Abstract
Placenta growth factor (PlGF) is released by immature erythrocytes and is elevated in sickle cell disease (SCD). Previous data generated in vitro suggest that PlGF may play a role in the pathophysiology of SCD-associated pulmonary hypertension (PHT) by inducing the release of the vasoconstrictor, endothelin-1. In this cross-sectional study of 74 patients with SCD, we confirm that PlGF is significantly elevated in SCD compared with healthy control subjects. We found significantly higher levels of PlGF in SCD patients with PHT but observed no association of PlGF with the frequency of acute pain episodes or history of acute chest syndrome. The observed correlation between PlGF and various measures of red cell destruction suggests that hemolysis, and the resultant erythropoietic response, results in the up-regulation of PlGF. Although relatively specific, PlGF, as well as N-terminal pro-brain natriuretic peptide and soluble vascular cell adhesion molecule, has low predictive accuracy for the presence of PHT. Prospective studies are required to conclusively define the contribution of PlGF to the pathogenesis of PHT and other hemolytic complications in SCD.
Introduction
Patients with sickle cell disease (SCD) exhibit elevated leukocyte counts and abnormal activation of granulocytes, monocytes, and endothelial cells.1-3 They also manifest increased thrombin and fibrin generation,4,5 increased tissue factor procoagulant activity,6,7 and increased platelet activation even when they are in the noncrisis, steady state.5,8,9 Furthermore, increased levels of multiple inflammatory mediators are observed in patients with SCD.10,11 Indeed, the baseline leukocyte count is a strong independent risk factor for disease severity. Leukocytosis is a risk factor for increased mortality,12 acute chest syndrome, hemorrhagic stroke,13 and vasoocclusive crises.14,15 As a result, SCD is increasingly referred to as a chronic inflammatory state.16
Placenta growth factor (PlGF), an angiogenic growth factor belonging to the vascular endothelial growth factor (VEGF) family,17,18 is produced not only by placental trophoblasts and umbilical vein endothelial cells during pregnancy but also by maturing erythroblasts.19 Plasma levels of PlGF are higher in patients with SCD than in healthy control subjects and have been reported to correlate with the frequency of acute pain episodes.20 Plasma levels of PlGF may also increase during acute pain episodes in SCD.21 The higher PlGF levels in patients with SCD may be due to hypoxia,22 increased erythropoiesis,19 and increased erythropoietin concentrations that follow anemia in these patients.20 Although PlGF binds to the VEGF-1 class of receptors,23,24 this cytokine elicits its own unique proinflammatory and arteriogenic effects.25 Perelman et al20 have demonstrated ex vivo that PlGF activates monocytes and promotes the release of interleukin-1β, interleukin-8, monocyte chemoattractant protein-1, and VEGF from these cells. PlGF also induces leukotriene formation in SCD.26 These data suggest a clinical role for PlGF in inflammation in SCD.
PlGF may play a role in pulmonary hypertension (PHT) in SCD. Data generated in vitro suggest that PlGF induces the release of the vasoconstrictor, endothelin-1 (ET-1) from pulmonary microvascular endothelial cells.27 In addition, treatment of these cells with PlGF induced expression of the endothelin B receptor, suggesting that PlGF may contribute to the pathogenesis of SCD-associated PHT. Furthermore, the arteriogenic effects of PlGF suggests a role for this cytokine in the plexiform lesions noted in PHT, the formation of which may be monocyte-dependent.25,28
In this study, we sought to evaluate the association of PlGF with measures of both inflammation and hemolysis in patients with SCD and to examine the association of PlGF with specific clinical complications in a cohort of patients followed at an adult sickle cell clinic.
Methods
Patients and study design
The study patients represent a cohort followed at the Sickle Cell Clinic at the University of North Carolina, Chapel Hill. Consecutive patients seen in the clinic for routine follow-up were evaluated. Seventy-four patients with SCD and an additional 19 healthy, race-matched control subjects were included in the analyses. Patients were assessed while in the noncrisis, steady state; had not experienced an episode of acute chest syndrome in the 4 weeks preceding enrollment; and had no clinical evidence of congestive heart failure. None of the study patients were on chronic red blood cell transfusion. This study was approved by the Institutional Review Board at University of North Carolina, Chapel Hill, and all subjects gave written informed consent to participate in accordance with the Declaration of Helsinki.
Echocardiography and PHT determination
Transthoracic Doppler echocardiography was performed in all study subjects with the use of a Philips Sonos 5500 ultrasound system as previously described.29 All the echocardiograms were interpreted by a single cardiologist blinded to all patient data. The pulmonary artery systolic pressure (PASP) was calculated using the modified Bernoulli equation (PASP = 4V2 + right atrial pressure), with the right atrial pressure assumed to be 10 mm Hg. Patients with no detectable tricuspid regurgitant jet were assumed to have a normal PASP if they had no other findings suggestive of PHT and were assigned a tricuspid regurgitant jet velocity that was lower than any measured in the study (1.8 m/s). The diagnosis of PHT in our study was based on PASP values adjusted for age, sex, and body mass index.30
SCD-related clinical complications
The presence (or history) of clinical complications was ascertained from a history obtained at the time of evaluation, combined with a detailed review of the patients' medical records. Acute pain episodes (or pain crises), acute chest syndrome, stroke, and other complications were defined with the use of standard definitions in patients with SCD.13,31-33
Measurement of laboratory variables
Blood samples were obtained by venipuncture and drawn into citrate-containing tubes. To minimize any artifactual platelet activation and release during processing, 1μM indomethacin and 0.05 μg/mL prostaglandin I2 (final concentration) were mixed gently with citrated whole blood. The sample was incubated for 15 minutes at 37°C and centrifuged at 150g for 15 minutes. Indomethacin and prostaglandin I2 were added to the extracted plasma sample, followed by centrifugation at 400g for 15 minutes to prepare platelet-poor plasma. The plasma samples were then divided into aliquots and frozen immediately at −80°C for subsequent analysis. Quantification of PlGF, ET-1, and human soluble vascular cell adhesion molecule-1 (VCAM) was accomplished with the use of commercially available enzyme-linked immunoabsorbent assay kits from R&D Systems. Samples were assayed in duplicate and according to manufacturer's instructions. Measurements of N-terminal pro-brain natriuretic peptide (NT-proBNP) and other routine laboratory studies were performed by the McClendon Clinical Laboratory at the University of North Carolina Hospitals. Values of NT-proBNP that were below the measurable limit (< 50 pg/dL) were assigned a value of 49 pg/dL.
Statistical analysis
Nonparametric Mann-Whitney 2-sided t tests were used to compare continuous variables, and categorical variables were compared with the use of a χ2 test. Spearman correlations were used to identify associations between PlGF and specified variables (α = 0.05). Bonferroni adjustments were made for multiple comparisons (PlGF and measures of hemolysis, such as lactate dehydrogenase level, reticulocyte count, and hemoglobin level; and PlGF and measures of inflammation such as soluble VCAM, absolute neutrophil count, and absolute monocyte count). Logistic regression analysis was used to explore whether the observed bivariable relationship between PlGF and PHT might be confounded by other specified variables (ie, reticulocyte count, lactate dehydrogenase level, and hemoglobin level). Receiver-operator characteristic curves (ROCs) for PlGF, NT-proBNP, soluble VCAM, and PHT were generated with the use of Analyze It for Microsoft Excel. Other statistical analyses were conducted by Prism software (Version 5.00 for Windows; GraphPad Software Inc).
Results
Demographic and laboratory characteristics
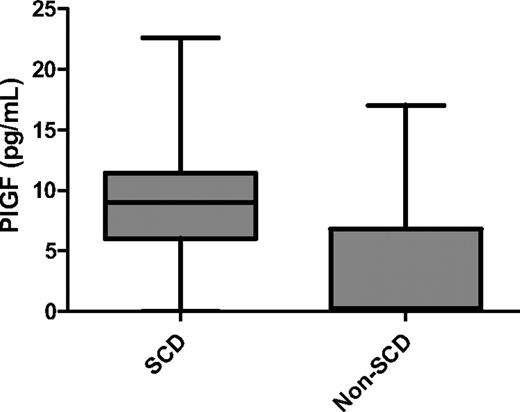
The demographic and laboratory characteristics of all the study subjects are shown in Table 1. Sixty-two patients (83.7%) had sickle cell anemia (HbSS), 8 had HbSC disease (10.8%), 3 had sickle β0 thalassemia (4.1%), and 1 had sickle β+ thalassemia (1.4%). All the healthy control subjects (18 HbAA and 1 HbAC) had normal PASP values. As expected, patients with SCD had higher white blood cell counts (9.4 × 109/L vs 7.2 × 109/L; P = .001), platelet counts (426 × 109/L vs 284 × 109/L; P < .001), and reticulocyte counts (6.9% vs 1.5%; P < .001) than did healthy control subjects. In addition, patients with SCD had significantly lower hemoglobin levels (8.9 g/dL vs 12.4 g/dL; P < .001) and higher total bilirubin (2.2 mg/dL vs 0.3 mg/dL; P < .001), lactate dehydrogenase (898 U/L vs 488 U/L; P < .001), and NT-proBNP (133.5 pg/mL vs 49 pg/mL; P < .001) levels than did healthy controls. Plasma levels of PlGF (9.03 pg/mL vs 0.23 pg/mL; P < .001) were significantly higher in patients with SCD than in healthy control subjects (Figure 1).
Demographic and laboratory characteristics of study subjects
| Characteristic . | SCD (n = 74) . | Control (n = 19) . | P . |
|---|---|---|---|
| Median age, y (IQR) | 41 (30-48.3) | 33 (22-50) | NS |
| Female sex, n (%) | 47 (64) | 15 (79) | NS |
| Genotype, n | — | ||
| SS | 62 | — | |
| SC | 8 | — | |
| Sβ0 | 3 | — | |
| Sβ+ | 1 | — | |
| PASP, mm Hg, median (IQR) | 31 (23-39) | 26 (23-28) | < .001 |
| TRjet, m/s, median (IQR) | 2.3 (1.8-2.7) | 2.0 (1.8-2.1) | .009 |
| White blood cell count, ×109/L, median (IQR) | 9.4 (7.4-11.5) | 7.2 (6.1-9.7) | .001 |
| Hemoglobin level, g/dL, median (IQR) | 8.9 (7.6-10.2) | 12.4 (11.9-13.5) | < .001 |
| Platelet count, ×109/L, median (IQR) | 426 (325.5-512) | 284 (265-322) | < .001 |
| Reticulocyte count, %, median (IQR percentile) | 6.9 (4.5-10.5) | 1.5 (1.3-1.9) | < .001 |
| Absolute neutrophil count, ×109/L, median (IQR) | 5.1 (4-6.2) | 4.7 (4-6.5) | NS |
| Absolute monocyte count, ×109/L, median (IQR) | 0.5 (0.3-0.7) | 0.4 (0.3-0.4) | < .001 |
| Hydroxyurea use, n (%) | 39 (53) | — | — |
| NT-proBNP level, pg/dL, median (IQR) | 133.5 (60.3-326) | 49 (49-59) | < .001 |
| Creatinine level, mg/dL, median (IQR) | 0.75 (0.6-1) | 0.8 (0.7-0.9) | NS |
| Total bilirubin level, mg/dL, median (IQR) | 2.2 (1.2-2.9) | 0.3 (0.2-0.4) | < .001 |
| Direct bilirubin level, mg/dL, median (IQR) | 0.09 (0.09-0.1) | 0.09 (0.09-0.09) | NS |
| LDH level, U/L, median (IQR) | 898 (658-1199) | 488 (413-516) | < .001 |
| PlGF level, pg/mL, median (IQR) | 9.03 (6-11.4) | 0.23 (0-6.8) | < .001 |
| Characteristic . | SCD (n = 74) . | Control (n = 19) . | P . |
|---|---|---|---|
| Median age, y (IQR) | 41 (30-48.3) | 33 (22-50) | NS |
| Female sex, n (%) | 47 (64) | 15 (79) | NS |
| Genotype, n | — | ||
| SS | 62 | — | |
| SC | 8 | — | |
| Sβ0 | 3 | — | |
| Sβ+ | 1 | — | |
| PASP, mm Hg, median (IQR) | 31 (23-39) | 26 (23-28) | < .001 |
| TRjet, m/s, median (IQR) | 2.3 (1.8-2.7) | 2.0 (1.8-2.1) | .009 |
| White blood cell count, ×109/L, median (IQR) | 9.4 (7.4-11.5) | 7.2 (6.1-9.7) | .001 |
| Hemoglobin level, g/dL, median (IQR) | 8.9 (7.6-10.2) | 12.4 (11.9-13.5) | < .001 |
| Platelet count, ×109/L, median (IQR) | 426 (325.5-512) | 284 (265-322) | < .001 |
| Reticulocyte count, %, median (IQR percentile) | 6.9 (4.5-10.5) | 1.5 (1.3-1.9) | < .001 |
| Absolute neutrophil count, ×109/L, median (IQR) | 5.1 (4-6.2) | 4.7 (4-6.5) | NS |
| Absolute monocyte count, ×109/L, median (IQR) | 0.5 (0.3-0.7) | 0.4 (0.3-0.4) | < .001 |
| Hydroxyurea use, n (%) | 39 (53) | — | — |
| NT-proBNP level, pg/dL, median (IQR) | 133.5 (60.3-326) | 49 (49-59) | < .001 |
| Creatinine level, mg/dL, median (IQR) | 0.75 (0.6-1) | 0.8 (0.7-0.9) | NS |
| Total bilirubin level, mg/dL, median (IQR) | 2.2 (1.2-2.9) | 0.3 (0.2-0.4) | < .001 |
| Direct bilirubin level, mg/dL, median (IQR) | 0.09 (0.09-0.1) | 0.09 (0.09-0.09) | NS |
| LDH level, U/L, median (IQR) | 898 (658-1199) | 488 (413-516) | < .001 |
| PlGF level, pg/mL, median (IQR) | 9.03 (6-11.4) | 0.23 (0-6.8) | < .001 |
SCD indicates sickle cell disease; IQR, interquartile range; NS, not significant; PASP, pulmonary arterial systolic pressure; TRjet, tricuspid regurgitant jet velocity; NT-proBNP, N terminal pro-brain natriuretic peptide; LDH, lactate dehydrogenase; and PlGF, placenta growth factor.
PlGF levels in patients with SCD and healthy controls. Plasma levels of PlGF in patients with SCD were significantly higher than in healthy control subjects (9.03 pg/mL vs 0.23 pg/mL; P < .001). Data are shown as median with range.
PlGF levels in patients with SCD and healthy controls. Plasma levels of PlGF in patients with SCD were significantly higher than in healthy control subjects (9.03 pg/mL vs 0.23 pg/mL; P < .001). Data are shown as median with range.
Association of PlGF with SCD-related clinical complications
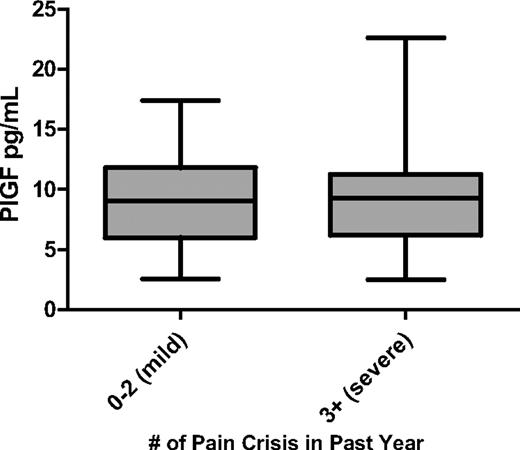
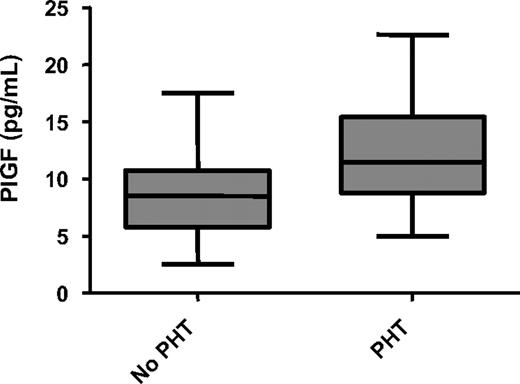
We found no significant correlation between PlGF levels and the number of acute pain episodes in the past year in patients with SCD (Figure 2). However, we observed that plasma levels of PlGF were significantly higher in patients with PHT than in patients without PHT (Figure 3; Table 2). Median plasma levels of PlGF also appeared to be higher in patients with a history of thrombotic stroke than in patients without this complication, although the difference did not achieve statistical significance (11.0 pg/mL vs 8.8 pg/mL; P = .08). No differences were observed in PlGF levels when patients with a history of acute chest syndrome, leg ulcers, priapism, or retinopathy were compared with patients without these complications (data not shown). In addition, there was no significant difference in PlGF levels in patients on hydroxyurea compared with patients not on hydroxyurea. Although adherence to hydroxyurea was not evaluated in this study, patients identified as being on hydroxyurea had significantly higher mean corpuscular volume values (104 fl; interquartile range [IQR], 99-110 fl vs 88 fl; IQR, 79-96 fl; P < .001) and HbF levels (10.4%; IQR, 5.2%-17.5% vs 4.6%; IQR, 1.7%-6.1%; P = .002) compared with patients not on hydroxyurea. There were, however, no significant differences in white blood cell counts and hemoglobin levels in patients receiving hydroxyurea compared with patients not on hydroxyurea.
PlGF is not associated with the frequency of acute pain episodes. Data are shown as median with range and stratified according to disease severity (mild vs severe).
PlGF is not associated with the frequency of acute pain episodes. Data are shown as median with range and stratified according to disease severity (mild vs severe).
PlGF levels in PHT in SCD. PlGF is elevated in PHT (11.5 pg/mL vs 8.5 pg/mL; P = .009). Data are shown as median with range.
PlGF levels in PHT in SCD. PlGF is elevated in PHT (11.5 pg/mL vs 8.5 pg/mL; P = .009). Data are shown as median with range.
Measures of hemolysis and inflammation in PHT and stroke
| Variable* . | PHT (n = 20) . | No PHT (n = 54) . | P . | Stroke (n = 11) . | No stroke (n = 62) . | P . |
|---|---|---|---|---|---|---|
| Hb level, g/dL | 7.6 (6.3-8.8) | 9.4 (8.3-10.2) | < .001 | 7.5 (6.9-9.4) | 9 (7.8-10.2) | .03 |
| LDH level, U/L | 1077 (879-1364) | 808 (620-1079) | .03 | 1136 (947-1367) | 840 (646-1123) | .05 |
| sVCAM level, ng/mL | 1123 (758-1346) | 624 (486-947) | < .001 | 1135 (813-1563) | 753 (515-1044) | .05 |
| PlGF level, pg/mL | 11.5 (8.8-15.4) | 8.5 (5.7-10.8) | .009 | 11.0 (8.4-16.0) | 8.8 (6.0.-11.3) | .08 |
| Variable* . | PHT (n = 20) . | No PHT (n = 54) . | P . | Stroke (n = 11) . | No stroke (n = 62) . | P . |
|---|---|---|---|---|---|---|
| Hb level, g/dL | 7.6 (6.3-8.8) | 9.4 (8.3-10.2) | < .001 | 7.5 (6.9-9.4) | 9 (7.8-10.2) | .03 |
| LDH level, U/L | 1077 (879-1364) | 808 (620-1079) | .03 | 1136 (947-1367) | 840 (646-1123) | .05 |
| sVCAM level, ng/mL | 1123 (758-1346) | 624 (486-947) | < .001 | 1135 (813-1563) | 753 (515-1044) | .05 |
| PlGF level, pg/mL | 11.5 (8.8-15.4) | 8.5 (5.7-10.8) | .009 | 11.0 (8.4-16.0) | 8.8 (6.0.-11.3) | .08 |
PHT indicates pulmonary hypertension; Hb, hemoglobin; LDH, lactate dehydrogenase; sVCAM, soluble vascular cell adhesion molecule-1; and PlGF, placenta growth factor.
Data are presented as medians and interquartile ranges (25th-75th percentiles).
Correlation of PlGF and laboratory variables
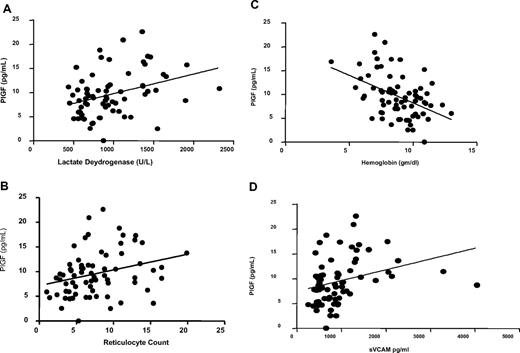
Although PlGF has been reported to induce ET-1 release in vitro,27 we found no correlation between PlGF and ET-1 (r = −0.136, P = .3). However, we observed significant correlations between PlGF levels and both lactate dehydrogenase level (r = 0.36, P = .002; Figure 4A) and reticulocyte count (r = 0.32, P = .007; Figure 4B) and a negative correlation between PlGF and hemoglobin levels (r = −0.44, P < .001; Figure 4C). In addition, we observed a correlation between PlGF and soluble VCAM levels (r = 0.42, P < .001; Figure 4D; Table 3).
Correlation between PlGF and markers of hemolysis and inflammation. (A) PlGF is correlated with lactate dehydrogenase levels (r = 0.36, P = .002). (B) PlGF is correlated with reticulocyte count (r = 0.32, P = .007). (C) PlGF is inversely correlated with hemoglobin levels (r = −0.44, P = .001). (D) PlGF is correlated with soluble VCAM (r = 0.42, P < .001). Data shown with regression line. Spearman correlation data are shown in Table 2.
Correlation between PlGF and markers of hemolysis and inflammation. (A) PlGF is correlated with lactate dehydrogenase levels (r = 0.36, P = .002). (B) PlGF is correlated with reticulocyte count (r = 0.32, P = .007). (C) PlGF is inversely correlated with hemoglobin levels (r = −0.44, P = .001). (D) PlGF is correlated with soluble VCAM (r = 0.42, P < .001). Data shown with regression line. Spearman correlation data are shown in Table 2.
Association of PlGF and measures of hemolysis and inflammation
| Variable . | r . | P . |
|---|---|---|
| Hemoglobin level | −0.44 | < .001 |
| Reticulocyte count | 0.32 | .007 |
| Lactate dehydrogenase level | 0.36 | .002 |
| sVCAM level | 0.42 | < .001 |
| Absolute neutrophil count | 0.18 | .13 |
| Absolute monocyte count | 0.18 | .14 |
| Variable . | r . | P . |
|---|---|---|
| Hemoglobin level | −0.44 | < .001 |
| Reticulocyte count | 0.32 | .007 |
| Lactate dehydrogenase level | 0.36 | .002 |
| sVCAM level | 0.42 | < .001 |
| Absolute neutrophil count | 0.18 | .13 |
| Absolute monocyte count | 0.18 | .14 |
PlGF indicates placenta growth factor; and sVCAM, soluble vascular cell adhesion molecule-1.
Association of ET-1 with PHT
There was a trend toward a higher level of ET-1 in patients with PHT than in patients without this complication (0.07 pg/mL vs 0.057 pg/mL, P = .07). Although there was a weak correlation between ET-1 and NT-proBNP (r = 0.102, P = .015), we did not observe any association between ET-1 and hemoglobin level, lactate dehydrogenase level, or reticulocyte count.
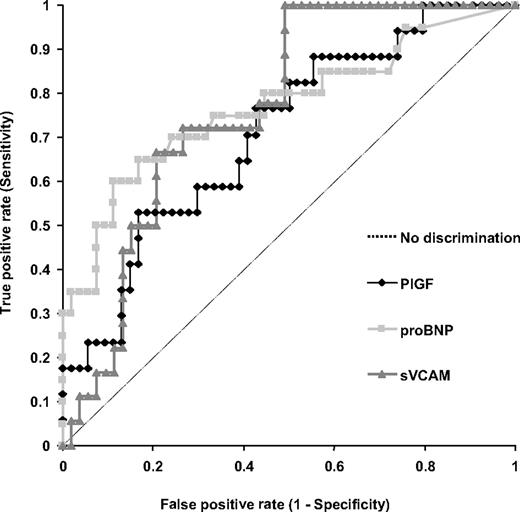
Predictive accuracy of PlGF for PHT
When the PASP was adjusted for age, sex, and body mass index, the area under the ROC with the use of PlGF to diagnose PHT was 0.71 (Figure 5). We observed a correlation of PlGF with NT-proBNP (r = 0.25, P = .03), a laboratory marker of cardiac dysfunction and an established predictor of PHT in SCD.34 The area under the ROC with the use of soluble VCAM and NT-proBNP to diagnose PHT was 0.77 for each biomarker (Figure 5). In addition, there was a significant correlation between soluble VCAM and NT-proBNP (r = 0.46, P < .001). Using the 75th percentile for each variable as the threshold level, we observed no substantial difference in the predictive accuracies of the 3 biomarkers for the diagnosis of PHT in our cohort (Table 4). The sensitivity of PlGF for the presence of PHT was 53%, compared with sensitivities of 60% for NT-proBNP and 50% for soluble VCAM; the specificity of PlGF was 83%, compared with 87% for NT-proBNP and 85% for soluble VCAM; and the likelihood ratio for PlGF was 3.18, compared with 4.63 for NT-proBNP and 3.31 for soluble VCAM.
Potential predictive accuracy of biomarkers in PHT. ROC curves of PlGF, soluble VCAM, and NT-proBNP in patients with SCD.
Potential predictive accuracy of biomarkers in PHT. ROC curves of PlGF, soluble VCAM, and NT-proBNP in patients with SCD.
Summary of ROC curves for PlGF, NT-proBNP, and sVCAM
| Variable . | AUC . | 95% CI . | 75th percentile . | Sensitivity, % . | Specificity, % . | Likelihood ratio . | Positive predictive value, % . |
|---|---|---|---|---|---|---|---|
| PlGF, pg/mL | 0.71 | 0.57-0.85 | 11.5 | 53 | 83 | 3.18 | 59 |
| NT-proBNP, pg/mL | 0.77 | 0.63-0.91 | 326 | 60 | 87 | 4.63 | 60 |
| sVCAM, ng/mL | 0.77 | 0.65-0.88 | 1199 | 50 | 85 | 3.31 | 50 |
| Variable . | AUC . | 95% CI . | 75th percentile . | Sensitivity, % . | Specificity, % . | Likelihood ratio . | Positive predictive value, % . |
|---|---|---|---|---|---|---|---|
| PlGF, pg/mL | 0.71 | 0.57-0.85 | 11.5 | 53 | 83 | 3.18 | 59 |
| NT-proBNP, pg/mL | 0.77 | 0.63-0.91 | 326 | 60 | 87 | 4.63 | 60 |
| sVCAM, ng/mL | 0.77 | 0.65-0.88 | 1199 | 50 | 85 | 3.31 | 50 |
ROC indicates receiver-operator characteristic curve; PlGF, placenta growth factor; NT-proBNP, N-terminal pro-brain natriuretic peptide; sVCAM, soluble vascular cell adhesion molecule-1; and AUC, area under the curve.
Logistic regression analysis
To assess whether PlGF is an independent predictor of PHT in SCD, we used logistic regression analyses to explore whether controlling for reticulocyte count, lactate dehydrogenase, and hemoglobin, separately, affected the observed association between PlGF and PHT. Because of the small number of participants with PHT in our cohort, it is not possible to adequately control for potential confounding variables, especially more than one at a time. Controlling for either reticulocyte count or lactate dehydrogenase had little effect on the magnitude or significance of the estimated odds ratio associated with a one-unit change in PlGF (Table 5). However, when we controlled for hemoglobin, the estimate of the odds ratio for PlGF was closer to one and was no longer statistically significant (P = .43).
Estimates of the unadjusted and adjusted odds ratio for PlGF and PHT
| Markers of hemolysis . | Odds ratio estimate for PlGF in PHT . | 95% CI . | P . |
|---|---|---|---|
| None | 1.19 | (1.04-1.35) | .01 |
| Lactate dehydrogenase level | 1.17 | (1.02-1.34) | .03 |
| Reticulocyte count | 1.19 | (1.04-1.37) | .01 |
| Hemoglobin level | 1.06 | (0.92-1.23) | .4 |
| Markers of hemolysis . | Odds ratio estimate for PlGF in PHT . | 95% CI . | P . |
|---|---|---|---|
| None | 1.19 | (1.04-1.35) | .01 |
| Lactate dehydrogenase level | 1.17 | (1.02-1.34) | .03 |
| Reticulocyte count | 1.19 | (1.04-1.37) | .01 |
| Hemoglobin level | 1.06 | (0.92-1.23) | .4 |
PlGF indicates placenta growth factor; and PHT, pulmonary hypertension.
Discussion
The elevated levels of PlGF in our study patients compared with healthy control subjects further confirms the inflammatory nature of SCD. Despite the abundant evidence of inflammation in patients with SCD, its contribution to the pathophysiology of specific complications remains poorly defined. The correlation of PlGF with both lactate dehydrogenase levels and reticulocyte count, as well as its inverse correlation with hemoglobin levels, suggests that hemolysis plays a role in the up-regulation of PlGF (and inflammation) in patients with SCD. Because hemolysis results in increased erythropoietin production and erythroid hyperplasia, our finding is consistent with the previous report of increased PlGF expression in erythroid cells after the addition of erythropoietin.20
Our finding that PlGF is elevated in patients with SCD with PHT and possibly in thrombotic stroke, but not with such vasoocclusive complications such as acute chest syndrome or acute pain episodes, is contrary to a previous report that suggested a correlation between PlGF levels and the frequency of acute pain episodes.20 In our study, PHT and history of stroke do not appear to be independent (data not shown), although any inference of dependence is limited by the relatively small number of subjects with these events in this study cohort. Every single one of the patients with a history of stroke also had PHT, and 55% of those patients with PHT had a history of stroke. There is an increasing body of evidence that hemolysis is associated with complications such as PHT, leg ulcers, priapism, and possibly stroke but not with acute pain episodes or acute chest syndrome. Hemolysis may predispose to certain SCD complications by decreasing the bioavailability of nitric oxide.35 This reduced bioavailability is thought to impair downstream effects of nitric oxide, such as inhibition of platelet activation,36 coagulation activation,29 and transcriptional repression of cell adhesion molecules, VCAM-1, intracellular adhesion molecule-1, P-selectin, and E-selectin.29,37 The correlation of PlGF with soluble VCAM suggests that PlGF may also contribute to the endothelial activation observed in patients with SCD.
The finding that PlGF is independent of either lactate dehydrogenase level or reticulocyte count as a predictor of PHT, but not independent of hemoglobin level, suggests that PlGF may predispose to PHT by a mechanism independent of hemolysis. The dependence of PlGF on hemoglobin level in predicting for PHT may be related to the decreased oxygen-carrying capacity associated with anemia. In view of our relatively small sample size, more studies are needed to fully validate the role of PlGF in the pathogenesis of SCD-associated PHT.
Despite the observed correlations, our data do not provide a causal link between PlGF and either PHT or stroke in SCD. It is particularly interesting that no significant correlation was observed between PlGF and ET-1. Markedly elevated PlGF levels during pregnancy have been reported to be associated with a decline in the level of ET-1 in the first trimester of pregnancy, and these levels remain low throughout gestation.38 Although there is a suggestion that ET-1 is associated with PHT in our study, elevated PlGF levels may produce a vasculopathy by an inflammatory mechanism not dependent on ET-1.
Although the observed elevation of PlGF levels in our study patients with PHT and stroke are relatively modest, being approximately 1.4- and 1.2-fold, respectively, compared with SCD patients without these complications, the presence of chronic, low-level elevation of this cytokine may be sufficient to produce vasculopathic changes in SCD patients, possibly in combination with increased levels of other angiogenic cytokines such as VEGF, erythropoietin, and angiopoietin.21 Elevated PlGF levels may also play a role in the formation of fragile, tortuous blood vessels in the brain (Moyamoya), a risk factor for stroke.39 Furthermore, PlGF-induced remodeling of the pulmonary vasculature may play a role in the formation of the plexiform lesions noted in PHT.40
Doppler echocardiography is usually used to screen for PHT. However, only approximately 80% to 90% of patients with increased right ventricular systolic pressures have a Doppler tricuspid regurgitant signal that is sufficient to predict pulmonary artery systolic pressure.41 The availability of biomarkers that predict the presence of PHT may be complementary to Doppler echocardiography in the diagnosis of this life-threatening complication in SCD. Furthermore, these biomarkers may provide prognostic information, as has been shown for NT-proBNP.34 Our current data show that, although relatively specific, PlGF, NT-proBNP, and soluble VCAM have low predictive accuracies for the presence of PHT, with low sensitivities and positive predictive values. The predictive accuracies of currently available biomarkers may however be enhanced by using statistical modeling techniques that combine biomarkers as well as other types of measurements.
In view of the potential role of hemolysis in the up-regulation of PlGF, therapeutic strategies that reduce red cell destruction or increase the oxygen-carrying capacity of the blood, including chronic red blood cell transfusion or treatment with compounds such as the investigational Gardos channel blocker, senicapoc,42 may be beneficial in decreasing levels of PlGF. It is interesting that no differences in PlGF levels were observed in patients on hydroxyurea compared with those patients not on hydroxyurea. Despite the increased mean corpuscular volume and HbF values in patients on hydroxyurea, there were no significant differences in hemoglobin levels between those patients on hydroxyurea and those patients not on the drug, potentially accounting for the similarities in PlGF levels between the 2 groups.
Because our study had a cross-sectional design, longer term prospective studies are required to validate the clinical significance of the modest elevation of PlGF levels observed in SCD-associated PHT and to further define the role of PlGF in SCD.
In conclusion, PlGF is higher in patients with SCD than in healthy control subjects and is correlated with the presence of PHT. The association of PlGF with measures of hemolysis suggests that treatments that improve survival of red blood cells may be beneficial in the treatment or prevention of SCD-associated vasculopathy. Prospective studies evaluating PlGF are required to conclusively define its contribution to the pathogenesis of SCD.
Parts of this work were presented in abstract form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 7, 2008.43
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sang Lee for his technical work on this project and Rosalie Dominik, DrPH, for help with statistical analyses. J.E.B. is an Office of Research on Women's Health BIRCWH scholar.
This work was supported by National Institutes of Health grants K12 HD01441 (J.E.B.), UL1RR025747, HL091265 (K.I.A.), and HL079915 (M.J.T. and K.I.A.) and by an award from the North Carolina State Sickle Cell Program.
National Institutes of Health
Authorship
Contribution: J.E.B. and K.I.A. designed and performed the research, analyzed and interpreted data, and wrote the paper; B.H. performed the research; S.K.J. and D.S. collected data; L.D.C. and E.P.O. interpreted data; M.J.T. interpreted data and contributed to the manuscript; and A.H. collected data, performed the research, and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth I. Ataga, Division of Hematology/Oncology, University of North Carolina at Chapel Hill, Rm 3149, Physicians Office Bldg, CB# 7305, Chapel Hill, NC 27599-7305; e-mail: kataga@med.unc.edu.
References
Author notes
*J.E.B. and K.I.A. contributed equally to this manuscript.