To the editor:
We read with interest the article by Adamkiewicz et al1 wherein the authors showed that serum ferritin (SF) level and liver iron concentration (LIC) do not rise linearly with transfusion load as measured by total number of transfusions received or transfusion iron load (TIL). We herein hypothesize that if the authors had considered transfusion rate instead of transfusion load, a more linear correlation may be found.
To test our hypothesis we analyzed data from 52 patients with sickle cell disease (SCD) attending the Rafik Hariri University Hospital (RHUH) and Nini Hospital, Lebanon. Written informed consent was provided by all patients in accordance with the Declaration of Helsinki and approval was obtained from ethical committees of both institutions. The mean age of the patients was 18.54 plus or minus 9.0 years (range, 4-49 years) with a male-to-female ratio of 20:32. All patients were chelation-naive, and none of the patients had evidence of hepatitis B or C infection or elevated liver enzyme levels. Patient charts were reviewed for data on the number of total lifetime transfusions (TLT) and transfusion rate (TR). TR was calculated according to the following formula: [TLT (units)/years receiving transfusion]. All transfusions were non-exchange with an approximate volume of 300 mL per unit. Blood samples were obtained for the assessment of steady-state SF levels. Direct determination of LIC was performed using R2 magnetic resonance imaging using established methodology.2
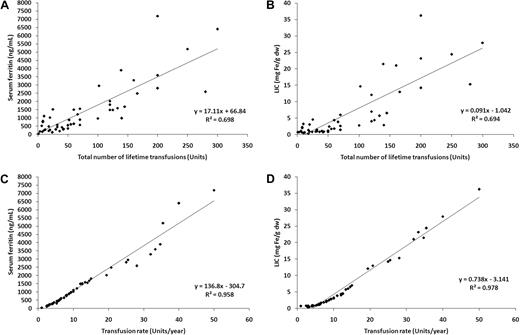
The mean plus or minus SD of SF and LIC were 1459.8 plus or minus 1552.0 ng/mL and 6.1 plus or minus 8.3 mg Fe/g dry weight (dw); respectively. SF was correlated with LIC (linear regression coefficient [R2] = 0.958; P < .001). TLT was a fair predictor of iron overload for both SF (R2 = 0.698; P < .001; Figure 1A) and LIC (R2 = 0.694; P < .001; Figure 1B). In contrast, TR was a much stronger predictor of SF (R2 = 0.958; P < .001, Figure 1C) and LIC (R2 = 0.978; P < .001, Figure 1D). No patient with a TR less than 10 units/year had a SF more than 1000 ng/mL or LIC more than 3 mg Fe/g dw; once this threshold was exceeded, SF and LIC rose linearly with TR.
Linear regressions. Linear regressions for (A) serum ferritin and total lifetime transfusions; (B) liver iron concentration (LIC) and total lifetime transfusions; (C) serum ferritin and transfusion rate; and (D) LIC and transfusion rate.
Linear regressions. Linear regressions for (A) serum ferritin and total lifetime transfusions; (B) liver iron concentration (LIC) and total lifetime transfusions; (C) serum ferritin and transfusion rate; and (D) LIC and transfusion rate.
Our data demonstrate that TR is a better predictor of SF and LIC than TLT. Moreover, intermittent transfusions (< 10 units/year) do not seem to produce significant iron overload in SCD patients, suggesting that spontaneous iron losses may be occurring simultaneously. Iron excretion rates of no more than 2 mg/day are usually quoted in normal subjects.3 SCD patients, however, exhibit intravascular hemolysis, heme-scavenging, heme filtration, and renal tubular absorption, which could potentially increase renal and fecal iron losses. In fact, iron elimination rates as high as 10.8 mg/kg/day have been described in patients with mechanical hemolysis.4,5 This may offer another mechanism to explain why SCD patients may be protected from organ-specific siderosis.6 Finally, TR predicts LIC changes in response to chelation therapy in patients with thalassemia major7 ; whether this applies to SCD patients merits prospective evaluation.
Authorship
Contribution: All authors were responsible for conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript for submission.
Conflict-of-interest disclosure: A.I. and A.T.T. are members of the Novartis Speakers' Bureau. J.C.W. received research funding from Novartis. The authors declare no other competing financial interests.
Correspondence: Adlette Inati, MD, Head, Division of Pediatric Hematology & Oncology; Medical Director, Children's Center for Cancer and Blood Diseases; Rafik Hariri University Hospital, Bir Hasan, Beirut 2034-7304, Lebanon; e-mail: khorina@dm.net.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal