Abstract
Although the involvement of plasminogen activator inhibitor-1 (PAI-1) in fibrotic diseases is well documented, its role in cardiac fibrosis remains controversial. The goal of this study was to determine the effect of a PAI-1 deficiency (PAI-1−/−) on the spontaneous development of cardiac fibrosis. PAI-1−/− mice developed pervasive cardiac fibrosis spontaneously with aging, and these mice displayed progressively distorted cardiac architecture and markedly reduced cardiac function. To mechanistically elucidate the role of PAI-1 in cardiac fibrosis, 12-week-old mice were chosen to study the biologic events leading to fibrosis. Although fibrosis was not observed at this early age, PAI-1−/− hearts presented with enhanced inflammation, along with increased microvascular permeability and hemorrhage. A potent fibrogenic cytokine, transforming growth factor-β (TGF-β), was markedly enhanced in PAI-1−/− heart tissue. Furthermore, the expression levels of several relevant proteases associated with tissue remodeling were significantly enhanced in PAI-1−/− hearts. These results suggest that PAI-1 is cardioprotective, and functions in maintaining normal microvasculature integrity. Microvascular leakage in PAI-1−/− hearts may provoke inflammation, and predispose these mice to cardiac fibrosis. Therefore, a PAI-1 deficiency contributes to the development of cardiac fibrosis by increasing vascular permeability, exacerbating local inflammation, and increasing extracellular matrix remodeling, an environment conducive to accelerated fibrosis.
Introduction
Cardiac fibrosis, defined pathologically as deposition of excess extracellular matrix, is increasingly recognized as a common and important feature in many cardiomyopathies, including hypertrophic cardiomyopathy, ischemic cardiomyopathy, and restrictive cardiomyopathy. Cardiac fibrosis is often progressive in nature, leading to a gradual distortion of normal tissue architecture with consequent compromised cardiac function, and ultimately heart failure.1 The prevalence of chronic cardiac fibrosis, the lack of effective treatment, and its associated high mortality have spurred extensive research into identifying the cellular, molecular, and genetic basis of the disease. However, the mechanisms associated with the development of this disease remain largely unknown.
It has been well established that many components of the coagulation and fibrinolytic pathways play critical regulatory roles in a wide variety of pathologic processes, including inflammatory responses2,3 and tumor growth.4 Several reports have emerged that implicate hemostasis proteins in cardiac fibrosis. For example, mice with low expression levels of either tissue factor (TF) or factor VII (FVII; < 1% of wild-type [WT] levels) developed spontaneous cardiac fibrosis.5,6 In the case of low TF-induced cardiac fibrosis, differential regulation of urokinase-type plasminogen activator (uPA) was involved in this process.7 Furthermore, mice that overexpress uPA develop spontaneous cardiac fibrosis,8
Plasminogen activator inhibitor-1 (PAI-1) is a member of the serine protease inhibitor (SERPIN) family, and is the main physiologic inhibitor of tissue-type plasminogen activator (tPA) and uPA. Elevated PAI-1 levels in humans with fibrotic lung disorders suggest an involvement of PAI-1 in fibrotic diseases. The most widely used model to recapitulate the disease condition in humans is the systemic or intratracheal administration of bleomycin, which initially induces potent inflammatory responses in pulmonary alveolar tissues, followed by the formation of pulmonary fibrosis. In this model, less collagen and fibrin deposition has been observed in PAI-1–deficient (PAI-1−/−) mice. Conversely, the opposite is seen in transgenic mice overexpressing PAI-1.9,10 These studies indicate a causal role for PAI-1 in inducing pulmonary fibrosis.
Compared with the role of PAI-1 in lung fibrosis, its function in cardiac fibrosis is not as extensively studied, and remains controversial. For example, a profibrotic effect of PAI-1 in the heart has been described, with enhanced fibrosis in PAI-1–overexpressing mice,11 and less fibrosis in PAI-1−/− mice.12 However, exacerbated cardiac fibrosis in PAI-1−/− mice was also reported.13 In the present study, a thorough characterization of the temporal development of spontaneous cardiac fibrosis in PAI-1−/− mice was made. Moreover, contributing mechanisms that lead to fibrosis formation before the appearance of fibrotic tissue in hearts were identified. The results are summarized herein.
Methods
Mice
Both wild-type (WT) and PAI-1−/− mice14 were bred to at least 8 generations into the C57Bl/6 background. Only male mice were used in these experiments. Mice were housed in microisolation cages on a 12-hour day/night cycle. The protocols used were approved by the Institutional Animal Care and Use Committee, University of Notre Dame.
Echocardiography
Mice were lightly sedated with 1.5% isoflurane inhalation, and echocardiography images were acquired using a Vevo 770 system (VisualSonics), as previously described.6 Echocardiographic images were obtained with either a 30-MHz or 40-MHz transducer, at a focal depth range of 12.7 to 6.0 mm, a temporal resolution of more than 150 frames/second, and a spatial resolution of less than 40 μm. Left ventricular (LV) diameters were measured using a parasternal short-axis view at the papillary muscle level. Calculations were made within the guidelines of the American Society of Echocardiography leading-edge convention.15
Vascular permeability in the heart
Vascular permeability was assessed by retro-orbital injection of Evans blue (EB) dye (1% solution, 0.1 mL/g body weight). After 24 hours, blood was collected from the inferior vena cava. Mice were then extensively perfused from the heart with saline, after which the heart was harvested. EB in the tissue was extracted with formamide for 3 days, and dry weights were determined after dehydration. EB concentrations in plasma and tissues were quantified by dual-wavelength spectrophotometer at 620 nm and 740 nm.16 The absorbance of heme pigments was corrected using the following formula: corrected absorbance = OD620 − (1.426 × OD740 + 0.03). The readings were normalized using the dry weight of organs.
mRNA and protein determinations
Total RNA was purified with the RNeasy Mini Kit (QIAGEN). Quantitative reverse-transcription–polymerase chain reaction (Q-RT-PCR) was performed using TaqMan chemistry with primers and probes. Gene expression levels were obtained using individual values normalized to RPL-19 as a housekeeping gene.17 PAI-1 antigen levels in plasma were determined by enzyme-linked immunosorbent assay (Molecular Innovation).
Histology
Sections were prepared from fixed paraffin-embedded tissues, and stained with hematoxylin and eosin (H&E), Gomori Prussian blue stain for iron, and Masson trichrome for collagen. Primary antibodies for immunohistochemical stains were rat anti–mouse CD45 for leukocytes (BD PharMingen) and rat anti–mouse Mac-3/F480 for macrophages (Serotec). A series of bright-field images, compiling whole heart images, was acquired at a magnification of 200× using a Nikon Eclipse TE2000U microscope, with MicroPublisher 5.0RTV color camera (QImaging). The images were also converted into digital signal by ScanScope scanner (Aperio). Detailed protocols for histology and immunohistochemistry were previously described.6,17
Radiotelemetric measurements of central blood pressures and heart rates
Male mice (12 and 48 weeks old) were anesthetized with a cocktail of 0.075 mg of ketamine/0.015 mg of xylazine/0.0025 mg of acepromazine per gram of body weight via intraperitoneal injection. Radiotelemetric catheters (TA-F20 Mouse Transmitter; Data Sciences International) were implanted in the thoracic aortas. After the implantation surgeries, mice were kept warm on a heating pad and monitored for 1 week to allow full recovery. At this time, the cages were placed on telemetric receivers and the radiotelemeters were magnetically activated. Pulse rate, systolic and diastolic blood pressures, mean arterial pressure, heart rate, and physical activity were recorded every 5 minutes for at least 3 days.
Peritoneal inflammation model
Mice were injected intraperitoneally with 1 mL of a 4% Brewer thioglycollate medium solution (Difco). At various times after stimulation, the mice were killed by cervical dislocation. The peritoneal cavity was then exposed and the exudates were collected by washing the cavity with 3 mL of sterile phosphate-buffered saline, using an 18-gauge catheter.
Statistics
Data are reported as mean plus or minus SEM. Student t tests were performed using a P value of .05 or less to void the null hypothesis in comparison of WT and PAI-1−/− mice at each time point.
Results
A PAI-1 deficiency causes spontaneous interstitial cardiac fibrosis
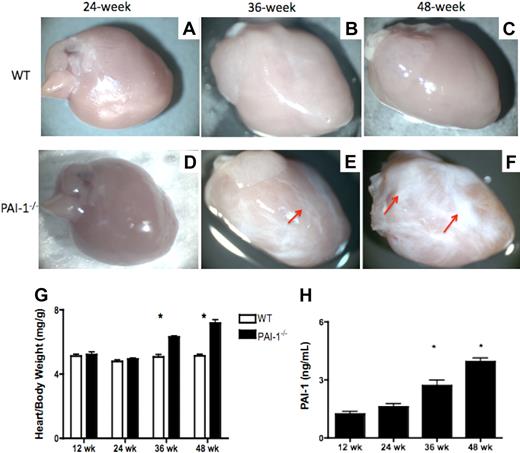
To investigate the role of PAI-1 in spontaneous cardiac fibrosis, WT and PAI-1−/− mice, fed a regular chow diet, were aged without challenge. Hearts from these mice were harvested at 12, 24, 36, and 48 weeks of age. No evidence for cardiac fibrosis was observed in the WT mice at any time point (Figure 1A-C). Although at 24 weeks, cardiac fibrosis was not macroscopically observed in the majority of PAI-1−/− mice (Figure 1D), approximately 50% of the 36-week-old PAI-1−/− mice developed obvious cardiac fibrotic patches (Figure 1E). The fibrotic tissue showed a loss of normal architecture, paucity of stromal cells, and replacement of essential parenchymal structures by dense, pale, homogeneous, and increasingly rigid connective tissue. At 48 weeks, fibrotic tissue was even more overt in PAI-1−/− hearts (Figure 1F). The progression of this phenotype with age led to a progressive distortion of cardiac tissue architecture in PAI-1−/− hearts. Both 36- and 48-week-old PAI-1−/− mice had enlarged hearts, compared with WT hearts, as indicated by their significantly increased heart–body weight ratios at necropsy (Figure 1G). Further, the antigen level of PAI-1 in plasma, determined by enzyme-linked immunosorbent assay, increased progressively with aging in WT mice (Figure 1H).
PAI-1 deficiency causes spontaneous cardiac fibrosis formation. (A-F) Macroscopic views of hearts from WT and PAI-1−/− mice. (A-C) WT hearts at 24, 36, and 48 weeks, respectively. None of the WT hearts at any time point examined developed fibrosis. (D-F) PAI-1−/− hearts at 24, 36, and 48 weeks, respectively. PAI-1−/− mice at 36 and 48 weeks developed obvious cardiac fibrosis. The fibrotic patches (red arrows) show a loss of normal architecture, paucity of stromal cells, and replacement of essential parenchymal structures by dense, pale, and increasingly rigid tissue. (G) Cardiac fibrosis formation caused an increase in the heart–body weight ratio (mg/g) in PAI-1−/− mice with aging, compared with WT mice. (H) PAI-1 antigen levels in plasma. n ≥ 8 for each age group; *P < .05.
PAI-1 deficiency causes spontaneous cardiac fibrosis formation. (A-F) Macroscopic views of hearts from WT and PAI-1−/− mice. (A-C) WT hearts at 24, 36, and 48 weeks, respectively. None of the WT hearts at any time point examined developed fibrosis. (D-F) PAI-1−/− hearts at 24, 36, and 48 weeks, respectively. PAI-1−/− mice at 36 and 48 weeks developed obvious cardiac fibrosis. The fibrotic patches (red arrows) show a loss of normal architecture, paucity of stromal cells, and replacement of essential parenchymal structures by dense, pale, and increasingly rigid tissue. (G) Cardiac fibrosis formation caused an increase in the heart–body weight ratio (mg/g) in PAI-1−/− mice with aging, compared with WT mice. (H) PAI-1 antigen levels in plasma. n ≥ 8 for each age group; *P < .05.
Pervasive interstitial collagen accumulation in PAI-1−/− hearts
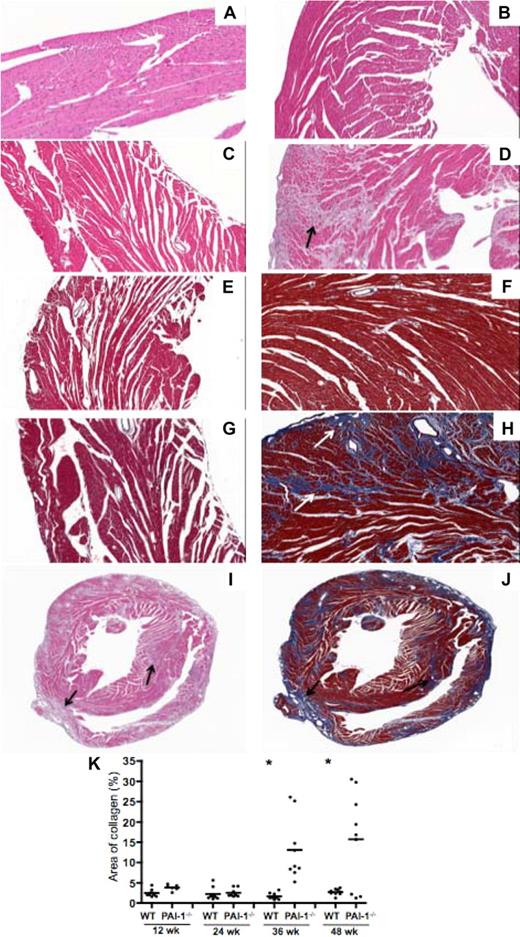
Histologic analysis by H&E staining showed that the structures of WT hearts were largely intact from 12 weeks (Figure 2A) to 48 weeks (Figure 2B; only hearts from 12- and 48-week-old WT and PAI-1−/− mice are shown in this figure). In contrast, extensive fibrillar interstitial collagen accumulation in 36- and 48-week PAI-1−/− hearts was observed. H&E staining revealed loss of cardiomyocytes and deposition of interstitial connective tissue in 48-week PAI-1−/− hearts (Figure 2D), but not in 12-week PAI-1−/− hearts (Figure 2C). Masson trichrome staining showed minimal collagen deposition in both 12-week WT (Figure 2E) and PAI-1−/− (Figure 2G) hearts. However, a significantly greater amount of interstitial collagen deposition (blue staining) in 48-week PAI-1−/− hearts (Figure 2H) was found, whereas minimal staining was observed in their WT counterparts (Figure 2F; only normal distribution around vasculature). Progressive temporal development of cardiac fibrosis in PAI-1−/− hearts was observed, as significantly more fibrosis was detected in older PAI-1−/− hearts than at the earlier time points (data not shown). Both H&E and Masson trichrome staining of 48-week PAI-1−/− hearts revealed that the fibrosis was not localized, but rather pervasive throughout the entire myocardium, including left/right ventricle and atrium, and interventricular septum (Figure 2I-J). Collagen deposition extended from the pericardium into the myocardium, with some distribution near the endocardium. Further, the amount of collagen deposition in hearts, as revealed by Masson trichrome staining, was quantified (Figure 2K). Both 36- and 48-week PAI-1−/− hearts had significantly more collagen deposition than WT counterparts.
Histochemical characterization of interstitial cardiac fibrosis in hearts of PAI-1−/− mice. H&E staining of WT hearts at (A) 12 and (B) 48 weeks, and PAI-1−/− hearts at (C) 12 and (D) 48 weeks. At 48 weeks, replacement of cardiomyocytes by connective tissue was observed in PAI-1−/− hearts (black arrow). Masson trichrome staining of WT hearts at (E) 12 and (F) 48 weeks, and PAI-1−/− hearts at (G) 12 and (H) 48 weeks revealed extensive interstitial collagen deposition (white arrow, blue staining) in 48-week PAI-1−/− hearts. Although 12-, 24-, 36-, and 48-week hearts were examined, only 12- and 48-week hearts are shown in this figure. Global view of 48-week PAI-1−/− hearts with H&E staining (I) and Masson trichrome staining (J) revealed that fibrosis formation (black arrow) was rather pervasive throughout the entire heart. (K) Quantification of collagen by Masson trichrome staining of hearts. Data points represent individual hearts, and bars are group means. n ≥ 4 for each age group; *P < .05.
Histochemical characterization of interstitial cardiac fibrosis in hearts of PAI-1−/− mice. H&E staining of WT hearts at (A) 12 and (B) 48 weeks, and PAI-1−/− hearts at (C) 12 and (D) 48 weeks. At 48 weeks, replacement of cardiomyocytes by connective tissue was observed in PAI-1−/− hearts (black arrow). Masson trichrome staining of WT hearts at (E) 12 and (F) 48 weeks, and PAI-1−/− hearts at (G) 12 and (H) 48 weeks revealed extensive interstitial collagen deposition (white arrow, blue staining) in 48-week PAI-1−/− hearts. Although 12-, 24-, 36-, and 48-week hearts were examined, only 12- and 48-week hearts are shown in this figure. Global view of 48-week PAI-1−/− hearts with H&E staining (I) and Masson trichrome staining (J) revealed that fibrosis formation (black arrow) was rather pervasive throughout the entire heart. (K) Quantification of collagen by Masson trichrome staining of hearts. Data points represent individual hearts, and bars are group means. n ≥ 4 for each age group; *P < .05.
Cardiac fibrosis in PAI-1−/− mice correlates to distorted cardiac architecture, impaired ventricular wall motion, compromised cardiac function, and restrictive cardiomyopathy
Echocardiography was used to investigate the impact of fibrosis on myocardial anatomy and function. WT and PAI-1−/− mice were lightly sedated at the time of measurements, and heart rates for both groups were kept similar (approximately 500 beats per minute [bpm]). Isoflurane, in conjunction with pure oxygen inhalation, was used as the anesthetic, because, in C57Bl/6 mice, this method leads to minimal cardiac depression, as well as the most stable measurements of fractional shortening and end-diastole dimensions.18
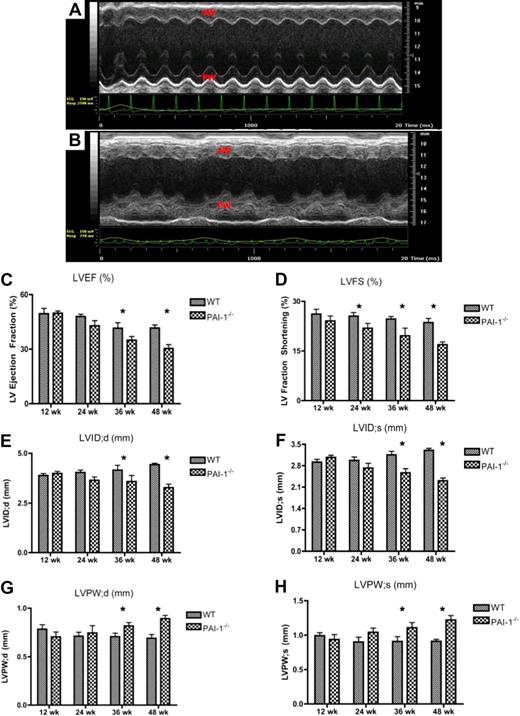
Echocardiographic M-mode images of the left ventricles (LVs) revealed, in contrast to 48-week-old WT mice (Figure 3A), substantial LV wall motion irregularities in 48-week PAI-1−/− hearts (Figure 3B). Severe reductions in both systolic and diastolic LV posterior and anterior wall movements were observed. The overall LV function, measured by both LV ejection fraction (LVEF) and fractioning shortening (LVFS), was diminished by a PAI-1 deficiency, especially after 36 weeks of age (Figure 3C-D). The internal diameter of the LV was also decreased in PAI-1−/− hearts, at both diastolic and systolic stages, especially after 36 weeks (Figure 3E-F). In addition, the LV posterior wall diameter was increased in PAI-1−/− hearts at both systole and diastole (Figure 3G-H). These measurements were consistent with the observation of enlarged hearts and restrictive cardiomyopathy in PAI-1−/− mice.
Echocardiographic evaluation of cardiac anatomy and cardiac function in WT and PAI-1−/− mice. Representative M-mode images of the short-axis view of the 48-week WT (A) and PAI-1−/− (B) LVs. Substantial LV wall motion irregularities were found in 48-week-old PAI-1−/− mice, demonstrated by severe reductions and odd movements in both systolic and diastolic LV posterior and anterior wall movements. AW indicates anterior wall; and PW, posterior wall. The overall left ventricular function, measured by left ventricle ejection fraction (LVEF; C) and left ventricle fractioning shortening (LVFS; D), was diminished by a PAI-1 deficiency, especially after 36 weeks of age. The internal diameters of LV at diastole and systole were decreased in PAI-1−/− mice, especially after 36 weeks (E-F). The LV posterior wall diameter was increased in PAI-1−/− mice at both systole and diastole (G-H). n ≥ 6 for each age group; *P < .05.
Echocardiographic evaluation of cardiac anatomy and cardiac function in WT and PAI-1−/− mice. Representative M-mode images of the short-axis view of the 48-week WT (A) and PAI-1−/− (B) LVs. Substantial LV wall motion irregularities were found in 48-week-old PAI-1−/− mice, demonstrated by severe reductions and odd movements in both systolic and diastolic LV posterior and anterior wall movements. AW indicates anterior wall; and PW, posterior wall. The overall left ventricular function, measured by left ventricle ejection fraction (LVEF; C) and left ventricle fractioning shortening (LVFS; D), was diminished by a PAI-1 deficiency, especially after 36 weeks of age. The internal diameters of LV at diastole and systole were decreased in PAI-1−/− mice, especially after 36 weeks (E-F). The LV posterior wall diameter was increased in PAI-1−/− mice at both systole and diastole (G-H). n ≥ 6 for each age group; *P < .05.
Assessment of hemodynamic parameters
Hypertension is often associated with the development of cardiac hypertrophy and fibrosis.19 To determine whether cardiac fibrosis in PAI-1−/− mice results from hypertension, the central blood pressures and heart rates in WT and PAI-1−/− mice were monitored continuously by radiotelemetry. The systolic, diastolic, and pulse pressures were slightly increased from 12- to 48-week-old mice in both genotypes (Figure 4A-C). However, they were similar between WT and PAI-1−/− mice at both 12 and 48 weeks. Heart rates for WT and PAI-1−/− mice were similar at around 650 bpm (Figure 4D). Taken together, these data exclude a hemodynamic influence on the vascular and myocardial changes observed in PAI-1−/− mice.
Hemodynamic parameters of WT and PAI−/− mice. The central arterial blood pressures and heart rates in 12- and 48-week-old WT and PAI-1−/− mice were monitored continuously for at least 3 days by radiotelemetry. The means of systolic pressure (A), diastolic pressure (B), and pulse pressure (C) were then calculated. Heart rates (D) were also monitored in each individual WT and PAI-1−/− mouse. The data represent means of at least 4 mice per group.
Hemodynamic parameters of WT and PAI−/− mice. The central arterial blood pressures and heart rates in 12- and 48-week-old WT and PAI-1−/− mice were monitored continuously for at least 3 days by radiotelemetry. The means of systolic pressure (A), diastolic pressure (B), and pulse pressure (C) were then calculated. Heart rates (D) were also monitored in each individual WT and PAI-1−/− mouse. The data represent means of at least 4 mice per group.
Alteration of vascular permeability and bleeding in PAI-1−/− hearts
In an effort to mechanistically elucidate the role of PAI-1 in cardiac fibrosis in vivo, 12-week-old WT and PAI-1−/− mice were chosen to study the pathologic processes leading to cardiac fibrosis. At this age, no overt fibrosis was observed in PAI-1−/− hearts, as indicated by gross appearance, heart–body weight ratios, and histologic examinations.
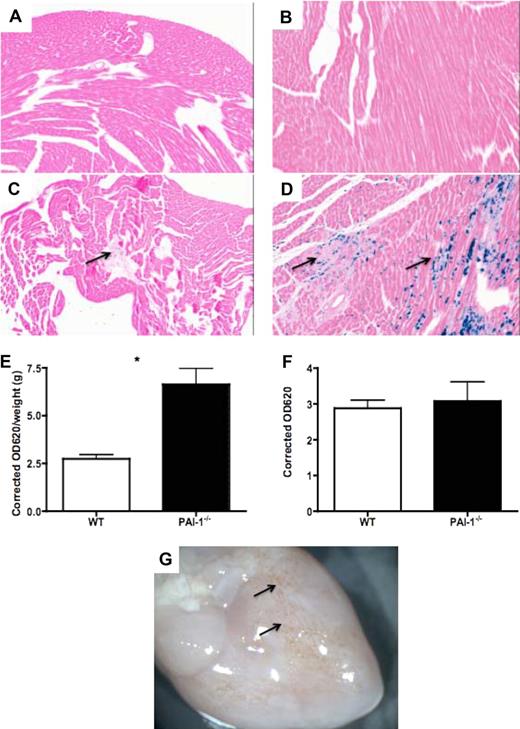
Prussian blue staining of WT and PAI-1−/− hearts was performed to examine interstitial hemosiderin granule deposition, indicative of prior bleeding. WT mice hearts were devoid of hemosiderin at all of the age groups examined (viz, 12, 24, 36, and 48 weeks; Figure 5A and B represent data for 12- and 48-week WT hearts, respectively). In contrast, abundant hemosiderin deposition was seen throughout the myocardium in 48-week PAI-1−/− hearts (Figure 5D). The hemorrhagic spots were noticeable macroscopically (Figure 5G arrow). Interestingly, PAI-1−/− mice as young as 12 weeks, before fibrosis formation, showed hemosiderin deposition as well (Figure 5C), indicating the occurrence of interstitial hemorrhages.
Spontaneous bleeding and increased vascular permeability in PAI-1−/− hearts. Prussian blue staining of 12- and 48-week WT (A-B) and PAI-1−/− (C-D) hearts revealed hemosiderin deposition in 12-week PAI-1−/− hearts (C, ↑), indicating bleeding before fibrosis formation. Hemosiderin deposition was overt in 48-week PAI-1−/− hearts (D). An in vivo vascular permeability assay revealed increased cardiac vascular leakage in 12-week PAI-1−/− hearts (E). The plasma levels of EB were the same between WT and PAI-1−/− mice (F). Gross photograph of PAI-1−/− hearts at 48 weeks is shown (G), where hemorrhage spots are observable macroscopically (↑). n ≥ 5 for each age group; *P < .05.
Spontaneous bleeding and increased vascular permeability in PAI-1−/− hearts. Prussian blue staining of 12- and 48-week WT (A-B) and PAI-1−/− (C-D) hearts revealed hemosiderin deposition in 12-week PAI-1−/− hearts (C, ↑), indicating bleeding before fibrosis formation. Hemosiderin deposition was overt in 48-week PAI-1−/− hearts (D). An in vivo vascular permeability assay revealed increased cardiac vascular leakage in 12-week PAI-1−/− hearts (E). The plasma levels of EB were the same between WT and PAI-1−/− mice (F). Gross photograph of PAI-1−/− hearts at 48 weeks is shown (G), where hemorrhage spots are observable macroscopically (↑). n ≥ 5 for each age group; *P < .05.
To address whether PAI-1 deficiency correlates with cardiac vascular leakage, we used an in vivo vascular permeability assay to measure extravasations of cell-impermeable Evans blue (EB) dye. PAI-1−/− mice at 12 weeks displayed increased cardiac vascular leakage compared with age-matched WT mice (Figure 5E). This difference was not attributed to an increased amount of EB in plasma (Figure 5F).
Elevated local inflammation and leukocyte infiltration in PAI-1−/− hearts
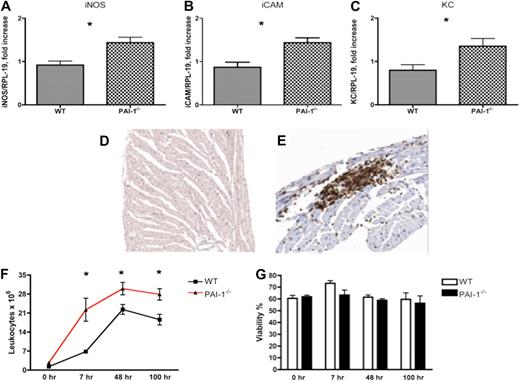
To examine whether increased microvascular leakage in PAI-1−/− mice is associated with inflammation, Q-RT-PCR analyses of mRNA levels encoding some key cytokines relevant to inflammation and cardiac fibrosis were performed. Elevated inflammation, measured by inducible nitric oxide synthase (iNOS), intercellular adhesion molecule (iCAM), and keratinocyte-derived chemokine (KC) expression levels, was detected in 12-week PAI-1−/− hearts (Figure 6A-C). Anti-CD45 immunostains of 12-week WT and PAI-1−/− hearts were performed to study leukocyte infiltration in myocardium. CD45+ cells were scarce and scattered within myocardia of WT mice (Figure 6D). In contrast, patches of CD45+ cells were observed in PAI-1−/− hearts (Figure 6E). The majority of infiltrated CD45+ cells were localized on, and adjacent to, the pericardium.
Assessments of inflammation in 12-week WT and PAI-1−/− hearts. An elevated inflammatory response, indicated by iNOS (A), iCAM (B), and KC (C) mRNA levels, was observed in 12-week PAI-1−/− hearts. Compared with WT heart (D), CD45 staining revealed enhanced local leukocyte infiltration in cardiac tissue of 12-week-old PAI-1−/− mice (E). Leukocyte infiltration and recruitment after peritoneal stimulation with thioglycollate were significantly enhanced in PAI-1−/− mice compared with WT mice (F). Comparison of peritoneal leukocyte viabilities indicated that the differences in leukocyte infiltration were not due to different rates of cell death (G). n ≥ 5 for each age group; *P < .05.
Assessments of inflammation in 12-week WT and PAI-1−/− hearts. An elevated inflammatory response, indicated by iNOS (A), iCAM (B), and KC (C) mRNA levels, was observed in 12-week PAI-1−/− hearts. Compared with WT heart (D), CD45 staining revealed enhanced local leukocyte infiltration in cardiac tissue of 12-week-old PAI-1−/− mice (E). Leukocyte infiltration and recruitment after peritoneal stimulation with thioglycollate were significantly enhanced in PAI-1−/− mice compared with WT mice (F). Comparison of peritoneal leukocyte viabilities indicated that the differences in leukocyte infiltration were not due to different rates of cell death (G). n ≥ 5 for each age group; *P < .05.
To further study the role of PAI-1 in leukocyte migration and infiltration in inflammation, a well-established peritoneal inflammation model was used. Leukocyte recruitment after peritoneal stimulation with thioglycollate was significantly enhanced in PAI-1−/− mice compared with WT mice (Figure 6F), consistent with the findings in PAI-1−/− hearts and a previous report.20 Blood leukocyte levels before and after stimulation were similar between WT and PAI-1−/− mice, suggesting that increased levels of peritoneal leukocytes in PAI-1−/− mice were not caused by an increased number of leukocytes in circulation. In addition, the percentage viable peritoneal leukocyte viability was comparable between WT and PAI-1−/− mice, indicating that the differences in leukocyte infiltration were not due to different rates of cell death (Figure 6G). Taken together, these data suggest that PAI-1 plays an important role in the regulation of inflammation by affecting leukocyte migration. Inflammation provoked by microvascular leakage in PAI-1−/− hearts may predispose them to cardiac fibrosis.
Elevated levels of cardiac remodeling in PAI-1−/− hearts
Transforming growth factor-β (TGF-β) is a potent profibrotic molecule, and its role in fibrosis has been well documented.21 In this study, it was found that the expression level of TGF-β in 12-week PAI-1−/− hearts was significantly higher than in age-matched WT mice (Figure 7A). The total protein level of TGF-β in 12-week PAI-1−/− hearts was also higher (Figure 7B). Active extracellular matrix (ECM) remodeling and imbalance of protease activities are key events leading to fibrosis formation. In this study, both matrix metalloproteinase-2 (MMP-2) and MMP-9 mRNA transcripts in 12-week PAI-1−/− hearts were higher than in WT hearts (Figure 7C-D). Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression levels were also elevated in the hearts of PAI-1−/− mice compared with WT mice (Figure 7E), indicating that this inhibitor of MMPs may be affecting dynamic cardiac extracellular matrix remodeling. uPA expression level was also elevated in PAI-1−/− hearts (Figure 7F). These data suggest an active environment for increased extracellular matrix remodeling in PAI-1−/− hearts.
Elevated level of cardiac ECM remodeling in PAI-1−/− mice. Both the mRNA level (A) and the protein level (B) of TGF-β in 12-week PAI-1−/− hearts were significantly higher than in WT hearts. Q-RT-PCR analysis of transcripts of proteins associated with tissue remodeling and fibrosis in 12-week WT and PAI-1−/− hearts, included MMP-2 (C), MMP-9 (D), TIMP-2 (E), and uPA (F). n ≥ 5 for each group; *P < .05.
Elevated level of cardiac ECM remodeling in PAI-1−/− mice. Both the mRNA level (A) and the protein level (B) of TGF-β in 12-week PAI-1−/− hearts were significantly higher than in WT hearts. Q-RT-PCR analysis of transcripts of proteins associated with tissue remodeling and fibrosis in 12-week WT and PAI-1−/− hearts, included MMP-2 (C), MMP-9 (D), TIMP-2 (E), and uPA (F). n ≥ 5 for each group; *P < .05.
Discussion
Aside from its traditional role in hemostasis, PAI-1 has been implicated as an important regulator of cardiovascular and tissue remodeling processes, such as neointima formation after vascular injury and atherosclerosis.22 Most studies have been conducted in challenge models using PAI-1−/− mice, and the function of PAI-1 in this regard remains controversial. For example, a central role of PAI-1 in ventricular remodeling has been proposed. Further, a high incidence of cardiac rupture occurs in PAI-1−/− mice subjected to coronary occlusion–induced acute myocardial infarction.23 It was additionally demonstrated that PAI-1−/− mice were resistant to vascular fibrosis in a model of cardiac remodeling by long-term nitric oxide synthase inhibition.24 In contrast, cardiac fibrosis was increased in PAI-1−/− mice compared with WT counterparts in a mouse model of angiotensin II– and/or aldosterone-induced vascular remodeling and cardiac fibrosis.13 In the present study, PAI-1−/− mice developed spontaneous cardiac fibrosis as a consequence of aging. Fibrosis formation was progressive, with less than 10% of 24-week-old PAI-1−/− mice examined displaying cardiac fibrosis. The occurrence of cardiac fibrosis increased to approximately 50% for 36-week-old mice, and 90% for 48-week-old mice. The progression of fibrosis in PAI-1−/− hearts led to a gradual distortion of cardiac tissue architecture. In direct contrast, none of the WT mice, at any time point, developed cardiac fibrosis.
Remarkably, the fibrotic patches in PAI-1−/− hearts, particularly after 36 weeks, were strikingly obvious even by gross examination. Histologic studies revealed that collagen was the main component of the fibrotic tissue. Interestingly, fibrosis formation in PAI-1−/− mice was rather pervasive throughout the entire heart, extending from the pericardium to the endocardium. Given the severity of fibrosis, it was not surprising to observe a concomitant occurrence of irregularities of ventricular wall movements and impaired cardiac function, as measured by echocardiography. Of note, the compromised cardiac functions in PAI-1−/− mice were not sufficiently large to cause shortened natural life spans, as no differences in survival between PAI-1−/− and WT mice were observed up to 48 weeks of age in this study. This underscored a large reservoir capacity of cardiac function. A small cohort of human PAI-1–deficient patients, mainly children of Amish descent, has been described.25 At present, it has not been determined whether these PAI-1–deficient persons develop cardiac fibrosis.
As a result of this study, a model is proposed implicating a functional role for PAI-1 in cardioprotection. The presence of PAI-1 at the interface of cardiomyocytes, endothelial cells, pericytes, and the ECM is critical in maintaining the stability and permeability of cardiac microvasculature. PAI-1 may serve as a neutralization protein in regulating protease activities at cell surfaces and at the cell-ECM barriers. In PAI-1−/− hearts, excessive protease activities, particularly through uPA, could cause excessive proteolytic damage to the basal membranes at the cell-ECM barrier, resulting in impaired microvascular integrity and hemorrhage. The presence of PAI-1 could provide a cardioprotective role in this manner. To directly test this hypothesis, a vascular permeability assay in vivo was performed to measure extravasation of cell-impermeable EB dye into the hearts. Results demonstrated that 12-week-old PAI-1−/− mice displayed increased cardiac vascular leakage compared with age-matched WT mice. In further support of this hypothesis, uPA overexpression in the heart caused fibrosis,8 and plasminogen was required for this process.26 In addition, the expression of PAI-1 in relevant cell types, such as endothelial cells, cardiomyocytes, vascular smooth muscle cells, and pericytes, has been reported.12,27,28 Furthermore, plasma levels of PAI-1 in WT mice were found to increase gradually and significantly with aging, implying an essential role of PAI-1 in maintaining microvasculature integrity, although often at the cost of increased thrombotic potential.
Of note, among many other major organs (eg, liver, kidney, spleen, and lung), only heart developed spontaneous fibrosis in PAI-1−/− mice, similar to the findings of cardiac fibrosis in both low TF- and low FVII-expressing mice.5,6 The most likely explanation for this organ specificity is the repetitive mechanical stress during heartbeat, which may cause cardiac vasculature damage more easily under pathologic conditions compared with other organs. Therefore, cardiac homeostasis is delicately maintained, and is sensitive to defects. In support of this view, it has been reported that very low levels of TF,5 FVII,6 FX,29 and FII30 in mice all resulted in cardiac-specific hemorrhage and fibrosis. Similar findings were also observed in mice with excessive uPA.7,26 These data suggest that many components of coagulation and fibrinolytic pathways are critical to maintain this balance, and the presence of these factors is essential to provide hemostatic protection to the heart. Interestingly, the intrinsic coagulation pathway does not seem to be important in this regard, as FVIII−/− and FIX−/− mice do not manifest cardiac pathologies. In addition, mortality due to ischemic heart diseases (but not cerebral stroke) in both hemophilia A (FVIII deficiency) and hemophilia B (FIX deficiency) patients is actually lower compared with the general male population.31,32
Data from the present study indicate that PAI-1 is also essential in maintaining cardiac homeostasis and microvascular integrity. Indeed, interstitial hemosiderin deposition, near the pericardium, as revealed by Prussian blue staining, was detected in younger PAI-1−/− hearts, but not in WT hearts. In older PAI-1−/− hearts, hemosiderin deposition was overt, and extended into the myocardium. Hemorrhagic spots were even observable macroscopically at 48 weeks in PAI-1−/− hearts. Microvascular hemorrhage and leakage are likely key contributors to the process of cardiac fibrosis, as this would, in turn, recruit leukocytes into cardiac tissue. Inflammation provoked by microvascular leakage in PAI-1−/− hearts may predispose them to cardiac fibrosis. The inflammatory responses are capable of inducing collagen synthesis and secretion by fibroblasts, and activating ECM remodeling, through several mechanisms. Such mechanisms may include, but are not limited to, cardiac fibroblast activation and transformation by cytokine stimulation, and enhanced cardiac protease activities. As a key component of the blood fibrinolytic cascade, it must be considered that a PAI-1 deficiency would cause impaired regulation of fibrinolysis, resulting in bleeding. However, mice with a fibrinogen deficiency did not demonstrate cardiac hemosiderin deposition up to 6 months of age,5 suggesting fibrin(ogen) is not essential in this setting. Furthermore, the additional absence of tissue factor pathway inhibitor in low TF mice presented with improved hemostasis, but did not affect the phenotype of cardiac fibrosis in low TF mice,33 providing another line of evidence against this hypothesis.
Additional studies were performed to further determine the in vivo mechanisms that lead to fibrosis formation before the appearance of fibrotic tissue in hearts. WT and PAI-1−/− mice at 12 weeks were used for this purpose, as none of the PAI-1−/− mice at this age developed cardiac fibrosis. It has long been thought that inflammation is a critical process resulting in fibrosis.34,35 Increased cardiac macrophage accumulation was associated with cardiac fibrosis in animal models and in humans.6,36 Indeed, several proinflammatory cytokines, including iNOS, iCAM, and KC, were up-regulated in hearts of young PAI-1−/− mice compared with WT counterparts. Leukocyte infiltration was also observed in PAI-1−/− hearts. These data suggest a proinflammatory environment in PAI-1−/− hearts. To further confirm the regulatory role of PAI-1 in leukocyte infiltration, we performed a peritoneal inflammation model using thioglycollate challenge. A significantly greater amount of leukocytes infiltrated into peritonea in PAI-1−/− mice, compared with WT mice, consistent with the findings in PAI-1−/− hearts and previous work.20 ECM remodeling and an imbalance of protease activities are key events leading to fibrosis formation.6,7 Evidence of active remodeling of the ECM in PAI-1−/− hearts was provided by the increased expression of MMP-2, MMP-9, TIMP-2, and uPA, which are important participants in matrix remodeling and fibrillar collagen catabolism.8 Furthermore, TGF-β is a key profibrotic molecule that plays a central role in promoting fibrosis.21 Among its pleiotropic functions, TGF-β may stimulate ECM production by fibroblasts,37 and/or induce cell proliferation, migration, and differentiation. In addition, PAI-1 has been demonstrated to regulate the TGF-β signaling pathway through αvβ3 integrin.38 In this study, 12-week PAI-1−/− hearts presented with significantly elevated levels of TGF-β transcript, as well as increased levels of TGF-β total protein, compared with WT mice.
Thus far, relationships between PAI-1 and cardiac fibrosis have been focused on the antiprotease activity of PAI-1. PAI-1 has been demonstrated to be a multifunctional protein, with high-affinity binding to ligands other than tPA and uPA. Those ligands include vitronectin and low-density lipoprotein receptor-related protein.39 Both PAI-1/vitronectin and PAI-1/lipoprotein receptor-related protein interactions have been demonstrated to have profound effects on cell functions, including cell adhesion, migration, proliferation, and cell signaling pathways.28,40 Therefore, it is conceivable that PAI-1 may affect cardiac fibrosis independent of its antifibrinolytic activities. Indeed, those activities have been shown to affect glomerular mesangial matrix accumulation in kidney.41 Because various ligand-binding properties have been ascribed to different regions of PAI-1, the multiple functions of PAI-1 could be dissected and separated through specific mutation knock-in approaches to further understand its role in fibrosis in vivo.39
In summary, results from this study demonstrate for the first time that the presence of PAI-1 is essential for maintaining normal cardiac architecture and vasculature integrity. A lack of PAI-1 in the heart results in spontaneous pervasive cardiac fibrosis formation, accompanied by compromised cardiac function and restrictive cardiomyopathy. The effects of a PAI-1 deficiency on cardiac fibrosis were mediated, at least in part, by increased vascular permeability and microvascular hemorrhage, increased local inflammation, enhanced leukocyte infiltration, and increased ECM remodeling, an environment conducive to accelerated fibrosis.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff of the Freimann Life Sciences facility at the University of Notre Dame for the breeding and husbandry of mice. We thank Ms Deborah Donahue for implanting blood pressure modules into the mice, and Ms Mayra Sandoval-Cooper and Annie Zhe Cheng for assisting with the histology. We also thank Dr David Brieger and Dr Wei Zhao for critique of the echocardiographic data.
This work was supported by National Institutes of Health grants HL063682 (V.A.P.) and HL073750 (F.J.C.).
National Institutes of Health
Authorship
Contribution: Z.X. and V.A.P. designed the experiments; Z.X. performed the experiments; and Z.X., F.J.C., and VA.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victoria A. Ploplis, W. M. Keck Center for Transgene Research, 230 Raclin-Carmichael Hall, University of Notre Dame, Notre Dame, IN 46556; e-mail: vploplis@nd.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal