protein), PF4-Cre/p110βflox/flox and p110βflox/flox platelet lysates were analyzed by Western blotting. A severe p110β deficiency was found in PF4-Cre/p110βflox/flox platelets (12.5% ± 6% of control; Figure 1A), indicating that the protein is either not produced or unstable. As expected, this deficiency is restricted to platelets (supplemental Figure 1) and has no impact on the expression of other class I PI3K catalytic subunits (p110α, p110γ, and p110δ). A significant reduction (56% ± 7%) in the level of the regulatory subunit p85, probably resulting from an instability of the free p85 protein, was observed (Figure 1A).12 However, the PI3K activity associated to p85 was not significantly different (Figure 1B), indicating an efficient association with p110α and δ. Only a residual lipid-kinase activity was detected in p110β immunoprecipitates, whereas the other class I PI3K catalytic subunits were highly active (not shown). Thus, this mouse model is a conditional specific deletion of p110β in platelets.
Genetic inactivation of p110β catalytic subunit specifically in megakaryocytes and platelets. (A) Western blot showing class I PI3K p110 isoforms and p85 expression in PF4-Cre− p110flox/flox control (Cre−) or PF4-Cre+ p110flox/flox p110β-null (PF4-Cre+) platelet lysates. ■ represent (Cre−) platelet lysates; □, (PF4-Cre+) platelet lysates. Average results are mean ± SEM for 4 experiments (right panel). (B) p110β and p85 subunits were immunoprecipitated with specific antibodies, and the associated lipid kinase activity was assayed with phosphatidylinositol as a substrate. ■ represent (Cre−) platelet lysates; □, (PF4-Cre+) platelet lysates. Average results are mean ± SEM for 3 experiments. (C) Platelets from control or PF4-Cre/p110βflox/flox mice were stimulated under nonaggregating conditions by indicated agonists, and the radioactivity of PIP3 was determined as described in “Lipid extraction and analysis.” Results are mean ± SEM of 3 independent experiments. (D) Platelets were stimulated under aggregating conditions by thrombin, U46619, collagen, or convulxin during 7 minutes at the indicated concentration. Lysates were submitted to immunoblotting with anti–Akt-Ser(P)473 or total Akt antibodies, as indicated. Quantification by densitometric analysis of the Western blots is shown (right panels), and data are expressed as percentage of P-Akt in response to thrombin (0.5 IU/mL), U46619 (1μM), collagen (10 μg/mL), and convulxin (10nM), and are mean ± SEM of 3 independent experiments. (E) Platelets were stimulated under aggregating or nonaggregating (integrilin) conditions by thrombin (0.5 IU/mL) during different times and analyzed as in panel D. (F) Platelets were stimulated under aggregating conditions by thrombin, U46619, collagen, or convulxin in the absence or presence of TGX-221 (0.5μM) and analyzed as in panel D. (G) Platelets from p110flox/flox control (Cre−) or PF4-Cre/p110flox/flox (PF4-Cre+) mice were stimulated under nonaggregating conditions with indicated agonists, and the radioactivity of phosphatidic acid (PtdOH) was determined as described in “Lipid extraction and analysis.” Results are mean ± SEM of 3 independent experiments. Statistical analysis: **P < .01.
Genetic inactivation of p110β catalytic subunit specifically in megakaryocytes and platelets. (A) Western blot showing class I PI3K p110 isoforms and p85 expression in PF4-Cre− p110flox/flox control (Cre−) or PF4-Cre+ p110flox/flox p110β-null (PF4-Cre+) platelet lysates. ■ represent (Cre−) platelet lysates; □, (PF4-Cre+) platelet lysates. Average results are mean ± SEM for 4 experiments (right panel). (B) p110β and p85 subunits were immunoprecipitated with specific antibodies, and the associated lipid kinase activity was assayed with phosphatidylinositol as a substrate. ■ represent (Cre−) platelet lysates; □, (PF4-Cre+) platelet lysates. Average results are mean ± SEM for 3 experiments. (C) Platelets from control or PF4-Cre/p110βflox/flox mice were stimulated under nonaggregating conditions by indicated agonists, and the radioactivity of PIP3 was determined as described in “Lipid extraction and analysis.” Results are mean ± SEM of 3 independent experiments. (D) Platelets were stimulated under aggregating conditions by thrombin, U46619, collagen, or convulxin during 7 minutes at the indicated concentration. Lysates were submitted to immunoblotting with anti–Akt-Ser(P)473 or total Akt antibodies, as indicated. Quantification by densitometric analysis of the Western blots is shown (right panels), and data are expressed as percentage of P-Akt in response to thrombin (0.5 IU/mL), U46619 (1μM), collagen (10 μg/mL), and convulxin (10nM), and are mean ± SEM of 3 independent experiments. (E) Platelets were stimulated under aggregating or nonaggregating (integrilin) conditions by thrombin (0.5 IU/mL) during different times and analyzed as in panel D. (F) Platelets were stimulated under aggregating conditions by thrombin, U46619, collagen, or convulxin in the absence or presence of TGX-221 (0.5μM) and analyzed as in panel D. (G) Platelets from p110flox/flox control (Cre−) or PF4-Cre/p110flox/flox (PF4-Cre+) mice were stimulated under nonaggregating conditions with indicated agonists, and the radioactivity of phosphatidic acid (PtdOH) was determined as described in “Lipid extraction and analysis.” Results are mean ± SEM of 3 independent experiments. Statistical analysis: **P < .01.
PI3Kβ is critical for PIP3 production and PKB/Akt activation downstream of both GPCR and ITAM signaling
We then tested the impact of PI3Kβ on the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in response to agonists binding either GPCR, such as thrombin receptor, or receptors bearing ITAM motif, such as glycoprotein VI (GPVI)–Fc receptor γ-chain (FcRγ) complex. Collagen has 2 receptors: GPVI and α2β1 integrin. The GPVI-FcRγ complex, specifically stimulated by the snake venom toxin convulxin, activates cell signaling via tyrosine kinases.13-15 Surprisingly, the PIP3 production was inhibited in platelets stimulated either by thrombin or by convulxin showing that both GPCR and ITAM-bearing receptor signaling involves p110β to produce this lipid second messenger (Figure 1C). Accordingly, on stimulation with thrombin, thromboxane A2 analog (U46619), convulxin, or collagen, p110β-null platelets showed an almost complete inhibition of PKB/Akt phosphorylation compared with control platelets. This defect of activation of this PI3K effector16,17 persisted even with high doses of agonists (90% ± 4%, 83% ± 11%, 92.4% ± 6%, and 88% ± 2.5%, respectively; Figure 1D) and was not the result of a shift in the time course of activation or a defect in aggregation (Figure 1E). Consistent with this, the selective PI3Kβ inhibitor, TGX-221, strongly inhibited protein kinase B (PKB)/Akt-phosphorylation in wild-type platelets stimulated with the different agonists (Figure 1F), showing that the catalytic function of p110β is responsible for PKB/Akt activation. These findings reveal a crucial involvement for PI3Kβ in PKB/Akt activation in response to the major physiologic platelet agonists and that PI3Kγ, PI3Kα, or PI3Kδ, which are present and catalytically active in these platelets, are unable to take over this function of p110β. The PI3K isoform activated by GPVI18 is mainly p110β because stimulation of both PKB/Akt and PLCγ (as shown by the decrease in phosphatidic acid production, Figure 1G) was nearly abolished in p110β-null platelets challenged by convulxin.
Deletion of p110β affects functional platelet responses
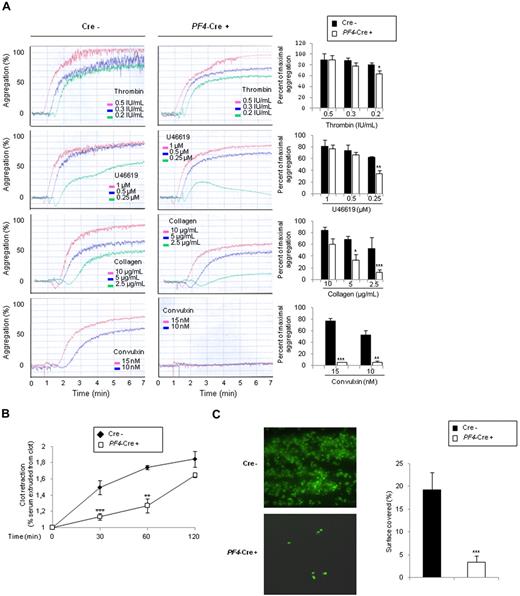
In contrast to control platelets, p110β-null platelets showed a slight defect in aggregation response to low concentration of thrombin and U46619. This defect was stronger in response to collagen (Figure 2A) and adenosine diphosphate (supplemental Figure 2B). Raising agonist concentrations increased aggregation but did not restore a normal response. Remarkably, p110β-null platelets were unable to aggregate on GPVI triggering by convulxin. Similar results were obtained with TGX-221-treated platelets (supplemental Figure 2A). These data suggest that platelet aggregation in response to GPCR agonists may be partially independent of PI3Kβ-mediated Akt activation, whereas PI3Kβ is mandatory for ITAM-mediated platelet activation. It is important to note that PI3Kβ is activated upstream of αIIbβ3-integrin engagement both in response to GPVI or GPCR triggering because blockage of αIIbβ3 by integrilin did not affect PIP3 production (Figure 1C) and Akt phosphorylation (not shown). However, evidence is accumulating that PI3Kβ is also acting downstream of αIIbβ3.19,20
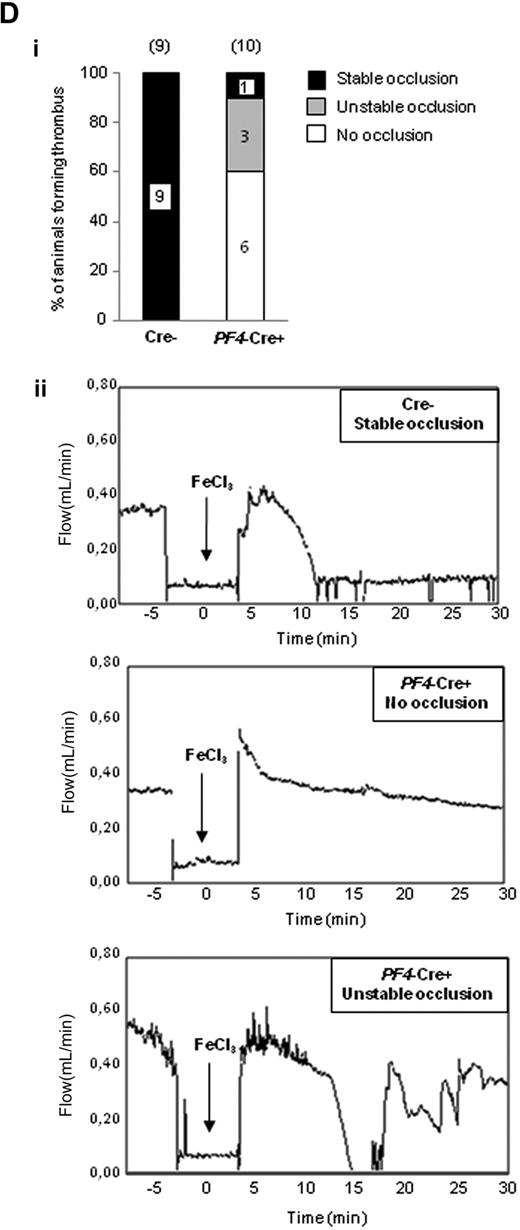
Role of PI3Kβ in functional platelet responses. (A) Role of PI3Kβ in promoting platelet aggregation. Platelets from p110flox/flox control (Cre−) or PF4-Cre/p110flox/flox (PF4-Cre+) mice were stimulated with thrombin, U46619, collagen, or convulxin, and aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900 rpm for 7 minutes. The profiles shown are representative of 5 independent experiments. Quantifications of the maximum of aggregation at 6 minutes are shown and are mean ± SEM of 5 independent experiments (right panels). (B) Role of PI3Kβ in promoting fibrin clot retraction. Photographs show the extent of clot retraction in wild-type and p110β-null platelet-rich plasma samples treated with 10 IU/mL thrombin. Quantification of the volume of serum extruded from the clot is shown. Results are the mean ± SEM of 3 independent experiments. (C) DIOC6-labeled platelets in whole blood from p110flox/flox control (Cre−) or PF4-Cre/p110flox/flox (PF4-Cre+) mice were perfused through fibrinogen-coated Bioflux plates at a shear rate of 1500 seconds for 5 minutes. Representative images at 5 minutes are shown. Images obtained with an epifluorescence microscope (Axiovert 200, Carl Zeiss; 40×/1.3 NA objective; 37°C) were captured with a CCD camera (CoolSnap HQ; Roper Scientific) and Metamorph software Version 6.2r6 (Universal Imaging Corp). Area covered by platelets was measured. Results shown are the mean ± SEM of 5 experiments. Statistical analysis: *P < .05; **P < .01; ***P < .005. (D) Thrombotic response of mice to ferric chloride injury of the carotid artery. Flow rates were measured in the carotid artery after exposure to 7% FeCl3 during 3 minutes. The experiment was stopped after 30 minutes. (i) For each genotype, the number of mice forming a stable occlusion is shown in black. The number of mice that formed an unstable occlusion that resolved is shown in gray. The number of mice that formed only a partial occlusion is shown in white. (ii) Representative flow traces for each case (stable occlusion, no occlusion, and unstable occlusion).
Role of PI3Kβ in functional platelet responses. (A) Role of PI3Kβ in promoting platelet aggregation. Platelets from p110flox/flox control (Cre−) or PF4-Cre/p110flox/flox (PF4-Cre+) mice were stimulated with thrombin, U46619, collagen, or convulxin, and aggregation was assessed using a Chrono-log dual-channel aggregometer under stirring at 900 rpm for 7 minutes. The profiles shown are representative of 5 independent experiments. Quantifications of the maximum of aggregation at 6 minutes are shown and are mean ± SEM of 5 independent experiments (right panels). (B) Role of PI3Kβ in promoting fibrin clot retraction. Photographs show the extent of clot retraction in wild-type and p110β-null platelet-rich plasma samples treated with 10 IU/mL thrombin. Quantification of the volume of serum extruded from the clot is shown. Results are the mean ± SEM of 3 independent experiments. (C) DIOC6-labeled platelets in whole blood from p110flox/flox control (Cre−) or PF4-Cre/p110flox/flox (PF4-Cre+) mice were perfused through fibrinogen-coated Bioflux plates at a shear rate of 1500 seconds for 5 minutes. Representative images at 5 minutes are shown. Images obtained with an epifluorescence microscope (Axiovert 200, Carl Zeiss; 40×/1.3 NA objective; 37°C) were captured with a CCD camera (CoolSnap HQ; Roper Scientific) and Metamorph software Version 6.2r6 (Universal Imaging Corp). Area covered by platelets was measured. Results shown are the mean ± SEM of 5 experiments. Statistical analysis: *P < .05; **P < .01; ***P < .005. (D) Thrombotic response of mice to ferric chloride injury of the carotid artery. Flow rates were measured in the carotid artery after exposure to 7% FeCl3 during 3 minutes. The experiment was stopped after 30 minutes. (i) For each genotype, the number of mice forming a stable occlusion is shown in black. The number of mice that formed an unstable occlusion that resolved is shown in gray. The number of mice that formed only a partial occlusion is shown in white. (ii) Representative flow traces for each case (stable occlusion, no occlusion, and unstable occlusion).
In agreement, we observed an important delay in fibrin clot retraction induced by p110β-null platelets, suggesting a role of this PI3K in organizing an efficient αIIbβ3-mediated contractility (Figure 2B). To better investigate the role of PI3Kβ in integrin αIIbβ3-dependent platelet adhesion, we performed flow-based adhesion assays over a fibrinogen matrix under arterial flow conditions using whole blood. Integrin αIIbβ3 outside-in signals stabilize and sustain αIIbβ3 adhesive bonds necessary for the maintenance of firm adhesion contacts under shear conditions.19,21 Our results show a critical role for PI3Kβ in the regulation of this process (Figure 2C). A role of PIP3 has been suggested in platelet adhesion to immobilized fibrinogen under flow.19 Here, we propose that the major PI3K involved in PIP3 production downstream of αIIbβ3 is the β isoform of class IA.
Consistent with our ex vivo data, during the reviewing of this study, a report highlighted the important role of PI3Kβ in mouse platelet regulation.20
Interestingly, our model of megakaryocyte/platelet-restricted p110β deficiency allowed us to investigate the role of this PI3K in vivo. Deletion of p110β did not significantly modify the bleeding time (2.6 ± 0.43 minutes for Cre− vs 3.40 ± 0.59 minutes for PF4-Cre+; supplemental Figure 3) and did not protect against acute thromboembolism induced by injection of a mixture of collagen and epinephrine (100% of mortality at 2 minutes for both Cre− and PF4-Cre+ on 0.3 mg/kg collagen and 60 μg/kg epinephrine). To investigate the relevance of PI3Kβ in pathologic occlusive thrombus formation in vivo, FeCl3 injury was induced on carotid artery and time to occlusion and blood flow were determined. Animals in which blood flow stopped, but then resumed, were scored as having an unstable thrombus. Whereas 100% of control mice presented a complete occlusion of the vessel 9 (± 1.1) minute after injury (mean occlusion time), 90% of megakaryocyte/platelets, -p110β null mice, partially occluded (17.95% ± 4.86% occlusion 30 minutes after injury); and among them, 30% presented an unstable thrombus. Only 10% of megakaryocyte/platelets, -p110β null mice, presented a complete occlusion (Figure 2D). These data suggest that PI3Kβ is critical for thrombus formation after arterial injury. This result is consistent with those from Jackson et al showing that inhibition of p110β by TGX-221 abolishes arterial occlusive thrombus formation in a modified “Folts-type” thrombosis model in rat.6 Altogether, these data suggest that PI3Kβ is a potentially interesting new target for antithrombotic drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Radeck Skoda for kindly providing us the PF4-Cre mice; the Zootechnie Core Facility (Aline Tridon and Maryline Calise) and the Transgenese Core Facility (Anne Huchenq-Champagne and Sylvie Pilipenko-Appolinaire) of IFR150; and Dr M. Beraud (Labtech-France) for his help with the BioFlux 200 system.
This work was supported by Inserm and ANR (program Jeunes Chercheurs, Jeunes Chercheuses; ANR-07-JCJC-0093-01). J.G.-G. was supported by Fondation pour la Recherche Médicale, France (FRM-SPE20051105175), EMBO (ALTF676-2005), and European Union (MEIF-CT-2006-039676).
Authorship
Contribution: V.M. and G.C. designed and performed most experiments and analyzed data; V.M., J.G.-G., B.V., and M.-P.G. produced the mice; C.C. performed carotid artery thrombosis; M.J.-P. purified Cvx; and M.P., B.P., and M.-P.G. designed research, supervised the work, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: B.V. is an advisor to Intellikine, San Diego. The remaining authors declare no competing financial interests.
Correspondence: Bernard Payrastre, Inserm U563, CHU Purpan, BP 3028, 31024 Toulouse Cedex 03, France; e-mail: bernard.payrastre@inserm.fr; or Marie-Pierre Gratacap, Inserm U563, CHU Purpan, BP 3028, 31024 Toulouse Cedex 03, France; e-mail: marie-pierre.gratacap@inserm.fr.
References
Author notes
*B.P. and M.-P.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal