Abstract
Toll-like receptors (TLRs) are known primarily as pathogen recognition receptors of the innate immunity, initiating inflammatory pathways to organize the immune defense. More recently, an involvement of TLRs in various physiologic and pathologic processes has been reported. Because many of these processes implicate angiogenesis, we here elucidated the role of a TLR2/6-dependent pathway on angiogenesis using the TLR2/6 agonist macrophage-activating lipopeptide of 2 kDa (MALP-2), a common bacterial lipopeptide. In vivo and in vitro Matrigel assays demonstrated that MALP-2 promoted angiogenesis in a TLR2/6-dependent manner. Moreover, MALP-2 induced endothelial cell proliferation and migration and a strong secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF release in response to MALP-2 from isolated vascular segments was completely prevented when the endothelium was removed. MALP-2 containing Matrigel implants exhibited vascular structures as well as CD45+ cells. MALP-2 induced migration of leukocytes and likewise GM-CSF release, particularly from the monocyte population. Inhibition of GM-CSF by siRNA or antibodies suppressed MALP-2-induced angiogenesis in vitro and in vivo. These results clearly identified a TLR2/6-dependent induction of angiogenesis by the bacterial lipopeptide MALP-2, which is mediated by GM-CSF. This might represent a general mechanism to enhance or restore blood flow and recruit immune cells for pathogen defense and tissue regeneration.
Introduction
Since their discovery more than a decade ago, mammalian homologs of the Drosophila Toll protein have long been considered exclusively as sentinels of the innate immunity recognizing invading pathogen.1 Since then, the number of known Toll-like receptors (TLRs) has increased to compose an entire receptor protein family with more than 10 members in mice and humans.2 During the immune response, TLRs sense a diversity of pathogen-associated molecular patterns to organize the body's immune defense via the activation of inflammatory pathways. Different receptor assemblies, eg, monodimerization and heterodimerization, as well as different adapter proteins were identified in this process.3 Their important role in dendritic cell maturation and T-cell activation established TLRs as a link between innate and adaptive immunity.4 Besides cells of the immune system, TLR expression in multiple tissues and cell types has been reported, which led to a more wide-ranging view on TLRs. Not only inflammatory disorders, such as atherosclerosis5 and liver disease,6 but also autoimmune diseases7 are critically influenced by TLRs. Furthermore, an involvement of TLRs in allograft acceptance or rejection during transplantation has been shown.8 Of interest, a new role for TLRs in wound healing9,10 and liver regeneration11 has recently been reported, suggesting also a regenerative aspect in TLR biology.
In this report, the N-terminal diacetylated lipopeptide macrophage-activating lipopeptide of 2 kDa (MALP-2) was used as a model TLR2 agonist from Gram-positive bacteria, which is now available as highly purified synthetic material. MALP-2 was originally discovered and isolated from Mycoplasma species,12 which are phylogenetically related to Gram-positive bacteria. MALP-2 is recognized by mammalian TLR2 cooperatively with TLR613,14 and is one of the few natural TLR2 ligands so far definitely identified. Of further importance, synthetic MALP-2 stimulation of the immune response has potential therapeutic implications, eg, as a mucosal adjuvant for vaccination15 or to induce lipopolysaccharide cross-tolerance.16 In addition, MALP-2 exhibits even regenerative potential because MALP-2 has been successfully used to accelerate dermal wound healing in diabetic mice.9 Wound repair is a tightly regulated scenario involving inflammatory processes as well as the reestablishment of a capillary network by endothelial cells, termed angiogenesis. Angiogenesis is initiated and maintained by factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and angiopoietins. Besides endothelial cells, the angiogenic process involves inflammatory cells as a major source for growth factors. These play a role under physiologic as well as pathologic conditions during development, pathogen defense, tissue regeneration, and tumor growth.17,18
This study is focused on the potential influence of synthetic MALP-2 on angiogenesis. For this purpose, we here used in vitro and in vivo Matrigel assays. The results presented here clearly demonstrate that MALP-2 promoted angiogenesis in a TLR2/6-dependent manner. Stimulation of TLR2/6 induced endothelial cell proliferation as well as migration of endothelial cells and leukocytes. Endothelial cells and the monocyte subpopulation secreted remarkable amounts of granulocyte-macrophage colony-stimulating factor (GM-CSF) in response to MALP-2. Blockade of GM-CSF by different strategies effectively prevented MALP-2-induced angiogenesis. Taken together, our results established a TLR2/6-dependent induction of angiogenesis by MALP-2 that is mediated by GM-CSF. This might represent a general mechanism to recruit immune cells for pathogen defense and tissue regeneration with additional implication for future angiogenic therapy.
Methods
Antibodies and reagents
All chemicals were obtained from Sigma-Aldrich unless otherwise specified. All cell-culture plates were from TTP. MALP-2 was synthesized and purified as described,12 dissolved in 30% 2-propanol/water to a 1-mg/mL stock solution, and diluted in cell-culture medium for in vitro applications and in 0.9% sodium chloride for in vivo applications, respectively. VEGF165 and GM-CSF were from PeproTech, bFGF was from CellSystems. All used antibodies, pharmacologic inhibitors, and primer sequences are listed in the supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Mice and cells
C57BL/6 mice (Charles River Laboratories) were maintained at the animal facility of the Hannover Medical School. All animal experiments were performed with the approval of the Animal Research Committee of Hannover Medical School. For experiments with aortic segments, mice were anesthetized with ketamine (400 mg/kg) and xylazine (5 mg/kg), killed, and opened. Subsequently, the aorta was perfused with phosphate-buffered saline and explanted. For some experiments, the endothelium was removed mechanically by repeated pull-through with a silicon catheter under suction. Afterward, the aorta was either embedded in optimum cutting temperature (OCT) medium or was cut in 1- to 2-mm segments with a scalpel and kept for further experiments in 96-well plates containing Dulbecco modified Eagle medium (DMEM) with 20% fetal calf serum (FCS). Human endothelial cells (human umbilical vein endothelial cells [HUVECs], human umbilical artery endothelial cells [HUAECs], human microvascular endothelial cells [HMVECs]) were obtained from Lonza. Cells were cultured in endothelial cell growth medium (EGM-2; Lonza) supplemented with 2% FCS and growth factors, and kept in endothelial basal medium (EBM-2, Lonza) with 0.5% FCS without growth factors during experiments. Cells between passage 2 and 4 were used for all experiments. Human leukocytes were isolated from the blood of healthy subjects. Erythrocytes were removed by hemolysis (155mM NH4Cl, 10mM KHCO3, 0.01% ethylenediaminetetraacetic acid; for 10 minutes) and blood leukocytes were resuspended in X-vivo 15 medium (Lonza) for further analysis. Leukocyte subpopulations were flow cytometrically sorted (FACSAria II; BD Biosciences) according to light scatter properties and CD14 expression. Purity of sorted lymphocyte, granulocyte, and monocyte populations was confirmed by staining with a mixture of antibodies against CD3, CD14, CD15, CD16, CD19, and CD56.
Matrigel plug angiogenesis assay in vivo
Ten- to 12-week-old C57BL/6 mice were anesthetized with ketamine (400 mg/kg) and xylazine (5 mg/kg) and received 0.5-mL injections of sterile chilled Matrigel (growth factor reduced; BD Biosciences) supplemented with MALP-2 (1 μg/mL), Pam2CSK4 (1 μg/mL), Pam3CAK4 (1 μg/mL), VEGF (50 ng/mL), or GM-CSF (100 ng/mL) subcutaneously into the abdominal wall. For some experiments, antibodies against TLR2 (25 μg/mL), TLR6 (25 μg/mL), GM-CSF (10 μg/mL), and appropriate IgG control (10 and 50 μg/mL) were additionally administered to the Matrigel. Control mice were injected with Matrigel without any supplements. Matrigel plugs were removed 6 days after implantation, photographed, and divided into 2 blocks. One Matrigel plug was digested with Dispase (BD Biosciences), and hemoglobin content was determined by the Drabkin method according to the manufacturer's protocol (Sigma-Aldrich). Absorbance was measured in a microplate reader (μQuant; Bio-Tek Instruments) at 540 nm. Hemoglobin concentration in the Matrigel plugs was calculated with the help of a hemoglobin (Sigma-Aldrich) standard curve and normalized to the plug weight. The other Matrigel block was fixed overnight in 3.7% formalin and embedded in paraffin. Sections (6 μm) were stained with hematoxylin and eosin. Sections were visualized and quantified using a microscope (DM 4000 B; Leica). Vascular structures were expressed as percentage vascular area per field.
Matrigel angiogenesis assay in vitro (tube formation)
HUVECs were cultured in EBM-2 without FCS overnight before being plated in 96-well plates (1 × 104 cells per well) previously coated with 25 μL of Matrigel (growth factor-reduced; BD Biosciences), in the presence of MALP-2 (0.01, 0.1, and 1.0 μg/mL), Pam2CSK4 (1 μg/mL), Pam3CAK4 (1 μg/mL), or bFGF (100 ng/mL) in 100 μL of EBM-2. For some experiments, HUVECs were treated with antibodies against TLR2 (25 μg/mL), TLR6 (25 μg/mL), GM-CSF (10 μg/mL), and appropriate control IgG (10 and 50 μg/mL) or siRNA against GM-CSF and control siRNA (10 nM), before MALP-2 stimulation. After 24 hours of culturing, tube-like structures were staining with the intracellular fluorescent dye 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (1:5000 in phosphate-buffered saline for 15 minutes) and subsequently visualized and quantified using an inverted fluorescence microscope (Axiovert 200M; Carl Zeiss).
Proliferation assay
HUVECs were cultured in gelatin-coated 96-well plates in EGM-2 to approximately 50% confluence and maintained overnight in EBM-2. Cell proliferation after 16 hours was measured on the basis of DNA synthesis by 5-bromo-2′-deoxyuridine (BrdU) incorporation in the presence of MALP-2 (0.01, 0.1, and 1.0 μg/mL) and 10% FCS. For some experiments, HUVECs were treated with antibodies against GM-CSF (10 μg/mL) or appropriate control IgG (10 μg/mL). The amount of BrdU incorporation was determined with a commercial colorimetric quantification kit (Roche Diagnostics) according to the manufacturer's protocol by measuring the absorbance at 450 nm with a plate reader (μQuant; Bio-Tek Instruments).
Cell migration assay
HUVECs (1 × 105) in 100 μL of EBM-2 were placed in the upper chamber of transwell cell culture inserts (8-μm pore size; Corning Life Sciences). The lower chamber contained 600 μL of EBM-2 supplemented with MALP-2 (0.01, 0.1, and 1.0 μg/mL) or 10% FCS. For some experiments, the supernatants of HUVEC stimulated for 24 hours with MALP-2 (1 μg/mL) alone or preabsorbed with antibodies against GM-CSF (10 μg/mL) or appropriate IgG control (10 μg/mL) were used. Human leukocytes (1 × 105) were treated in the same manner but kept in X-vivo 15 medium during experiments and transwell cell culture inserts with 5-μm pore size were used. Migration was carried out for 18 hours at 37°C and 5% CO2. Migrated cells into the lower chamber were quantified by counting in a Neubauer counting chamber using an inverted cell culture microscope (Olympus).
Immunohistochemistry and immunofluorescence staining
Cryosections (7 μm) of OCT-embedded Matrigel plugs were fixed in ice-cold acetone and stained with anti-CD31 (1:200), anti-CD45 (1:200), anti-TLR2 (1:100), anti-TLR6 (1:200), anti-MOMA-2 (1:100) followed by Alexa 488 and Alexa 555–conjugated secondary antibody (1:500), respectively. HUVECs were cultured to approximately 50% confluence on gelatin-coated coverslips in 24-well plates. After fixation with 3.7% formalin, cells were stained with anti-TLR2 and -TLR6 antibody (1:200) followed by Alexa 488 and Alexa 555–conjugated secondary antibody (1:2000), respectively. Sections (5 μm) of OCT-embedded segments of the aorta were fixated in ice-cold acetone and stained with anti-CD31 antibody (1:100) followed by Alexa 488–conjugated secondary antibody (1:400). Nuclei were counterstained with 4,6-diamidino-2-phenylindole. Samples were visualized using a microscope (DM 4000 B; Leica) or a laser scanning confocal microscope (DM IRB/TCS SP2 AOBS; Leica).
Protein array
Supernatants from HUVECs were analyzed with a commercial protein array for angiogenesis-related cytokines and growth factors (RayBiotech) according to the manufacturer's instructions. In brief, membranes were blocked for 30 minutes with blocking buffer and incubated with 2 mL of supernatants (1:2 diluted with blocking buffer) overnight at 4°C. After washing, biotin-conjugated antibody cocktail was added and again incubated overnight at 4°C, followed by a 2-hour incubation with streptavidin-conjugated peroxidase at room temperature. Membranes were incubated with peroxidase substrate and exposed to enhanced chemilumescence films (Hyperfilm ECL; GE Healthcare). Films were digitalized and quantified densitometrically using an image analysis system (GeneGenius) and the software Quantity One (Bio-Rad).
LDH cytotoxicity assay
HUVECs were grown to complete confluence in gelatin-coated 96-well plates. Cytotoxicity after MALP-2 stimulation was quantified by the detection of lactate dehydrogenase (LDH) in the supernatants using the LDH cytotoxicity detection kit (Roche Diagnostics) following the manufacturer's protocol with the help of a plate reader (μQuant; Bio-Tek Instruments).
ELISA and enzyme-linked immunosorbent spot assay
For enzyme-linked immunosorbent assay (ELISA), HUVECs, HUAECs, and HMVECs (1.5 × 104) were plated in gelatin-coated 96-well plates in EGM-2 for 24 hours and maintained overnight in EBM-2. For some experiments, HUVECs were treated with antibodies against TLR2 (10 μg/mL), TLR6 (10 μg/mL), and appropriate control IgG (20 μg/mL) or siRNA against GM-CSF (10 nM) and control siRNA (10 nM) or pharmacologic mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) inhibitors PD98059 (5 μM), PD169316 (20 μM), SP600125 (10 μM), and TMB-8 (20 μM) before MALP-2 stimulation. Human leukocytes (1.5 × 104 and 1.5 × 105) and lymphocytes, granulocytes, and monocytes (1.5 × 105) after cell sorting were plated in X-vivo 15 medium in 96-well plates and directly used for further experiments. Aortic segments were placed in 96-well plates containing DMEM with 20% FCS and maintained overnight in DMEM. For some experiments, aortic segments were treated with antibodies against TLR2 (10 μg/mL), TLR6 (10 μg/mL), or appropriate control IgG (20 μg/mL) before MALP-2 stimulation. Supernatants from cultured cells and aortic segments were analyzed for GM-CSF after MALP-2 stimulation using a commercial ELISA (R&D Systems) according to the manufacturer's protocol with the help of a plate reader (μQuant; Bio-Tek Instruments).
For enzyme-linked immunosorbent spot (ELISPOT) assay, HUVECs and human leukocytes (1.5 × 104) were plated in 96-well plates containing polyvinylidene difluoride membranes in EBM-2 and X-vivo 15 medium, respectively. GM-CSF secretion after MALP-2 stimulation was visualized using a commercial ELISPOT assay (MABTECH) according to the manufacturer's protocol.
siRNA knockdown
HUVECs were seeded in 6-well plates (100 000 cells per well) and cultured in EGM-2. To silence the expression of tumor necrosis factor receptor associated factor-6 (TRAF-6) and GM-CSF, cells were transfected 24 and 48 hours after seeding using HiPerFect Transfection reagent (QIAGEN) and siRNA (10 nM, TRAF-6 siRNA no. SI00066115, GM-CSF siRNA no. SI03037272, control siRNA no. 102728010; QIAGEN) for 6 hours in EBM-2. At day 3 after seeding, cells were used for further experiments. Tranfection efficiency (> 90%) was determined by transfecting Alexa 588–labeled siRNA. Effective silencing of TRAF-6 and GM-CSF siRNA was determined by Western blot and ELISA, respectively.
In silico promoter analysis
Statistical analysis
Data are presented as the mean plus or minus SEM of at least 3 independent experiments. Comparisons were made by the 2-tailed Student t test for independent samples or one-way analysis of variance and post hoc Scheffé test as appropriate. P values less than .05 were considered statistically significant.
Results
MALP-2 up-regulates TLR2/6 expression in endothelial cells
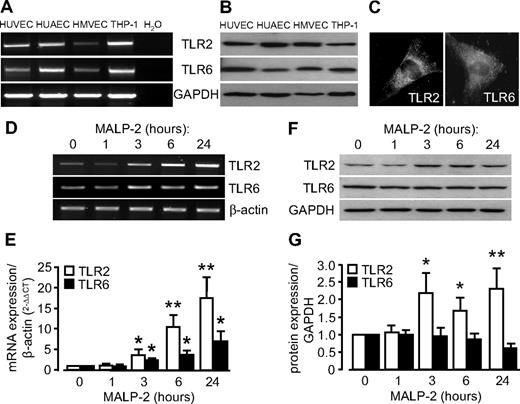
To rule out any potential cytotoxic effects of bacterial MALP-2 on endothelial cells (HUVECs), we measured cytotoxicity by LDH release. However, no cytotoxic effects of synthetic MALP-2 up to 1 μg/mL for 24 hours could be detected (supplemental Figure 1). Expression of the MALP-2 receptors TLR2 and TLR6 in endothelial cell of venous origin (HUVECs), arterial origin (HUAECs), and microvascular origin (HMVECs) was demonstrated by polymerase chain reaction (PCR) and Western blot (Figure 1A-B). Moreover, HUVECs were positive for immunofluorescence staining for both TLR2 and TLR6 (Figure 1C). MALP-2 (1 μg/mL) stimulation increased TLR2 and TLR6 mRNA expression (TLR2 ∼ 17 times, TLR6 ∼ 7 times, maximal at 24 hours) but solely TLR2 protein expression (∼ 2 times at 3, 6, and 24 hours), pointing to an activation or even sensitizing of endothelial cells toward MALP-2. Interestingly, beyond TLR2 and TLR6, mRNA expression of other TLRs, such as TLR5, TLR7, TLR8, and TLR9, was likewise significantly up-regulated in HUVECs after 24 hours of MALP-2 stimulation, which even indicates a more general activation of the TLR system by MALP-2 (supplemental Figure 2).
MALP-2 up-regulates TLR2/6 expression in endothelial cells. (A) TLR2 and TLR6 mRNA expression was determined by PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression is shown as loading control. Representative pictures of 3 independent experiments are shown. (B) TLR2 and TLR6 protein expression was demonstrated by Western blot. GAPDH expression is shown as loading control. Representative pictures of 3 independent experiments are shown. Human monocytic THP-1 cells were used as positive control. (C) Immunofluorescent staining of HUVECs with antibodies against TLR2 and TLR6. Representative pictures of 3 independent experiments are shown (Leica DM 4000B microscope, 40×/0.75 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (D) Products of real-time PCR were run on agarose gels showing TLR2 and TLR6 mRNA levels in HUVECs after MALP-2 (1 μg/mL) stimulation for the indicated time points. β-Actin mRNA expression is shown as loading control. Representative pictures are shown. (E) Quantification of TLR2 and TLR6 mRNA levels relative to β-actin mRNA levels by real-time PCR (n = 10). *P < .05, **P < .01 vs 0 hours. (F) Western blot showing TLR2 and TLR6 protein levels in HUVECs after MALP-2 (1 μg/mL) stimulation for the indicated time points. GAPDH expression is shown as loading control. Representative pictures are shown. (G) Quantification of TLR2 and TLR6 protein levels relative to GAPDH protein levels (n = 4-6). *P < .05, **P < .01 vs 0 hours. (E,G) Error bars represent mean ± SEM.
MALP-2 up-regulates TLR2/6 expression in endothelial cells. (A) TLR2 and TLR6 mRNA expression was determined by PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression is shown as loading control. Representative pictures of 3 independent experiments are shown. (B) TLR2 and TLR6 protein expression was demonstrated by Western blot. GAPDH expression is shown as loading control. Representative pictures of 3 independent experiments are shown. Human monocytic THP-1 cells were used as positive control. (C) Immunofluorescent staining of HUVECs with antibodies against TLR2 and TLR6. Representative pictures of 3 independent experiments are shown (Leica DM 4000B microscope, 40×/0.75 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (D) Products of real-time PCR were run on agarose gels showing TLR2 and TLR6 mRNA levels in HUVECs after MALP-2 (1 μg/mL) stimulation for the indicated time points. β-Actin mRNA expression is shown as loading control. Representative pictures are shown. (E) Quantification of TLR2 and TLR6 mRNA levels relative to β-actin mRNA levels by real-time PCR (n = 10). *P < .05, **P < .01 vs 0 hours. (F) Western blot showing TLR2 and TLR6 protein levels in HUVECs after MALP-2 (1 μg/mL) stimulation for the indicated time points. GAPDH expression is shown as loading control. Representative pictures are shown. (G) Quantification of TLR2 and TLR6 protein levels relative to GAPDH protein levels (n = 4-6). *P < .05, **P < .01 vs 0 hours. (E,G) Error bars represent mean ± SEM.
TLR2/6-dependent stimulation by MALP-2 promotes angiogenesis in vivo and in vitro
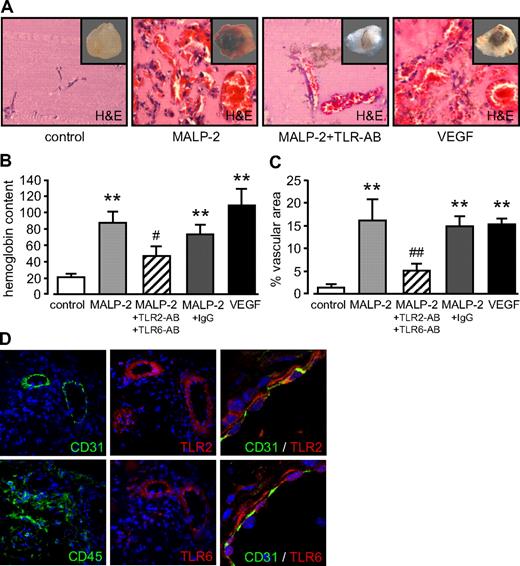
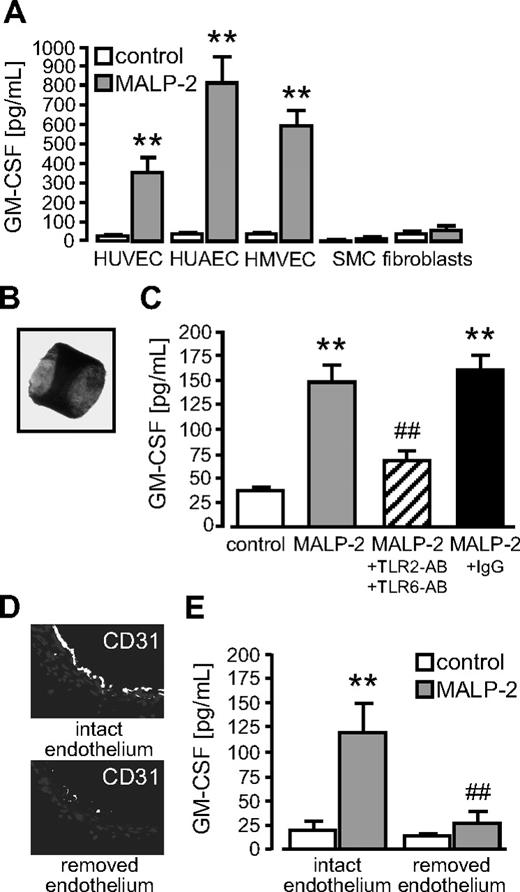
Incorporation of MALP-2 (1 μg/mL) in Matrigel, which was subsequently injected subcutaneously into mice, led to the development of vascular structures in Matrigel plugs within 6 days (Figure 2A). The extent of the angiogenesis in vivo was quantified by the plug's hemoglobin content (Figure 2B) and the determination of the plug's area covered by vascular structure filled with erythrocytes (Figure 2C) as described recently.21 The angiogenic response of MALP-2 was comparable with that of the positive control VEGF and could be blocked with antibodies against TLR2 and TLR6, whereas unspecific control IgG did not influence MALP-2-induced angiogenesis. To further characterize the angiogenic Matrigel plug after MALP-2 application, we performed immunofluorescence staining for endothelial cells and leucocytes as well as for the MALP-2 receptors TLR2 and TLR6. In consecutive sections, we detected a CD31+ endothelial monolayer lining the lumen of vascular structures, which were surrounded by CD45+ cells. Both cell types are important for angiogenesis. TLR2 and TLR6 were found to be predominantly expressed in vascular structures, and confocal microscopy revealed a colocalization of both receptors with the CD31+ endothelium (Figure 2D).
TLR2/6-dependent stimulation by MALP-2 promotes angiogenesis in vivo. (A) Representative pictures of hematoxylin and eosin-stained sections of Matrigel plugs after the administration of MALP-2 (1 μg/mL) for 6 days. VEGF (50 ng/mL) was used as positive control. AB indicates antibody (25 μg/mL each). Insets represent the total Matrigel plug. Vascular structures are filled with erythrocytes. (B) Hemoglobin content is given in micrograms per milligram of Matrigel. (C) Vascular structures are expressed as percentage vascular area per field. Unspecific IgG (50 μg/mL) was used as antibody control (n = 4-8). **P < .01 vs control. #P < .05, ##P < .01 vs MALP-2. (B-C) Error bars represent mean ± SEM. (D) Immunofluorescent staining of Matrigel plaques after the administration of MALP-2 (1 μg/mL) for 6 days with antibodies against CD31, CD45, TLR2, and TLR6. Colocalization of CD31 with TLR2 and TLR6, respectively, was visualized by confocal microscopy (right panels). Representative pictures of 3 independent experiments are shown. Image acquisition: (A and left/middle panels of D) Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software. (D right panels) Leica DM IRB microscope, 40×/1.25 NA oil objective, Leica immersion oil, TCS SP2 AOBS scanhead, Leica LCS confocal software, Version 1347.
TLR2/6-dependent stimulation by MALP-2 promotes angiogenesis in vivo. (A) Representative pictures of hematoxylin and eosin-stained sections of Matrigel plugs after the administration of MALP-2 (1 μg/mL) for 6 days. VEGF (50 ng/mL) was used as positive control. AB indicates antibody (25 μg/mL each). Insets represent the total Matrigel plug. Vascular structures are filled with erythrocytes. (B) Hemoglobin content is given in micrograms per milligram of Matrigel. (C) Vascular structures are expressed as percentage vascular area per field. Unspecific IgG (50 μg/mL) was used as antibody control (n = 4-8). **P < .01 vs control. #P < .05, ##P < .01 vs MALP-2. (B-C) Error bars represent mean ± SEM. (D) Immunofluorescent staining of Matrigel plaques after the administration of MALP-2 (1 μg/mL) for 6 days with antibodies against CD31, CD45, TLR2, and TLR6. Colocalization of CD31 with TLR2 and TLR6, respectively, was visualized by confocal microscopy (right panels). Representative pictures of 3 independent experiments are shown. Image acquisition: (A and left/middle panels of D) Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software. (D right panels) Leica DM IRB microscope, 40×/1.25 NA oil objective, Leica immersion oil, TCS SP2 AOBS scanhead, Leica LCS confocal software, Version 1347.
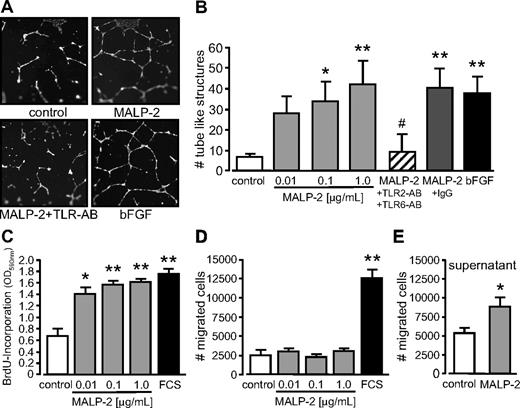
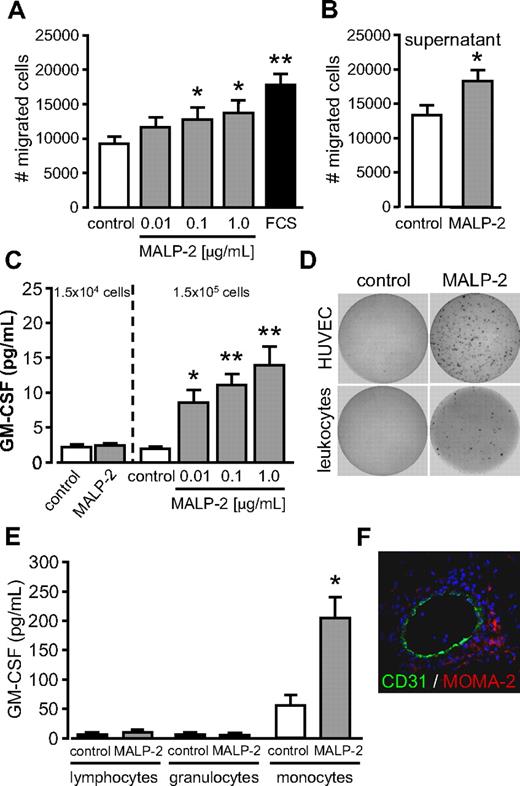
Angiogenesis describes the process of vessel sprouting from preexisting ones. Therefore, we determined the capacity of MALP-2 to generate tube-like structures from HUVECs in Matrigel in vitro. We observed a dose-dependent increase in the number of tube-like structures in response to MALP-2. In turn, the effect was comparable with the positive control bFGF and could be efficiently blocked by the use of antibodies against TLR2 and TLR6, whereas unspecific IgG did not show any influence (Figure 3A-B). Because angiogenesis critically depends on proliferation and migration of endothelial cells, we next addressed these issues in vitro. We found a dose-dependent increase in HUVEC proliferation after MALP-2 stimulation as assessed by BrdU incorporation (Figure 3C). By contrast, we did not observe any direct effects on HUVEC migration when MALP-2 was provided as a chemoattractant in transwell cell-culture inserts (Figure 3D). However, migration was significantly enhanced in response to cell culture supernatants obtained from HUVECs stimulated with 1 μg/mL MALP-2 for 24 hours compared with supernatants from unstimulated HUVECs (Figure 3E). These data suggest that the observed indirect effect on migration is mediated by autocrine factors.
TLR2/6-dependent stimulation by MALP-2 promotes angiogenesis in vitro as well as proliferation and migration of endothelial cells. (A) Pictures represent fluorescence of BCECF-AM-stained tube-like structures of HUVECs 24 hours after the administration of MALP-2 (1 μg/mL). Representative pictures are shown (Zeiss Axiovert 200M microscope, 20×/0.50 NA dry objective, Zeiss AxioCam MRc camera, AxiVision Version 4 software). (B) Quantification of the numbers (#) of tube-like structures of HUVECs. bFGF (100 ng/mL) was used as positive control. Unspecific IgG (50 μg/mL) was used as antibody control (n = 3-5). AB indicates antibody (25 μg/mL each). *P < .05, **P < .01 vs control. #P < .05 vs MALP-2. (C) Proliferation of HUVECs was determined by BrdU incorporation for 16 hours after stimulation with different concentrations of MALP-2 as indicated; 10% FCS was used as positive control (n = 6). *P < .05, **P < .01 vs control. (D) Migration of HUVECs (1 × 105) was carried out in transwell cell culture inserts for 24 hours after stimulation with different concentrations of MALP-2 as indicated; 10% FCS was used as positive control (n = 5-7). **P < .01 vs control. (E) Migration of HUVECs (1 × 105) was carried out in transwell cell culture inserts for 24 hours. As chemoattractant, supernatants from HUVECs after 24-hour stimulation with MALP-2 (1 μg/mL) were used and compared with control supernatant from unstimulated HUVECs (n = 5). *P < .05 vs control. (B-E) Error bars represent mean ± SEM.
TLR2/6-dependent stimulation by MALP-2 promotes angiogenesis in vitro as well as proliferation and migration of endothelial cells. (A) Pictures represent fluorescence of BCECF-AM-stained tube-like structures of HUVECs 24 hours after the administration of MALP-2 (1 μg/mL). Representative pictures are shown (Zeiss Axiovert 200M microscope, 20×/0.50 NA dry objective, Zeiss AxioCam MRc camera, AxiVision Version 4 software). (B) Quantification of the numbers (#) of tube-like structures of HUVECs. bFGF (100 ng/mL) was used as positive control. Unspecific IgG (50 μg/mL) was used as antibody control (n = 3-5). AB indicates antibody (25 μg/mL each). *P < .05, **P < .01 vs control. #P < .05 vs MALP-2. (C) Proliferation of HUVECs was determined by BrdU incorporation for 16 hours after stimulation with different concentrations of MALP-2 as indicated; 10% FCS was used as positive control (n = 6). *P < .05, **P < .01 vs control. (D) Migration of HUVECs (1 × 105) was carried out in transwell cell culture inserts for 24 hours after stimulation with different concentrations of MALP-2 as indicated; 10% FCS was used as positive control (n = 5-7). **P < .01 vs control. (E) Migration of HUVECs (1 × 105) was carried out in transwell cell culture inserts for 24 hours. As chemoattractant, supernatants from HUVECs after 24-hour stimulation with MALP-2 (1 μg/mL) were used and compared with control supernatant from unstimulated HUVECs (n = 5). *P < .05 vs control. (B-E) Error bars represent mean ± SEM.
TLR2/6-dependent stimulation by MALP-2 induces GM-CSF release from HUVECs
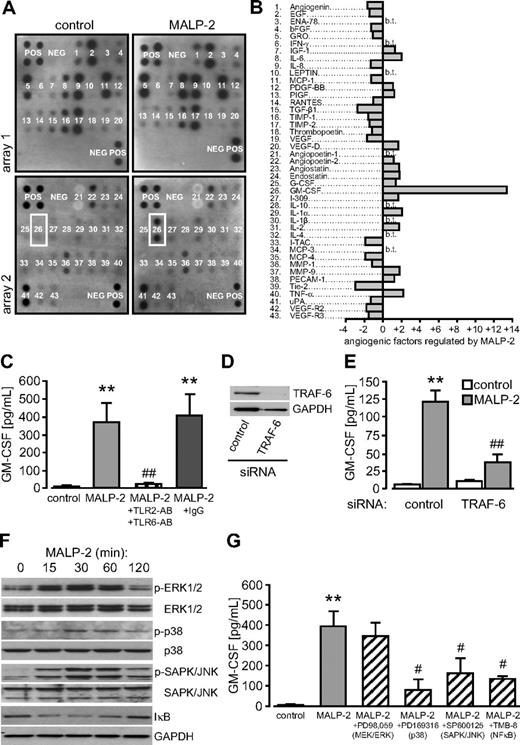
To investigate which factors are released by endothelial cells in response to MALP-2 and might be involved in angiogenesis, we used protein arrays specific for angiogenesis-related factors. To this end, we stimulated HUVECs with 1 μg/mL MALP-2 for 24 hours and subjected the collected supernatants to protein array analysis. Most of the factors represented on the array were hardly regulated by MALP-2 stimulation. However, the release of one factor was enhanced more than 10-fold and could be identified as GM-CSF (Figure 4A-B). ELISA analysis confirmed the protein data and revealed that GM-CSF was barely detectable under control conditions but accumulated to approximately 400 pg/mL 24 hours after MALP-2 stimulation. This effect was completely blocked by antibodies against TLR2 and TLR6 (Figure 4C). MALP-2–dependent GM-CSF release from HUVECs was found to be dose- and time-dependent (supplemental Figure 3A-B). In addition to inhibitory antibodies against TLR2 and TLR6, we established siRNA targeted against TRAF-6, a common signaling component downstream of TLRs (Figure 4D).22 Administration of this siRNA to HUVECs significantly inhibited GM-CSF release after MALP-2 stimulation (Figure 4E). TLR-dependent signaling involves major inflammatory pathways, such as the MAPK cascade and the NF-κB pathway (supplemental Figure 4A).22 Accordingly, we observed enhanced phosphorylation of the extracellular signal-regulated kinase 1/2, the p38 MAPK, and the stress-activated protein kinase/c-Jun N-terminal kinase after MALP-2 stimulation in HUVECs with a maximum at 30 minutes. In addition, we observed a transient degradation of the inhibitor κB after MALP-2, reflecting the activation of the NF-κB pathway (Figure 4F). In silico analysis of the human GM-CSF promoter using the MatInspector software20 predicted binding sites for transcription factors related to all of these pathways (supplemental Figure 4B). Using pharmacologic inhibitors for each pathway, we observed that inhibition of p38 (PD169316), stress-activated protein kinase/c-Jun N-terminal kinase (SP600125), and NF-κB (TMB-8), but not of MEK/extracellular signal-regulated kinase (PD98059), blocked MALP-2–induced GM-CSF release (Figure 4G). Thus, different inflammatory pathways seem to cooperate during MALP-2-dependent GM-CSF release in endothelial cells. Inhibitors alone did not enhance GM-CSF levels in HUVECs (data not shown).
TLR2/6-dependent stimulation by MALP-2 induces GM-CSF release from HUVECs. (A) Pictures represent protein array membranes specific for angiogenesis-related factors. Membranes were incubated with supernatants from HUVECs not stimulated (control) or stimulated with MALP-2 (1 μg/mL) for 24 hours. Representative pictures of 2 independent experiments are shown. The position of GM-CSF is indicated by a white box. POS indicates positive control; NEG, negative control. (B) Quantification of the dot intensity relative to the positive control (n = 2). b.t. indicates below threshold. (C) GM-CSF levels in supernatants of HUVECs after MALP-2 (1 μg/mL) stimulation for 24 hours as assessed by ELISA. Unspecific IgG (50 μg/mL) was used as antibody control (n = 4). AB indicates antibody (25 μg/mL each). **P < .01 vs control. ##P < .01 vs MALP-2. (D) Knockdown of TRAF-6 protein levels after siRNA application (10 nM) in HUVECs was confirmed by Western blot. GAPDH expression is shown as loading control. Representative pictures of 3 independent experiments are shown. (E) GM-CSF levels in supernatants of HUVECs after siRNA application (10 nM) and MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2 plus control siRNA. (F) Activation of the MAPK and the NF-κB pathway after MALP-2 (1 μg/mL) stimulation for the indicated time points was demonstrated by Western blot. Representative pictures of 3 independent experiments are shown. (G) GM-CSF levels in supernatants of HUVECs after MALP-2 (1 μg/mL) stimulation for 24 hours in the presence of pharmacologic inhibitors of the MAPK and the NF-κB pathway are assessed by ELISA (n = 5). **P < .01 vs control. #P < .01 vs MALP-2. (C,E,G) Error bars represent mean ± SEM.
TLR2/6-dependent stimulation by MALP-2 induces GM-CSF release from HUVECs. (A) Pictures represent protein array membranes specific for angiogenesis-related factors. Membranes were incubated with supernatants from HUVECs not stimulated (control) or stimulated with MALP-2 (1 μg/mL) for 24 hours. Representative pictures of 2 independent experiments are shown. The position of GM-CSF is indicated by a white box. POS indicates positive control; NEG, negative control. (B) Quantification of the dot intensity relative to the positive control (n = 2). b.t. indicates below threshold. (C) GM-CSF levels in supernatants of HUVECs after MALP-2 (1 μg/mL) stimulation for 24 hours as assessed by ELISA. Unspecific IgG (50 μg/mL) was used as antibody control (n = 4). AB indicates antibody (25 μg/mL each). **P < .01 vs control. ##P < .01 vs MALP-2. (D) Knockdown of TRAF-6 protein levels after siRNA application (10 nM) in HUVECs was confirmed by Western blot. GAPDH expression is shown as loading control. Representative pictures of 3 independent experiments are shown. (E) GM-CSF levels in supernatants of HUVECs after siRNA application (10 nM) and MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2 plus control siRNA. (F) Activation of the MAPK and the NF-κB pathway after MALP-2 (1 μg/mL) stimulation for the indicated time points was demonstrated by Western blot. Representative pictures of 3 independent experiments are shown. (G) GM-CSF levels in supernatants of HUVECs after MALP-2 (1 μg/mL) stimulation for 24 hours in the presence of pharmacologic inhibitors of the MAPK and the NF-κB pathway are assessed by ELISA (n = 5). **P < .01 vs control. #P < .01 vs MALP-2. (C,E,G) Error bars represent mean ± SEM.
Next, we addressed the question whether the observed effects on GM-CSF release and on angiogenesis are specific for TLR2/6 and MALP-2, respectively. Therefore, we used the synthetic TLR2/6 ligand Pam2CSK4 and the TLR2/1 ligand Pam3CSK4. Solely Pam2CSK4 stimulation induced GM-CSF release from HUVECs (supplemental Figure 5A) and significantly promoted angiogenesis in vitro (supplemental Figure 5B) and in vivo (supplemental Figure 5C-E). In this regard, our data demonstrate particularly the contribution of the TLR2/6 pathway.
Vascular GM-CSF released by TLR2/6-dependent MALP-2 stimulation exclusively occurs from the endothelium
To test whether effects of GM-CSF release were restricted to HUVECs, we investigated different other vascular cell types. We found an even stronger GM-CSF release in response to MALP-2 from endothelial cells of arterial and microvascular origin but hardly any GM-CSF release from smooth muscle cells and fibroblasts (Figure 5A). To study the whole vessel wall, we prepared segments of murine aortas (Figure 5B). Ex vivo stimulation of these segments with MALP-2 led to a remarkable release of GM-CSF, which could significantly be inhibited by the use of antibodies against TLR2 and TLR6 (Figure 5C). Next, we mechanically removed the endothelium lining the lumen of the vessel wall. The removal was confirmed by immunofluorescent staining for the endothelium-specific marker CD31 (Figure 5D). GM-CSF release in response to MALP-2 was completely abrogated when the endothelium was removed, demonstrating that the endothelium represents the exclusive source of vascular GM-CSF synthesis in this context (Figure 5E).
Vascular GM-CSF released by TLR2/6-dependent MALP-2 stimulation occurs exclusively from the endothelium. (A) GM-CSF levels in supernatants of different vascular cells after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 3-6). SMC indicates smooth muscle cells. **P < .01 vs control. (B) Picture represents a segment of the murine aorta (Zeiss Axiovert 200M microscope, 5×/0.25 NA dry objective, Zeiss AxioCam MRc camera, AxiVision Version 4 software). (C) GM-CSF levels in the supernatants of murine aortic segments after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA. Unspecific IgG (50 μg/mL) was used as antibody control (n = 4-6). AB indicates antibody (25 μg/mL each,  ). **P < .01 vs control. ##P < .01 vs MALP-2. (D) Immunofluorescent staining of murine aortic segments with antibodies against CD31. Representative pictures of segments with intact or removed endothelium are shown (Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (E) GM-CSF levels in supernatants of murine aortic segments with intact or removed endothelium after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2. (A,C,E) Error bars represent mean ± SEM.
). **P < .01 vs control. ##P < .01 vs MALP-2. (D) Immunofluorescent staining of murine aortic segments with antibodies against CD31. Representative pictures of segments with intact or removed endothelium are shown (Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (E) GM-CSF levels in supernatants of murine aortic segments with intact or removed endothelium after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2. (A,C,E) Error bars represent mean ± SEM.
Vascular GM-CSF released by TLR2/6-dependent MALP-2 stimulation occurs exclusively from the endothelium. (A) GM-CSF levels in supernatants of different vascular cells after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 3-6). SMC indicates smooth muscle cells. **P < .01 vs control. (B) Picture represents a segment of the murine aorta (Zeiss Axiovert 200M microscope, 5×/0.25 NA dry objective, Zeiss AxioCam MRc camera, AxiVision Version 4 software). (C) GM-CSF levels in the supernatants of murine aortic segments after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA. Unspecific IgG (50 μg/mL) was used as antibody control (n = 4-6). AB indicates antibody (25 μg/mL each,  ). **P < .01 vs control. ##P < .01 vs MALP-2. (D) Immunofluorescent staining of murine aortic segments with antibodies against CD31. Representative pictures of segments with intact or removed endothelium are shown (Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (E) GM-CSF levels in supernatants of murine aortic segments with intact or removed endothelium after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2. (A,C,E) Error bars represent mean ± SEM.
). **P < .01 vs control. ##P < .01 vs MALP-2. (D) Immunofluorescent staining of murine aortic segments with antibodies against CD31. Representative pictures of segments with intact or removed endothelium are shown (Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (E) GM-CSF levels in supernatants of murine aortic segments with intact or removed endothelium after MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2. (A,C,E) Error bars represent mean ± SEM.
TLR2/6-dependent stimulation by MALP-2 promotes migration and GM-CSF release of leukocytes
Angiogenesis not only involves endothelial cells but also leukocytes, such as granulocytes and monocytes, which attain via the bloodstream to sites of angiogenesis. Accordingly, we found that Matrigel plugs after MALP-2 administration contain beyond CD31+ vascular structures abundant CD45+ cells (Figure 2D). To investigate whether MALP-2 could act as chemoattractant for leukocytes, we isolated leukocytes from the blood of healthy donors. Those cells were highly positive for the leukocyte common antigen CD45 (data not shown). In transwell migration assays, a direct and dose-dependent effect of MALP-2 on leukocyte migration could be shown (Figure 6A). Similar to HUVECs, migration of leukocytes was significantly enhanced in response to cell-culture supernatants obtained from leukocytes stimulated with 1 μg/mL MALP-2 for 24 hours compared with supernatants from unstimulated leukocytes (Figure 6B). Our findings indicate that, contrary to HUVECs, MALP-2 represents a direct chemoattractant for leukocytes but could also induce autocrine migratory effects. Plating equivalent cell number compared with HUVECs (1.5 × 104 per well), we did not detect elevated GM-CSF levels in the supernatant of leukocytes in response to MALP-2. But a 10-fold increase in leukocyte cell number (1.5 × 105 per well) led to low but significant dose-dependent GM-CSF release from leukocytes on MALP-2 stimulation (Figure 6C). ELISPOT assays confirmed high GM-CSF secretion from HUVECs after MALP-2 stimulation. In contrast, just few spots were detected when leukocytes were treated with MALP-2 (Figure 6D). Separation of leukocytes by cell sorting revealed that the monocyte population already secreted considerable amounts of GM-CSF under basal conditions, which was significantly enhanced after MALP-2 stimulation. Lymphocytes and granulocytes did not secrete GM-CSF (Figure 6E). In this regard, we detected abundant monocytes/macrophages in close proximity to CD31+ vascular structures in the Matrigel plugs after MALP-2 administration (Figure 6F). In conclusion, in addition to endothelial cells, we could identify monocytes as a source for GM-CSF in response to MALP-2.
TLR2/6-dependent stimulation by MALP-2 promotes migration and GM-CSF release of leukocytes. (A) Migration of human leukocytes (1 × 105) was carried out in transwell cell-culture inserts for 24 hours after stimulation with different concentrations of MALP-2 as indicated; 10% FCS was used as positive control (n = 8). *P < .05, **P < .01 vs control. (B) Migration of human leukocytes (1 × 105) was carried out in transwell cell-culture inserts for 24 hours. As chemoattractant, supernatants from leukocytes after 24 hours stimulation with MALP-2 (1 μg/mL) were used and compared with control supernatant from unstimulated leukocytes (n = 6). *P < .05 vs control. (C) GM-CSF levels in supernatants of 1.5 × 104 and 1.5 × 105 human leukocytes after stimulation with MALP-2 (1 μg/mL) or with different concentrations of MALP-2 for 24 hours as indicated determined by ELISA (n = 7). *P < .05, **P < .01 vs control. (D) GM-CSF release from HUVECs (1.5 × 104) and human leukocytes (1.5 × 104) after stimulation with MALP-2 (1 μg/mL) for 24 hours assessed by ELISPOT. Representative pictures of 3 independent experiments are shown. (E) GM-CSF levels in sorted lymphocytes, granulocytes, and monocytes (1.5 × 105) after stimulation with MALP-2 (1 μg/mL) for 24 hours as determined by ELISA (n = 5). *P < .05 vs control. (F) Immunofluorescent staining of Matrigel plaques after the administration of MALP-2 (1 μg/mL) for 6 days with antibodies against CD31 and MOMA-2. Representative pictures of 3 independent experiments are shown (Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (A-C,E) Error bars represent mean ± SEM.
TLR2/6-dependent stimulation by MALP-2 promotes migration and GM-CSF release of leukocytes. (A) Migration of human leukocytes (1 × 105) was carried out in transwell cell-culture inserts for 24 hours after stimulation with different concentrations of MALP-2 as indicated; 10% FCS was used as positive control (n = 8). *P < .05, **P < .01 vs control. (B) Migration of human leukocytes (1 × 105) was carried out in transwell cell-culture inserts for 24 hours. As chemoattractant, supernatants from leukocytes after 24 hours stimulation with MALP-2 (1 μg/mL) were used and compared with control supernatant from unstimulated leukocytes (n = 6). *P < .05 vs control. (C) GM-CSF levels in supernatants of 1.5 × 104 and 1.5 × 105 human leukocytes after stimulation with MALP-2 (1 μg/mL) or with different concentrations of MALP-2 for 24 hours as indicated determined by ELISA (n = 7). *P < .05, **P < .01 vs control. (D) GM-CSF release from HUVECs (1.5 × 104) and human leukocytes (1.5 × 104) after stimulation with MALP-2 (1 μg/mL) for 24 hours assessed by ELISPOT. Representative pictures of 3 independent experiments are shown. (E) GM-CSF levels in sorted lymphocytes, granulocytes, and monocytes (1.5 × 105) after stimulation with MALP-2 (1 μg/mL) for 24 hours as determined by ELISA (n = 5). *P < .05 vs control. (F) Immunofluorescent staining of Matrigel plaques after the administration of MALP-2 (1 μg/mL) for 6 days with antibodies against CD31 and MOMA-2. Representative pictures of 3 independent experiments are shown (Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software). (A-C,E) Error bars represent mean ± SEM.
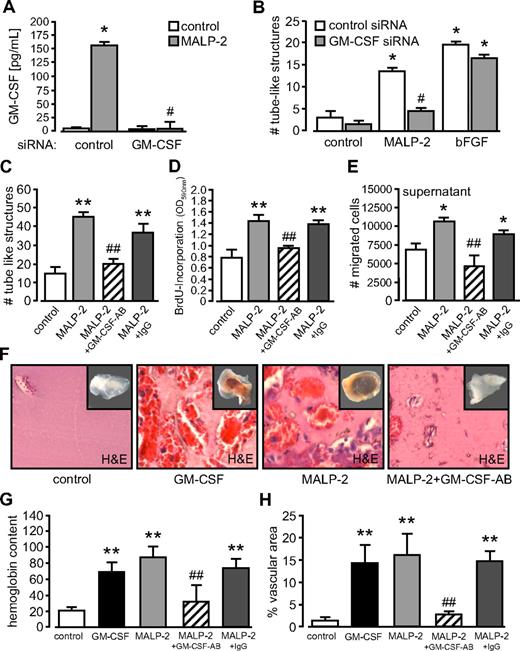
Blockade of GM-CSF inhibits MALP-2–induced angiogenesis
To elucidate the role of GM-CSF on angiogenesis after MALP-2 application, we used different strategies to block GM-CSF. First, for in vitro studies, we established siRNA targeted against GM-CSF. As determined in HUVECs, this siRNA efficiently blocked GM-CSF release in response to MALP-2 (Figure 7A). Next, we used GM-CSF siRNA to analyze tube formation of HUVECs in Matrigel. We observed a significant reduction in tube-like structures after the application of MALP-2 (Figure 7B). Of interest, GM-CSF blockage did not influence tube formation induced by the positive control bFGF. Similarly, an antibody against GM-CSF blocked MALP-2–dependent tube formation of HUVECs (Figure 7C). In addition, this antibody against GM-CSF inhibited proliferation of HUVECs in response to MALP-2 (Figure 7D) and completely blocked the migration of HUVECs toward supernatants of MALP-2–stimulated HUVECs (Figure 7E).
Blockade of GM-CSF inhibits MALP-2-induced angiogenesis. (A) GM-CSF levels in supernatants of HUVECs after siRNA application (10 nM) and MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 3). *P < .05 vs control. #P < .05 vs MALP-2. (B) Quantification of the numbers (#) of tube-like structures of HUVEC after application of siRNA (10 nM) and MALP-2 (1 μg/mL) for 24 hours. bFGF (100 ng/mL) was used as positive control (n = 4). *P < .05 vs control. #P < .05 vs MALP-2 plus control siRNA. (C) Quantification of the number (#) of tube-like structures of HUVEC after application of GM-CSF antibody and MALP-2 (1 μg/mL) for 24 hours. AB indicates antibody (10 μg/mL). Unspecific IgG (10 μg/mL) was used as antibody control (n = 4). **P < .01 vs control. ##P < .05 vs MALP-2. (D) Proliferation of HUVECs was determined by BrdU incorporation after application of GM-CSF antibody and MALP-2 (1 μg/mL) for 16 hours. AB indicates antibody (10 μg/mL). Unspecific IgG (10 μg/mL) was used as antibody control (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2. (E) Migration of HUVEC (1 × 105) was carried out in transwell cell-culture inserts for 24 hours. As chemoattractant, supernatants from unstimulated HUVEC or after 24-hour stimulation with MALP-2 (1 μg/mL) alone or pretreated with GM-CSF antibody were used (n = 4). AB indicates antibody (10 μg/mL). Unspecific IgG (10 μg/mL) was used as antibody control (n = 4). *P < .05 vs control. ##P < .01 vs MALP-2. (F) Representative pictures of hematoxylin and eosin–stained sections of Matrigel plugs 6 days after the administration of GM-CSF (100 ng/mL) and MALP-2 (1 μg/mL). AB indicates antibody (10 μg/mL each). Insets represent the total Matrigel plug. Vascular structures are filled with erythrocytes. Image acquisition: Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software. (G) Hemoglobin content is given as micrograms per milligram of Matrigel. (H) Vascular structures are expressed as percentage vascular area per field. Unspecific IgG (10 μg/mL) was used as antibody control (n = 4-8). **P < .01 vs control. ##P < .01 vs MALP-2. (A-E,G,H) Error bars represent mean ± SEM.
Blockade of GM-CSF inhibits MALP-2-induced angiogenesis. (A) GM-CSF levels in supernatants of HUVECs after siRNA application (10 nM) and MALP-2 (1 μg/mL) stimulation for 24 hours determined by ELISA (n = 3). *P < .05 vs control. #P < .05 vs MALP-2. (B) Quantification of the numbers (#) of tube-like structures of HUVEC after application of siRNA (10 nM) and MALP-2 (1 μg/mL) for 24 hours. bFGF (100 ng/mL) was used as positive control (n = 4). *P < .05 vs control. #P < .05 vs MALP-2 plus control siRNA. (C) Quantification of the number (#) of tube-like structures of HUVEC after application of GM-CSF antibody and MALP-2 (1 μg/mL) for 24 hours. AB indicates antibody (10 μg/mL). Unspecific IgG (10 μg/mL) was used as antibody control (n = 4). **P < .01 vs control. ##P < .05 vs MALP-2. (D) Proliferation of HUVECs was determined by BrdU incorporation after application of GM-CSF antibody and MALP-2 (1 μg/mL) for 16 hours. AB indicates antibody (10 μg/mL). Unspecific IgG (10 μg/mL) was used as antibody control (n = 4). **P < .01 vs control. ##P < .01 vs MALP-2. (E) Migration of HUVEC (1 × 105) was carried out in transwell cell-culture inserts for 24 hours. As chemoattractant, supernatants from unstimulated HUVEC or after 24-hour stimulation with MALP-2 (1 μg/mL) alone or pretreated with GM-CSF antibody were used (n = 4). AB indicates antibody (10 μg/mL). Unspecific IgG (10 μg/mL) was used as antibody control (n = 4). *P < .05 vs control. ##P < .01 vs MALP-2. (F) Representative pictures of hematoxylin and eosin–stained sections of Matrigel plugs 6 days after the administration of GM-CSF (100 ng/mL) and MALP-2 (1 μg/mL). AB indicates antibody (10 μg/mL each). Insets represent the total Matrigel plug. Vascular structures are filled with erythrocytes. Image acquisition: Leica DM 4000B microscope, 20×/0.50 NA dry objective, Leica DFC 320 camera, Leica QWin Version 3 software. (G) Hemoglobin content is given as micrograms per milligram of Matrigel. (H) Vascular structures are expressed as percentage vascular area per field. Unspecific IgG (10 μg/mL) was used as antibody control (n = 4-8). **P < .01 vs control. ##P < .01 vs MALP-2. (A-E,G,H) Error bars represent mean ± SEM.
In vivo studies with Matrigel in mice revealed that both GM-CSF and MALP-2 showed a comparable induction of angiogenesis. Finally, the application of the antibody against GM-CSF significantly reduced the angiogenesis in response to MALP-2 as assessed by the hemoglobin content and the vascular area of the Matrigel plug (Figure 7F-H). Taken together, these results indicate a crucial role for GM-CSF in the TLR2/6-dependent induction of angiogenesis by MALP-2, which is illustrated in supplemental Figure 6.
Discussion
In this study, we show that the lipopeptide and TLR2/6 ligand MALP-2 exhibits a considerable angiogenic potential. We further provide evidence that this angiogenic potential is mediated by GM-CSF, mainly released by endothelial cells and monocytes. We propose a general immunologic mechanism to enhance or restore blood flow to recruit immune cells for pathogen clearance and tissue regeneration. Angiogenesis describes the sprouting of new blood vessels from preexisting ones, whereas vasculogenesis characterizes the development of blood vessels by endothelial cells differentiated from progenitor cells.17 These processes are frequently subsumed under the more general term neovascularization. The development of the vascular network is a tightly controlled process, a process that is of great physiologic as well as pathophysiologic relevance. However, angiogenesis seems to be a double-edged sword: on the one hand, essential during development and crucial for wound healing but, on the other hand, detrimental during tumorigenesis.17 Angiogenesis is initiated and regulated by several growth factors. In this regard, VEGF, which was initially described to modify endothelial cell permeability,23 seems to be a predominant factor and was intensively studied in the last 2 decades. Furthermore, growth factors, such as bFGF24 and the hematopoietic growth factors granulocyte colony-stimulating factor and GM-CSF,25 have been shown to exhibit angiogenic potential. Inhibitory factors, such as angiostatin, may prevent the initiation of angiogenesis or keep overwhelming capillary growth in check.26 Therefore, different proangiogenic and antiangiogenic factors cooperate to regulate the angiogenic process.
The lipopeptide MALP-2 was initially described to stimulate macrophages via a complex of TLR2 and TLR6.13,14 The compound is synthetically available, excluding contaminations with other TLR agonists during extraction procedure or recombinant expression, which is a well-known problem in the field. MALP-2 enhanced dermal wound healing in experimental animals.9 These findings initiated the present study with the aim to investigate the role of MALP-2 in angiogenesis. Indeed, we were able to demonstrate a high angiogenic potential of MALP-2, which was mediated by GM-CSF because different strategies to inhibit GM-CSF prevented MALP-2–induced angiogenesis. Endothelial cells secreted high levels of GM-CSF in response to MALP-2. In addition, monocytes were shown to release GM-CSF on MALP-2 stimulation, which is in line with previous observations using MALP-2 and other TLR agonists.9,27 On a per-cell basis, endothelial cells released clearly more GM-CSF; however, monocytic cells are perhaps more abundant in angiogenic tissue. These 2 cell types probably cooperate during the angiogenic process in response to MALP-2. An angiogenic potency of GM-CSF has been known for some time.25 In addition, a comprehensive body of evidence now exits, demonstrating that GM-CSF, like granulocyte colony-stimulating factor, effectively mobilizes bone marrow–derived progenitor cells into the peripheral circulation with potential implications for vasculogenesis. Of note, we could not detect enhanced VEGF levels after MALP-2 stimulation in either the cell supernatants of endothelial cells or in those from inflammatory cells (data not shown). These data suggest that the major angiogenic factor VEGF is not responsible for the observed effects in our study.
TLRs were initially discovered as pattern recognition receptors to recognize invading pathogens to initiate inflammatory pathways of the innate immunity.1 Our observation that a TLR agonist induces angiogenesis may represent a general mechanism how the body reacts to an infection by the concerted action of initiating an innate immune response and restoring the blood flow to infected or wounded tissue. MALP-2 naturally occurs in cell wall-less Mycoplasma fermentans.12 Lipopeptides with similar structure and activity have been identified also in other Mycoplasma species, eg, in Mycoplasma hyorhinis28 and, of note, in Gram-positive bacteria, eg, Staphylococcus aureus.29 Therefore, MALP-2 could be considered as a model lipopeptide signaling via TLR2. Indeed, angiogenesis and microvascular remodeling are features of the chronic inflammation produced by Mycoplasma pulmonis infections of the respiratory tract.30 Our findings strongly suggest that effects of MALP-2–like lipopeptides represent the underlying mechanism of these symptoms. Our data are an extension of previous findings in other experimental systems regarding bacterial infection and angiogenesis. McCurdy et al identified bacterial peptidoglycan and yeast zymosan as potent GM-CSF inducers in mast cells.31 The cell wall components peptidoglycan and zymosan were regarded as TLR2 agonists. Whether these agents or rather contaminations with lipopeptides are the active TLR2 agonists is presently being questioned.32 Further support of the notion that TLR-dependent signaling induces angiogenesis comes from the study of Pollet et al, who were recently able to demonstrate a role for bacterial lipopolysaccharide, a TLR4 agonist, in initiating angiogenesis.33 For TLR2, the story is perhaps even more complex. We observed GM-CSF release as well as angiogenesis in response to the synthetic ligand Pam2CSK4 signaling just as MALP-2 via TLR2/6. In contrast, the TLR2/1 ligand Pam3CSK4 was completely ineffective to induce GM-CSF and angiogenesis. In this regard, our data underline particularly the contribution of the TLR2/6 pathway.
Based on the present findings, MALP-2 may have therapeutic potential. Topically applied MALP-2 accelerated wound closure in diabetic obese mice9 and proved to be well tolerable when applied to the skin of volunteers in a phase 1 clinical trial.34 Beneficial effects for wound healing are to be expected in view of the angiogenic potency of MALP-2. Possibly surprising were the beneficial effects of topical MALP-2 application in various tumor models and in a phase 1/2 study with patients having developed a pancreas adenocarcinoma.35–37 However, the underlying mechanism of tumor suppression is still open and needs further investigation. Enhanced angiogenesis and short-timed stimulatory effects of MALP-2 on macrophages and natural killer cells12,35 and the production of chemokines attracting other leukocytes possibly act in concert during antitumor immune response. Although MALP-2 did induce angiogenesis in our in vivo studies with Matrigel, it has not been established by now in any animal model to restore blood flow after ischemia, eg, in myocardial infarction or in peripheral arterial disease. In this regard, a potential use of MALP-2 for an angiogenic therapy after ischemic disorders seems suitable and needs future elucidation. Several reviews point out the therapeutic options of using GM-CSF38 or endothelial progenitor cells39 in this field. In this context, MALP-2-induced GM-CSF followed by progenitor cell mobilization may offer additional benefit for therapy.
In conclusion, our study demonstrates a novel mode of action for the lipopeptide and TLR2/6 ligand MALP-2. We observed that MALP-2 exhibits a considerable angiogenic potential. We further provide evidence that the induction of angiogenesis is mediated by GM-CSF, mainly released by endothelial cells and monocytes in a TLR2/6-dependent manner. In light of immune defense and tissue injury, our results may uncover a general mechanism to enhance or restore blood flow to recruit immune cells for pathogen clearance and tissue regeneration. Furthermore, application of MALP-2 may provide the opportunity for an angiogenic therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Silke Pretzer, Nathalie Stonka, Sandra Witting, and Mirja Sirisko for excellent technical assistance.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (KFO 136; Schie 386/7-2; K.G., B.S.).
Authorship
Contribution: K.G. and H.S. designed the experiment, analyzed the data, and prepared the manuscript; G.S. prepared angiogenesis models and analyzed the data; C.G. performed TLR immunostaining; J.J. performed siRNA transfection; and H.D., P.F.M., and B.S. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karsten Grote, Department of Cardiology and Angiology, Hannover Medical School, Carl-Neuberg Str. 1, 30165, Hannover Germany; e-mail: grote.karsten@mh-hannover.de.
References
Author notes
K.G. and H.S. contributed equally to this study.