Abstract
Neutrophil mobilization, the release of neutrophils from the bone marrow reserve into circulating blood, is important to increase peripheral neutrophil amounts during bacterial infections. Granulocyte colony-stimulating factor (G-CSF) and chemokines, such as macrophage-inflammatory protein-2 (MIP-2; CXCL2), can induce neutrophil mobilization, but the mechanism(s) they use remain unclear. Signal transducers and activator of transcription 3 (STAT3) is the principal intracellular signaling molecule activated upon G-CSF ligation of its receptor. Using a murine model with conditional STAT3 deletion in bone marrow, we demonstrated previously that STAT3 regulates acute G-CSF–responsive neutrophil mobilization and MIP-2–dependent neutrophil chemotaxis. In this study, we show STAT3 is also necessary for MIP-2–elicited neutrophil mobilization. STAT3 appears to function by controlling extracellular signal-regulated kinase (ERK) activation, which is important for MIP-2–mediated chemotaxis. In addition, we demonstrate that G-CSF stimulates the expression of the MIP-2 receptor via STAT3-dependent transcriptional activation of Il8rb. G-CSF treatment also induces STAT3-dependent changes in bone marrow chemokine expression levels which may further affect neutrophil retention and release. Taken together, our study demonstrates that STAT3 regulates multiple aspects of chemokine and chemokine receptor expression and function within the bone marrow, indicating a central role in the neutrophil mobilization response.
Introduction
Neutrophils are cells of the innate immune system that have critical roles in managing bacterial and fungal infections because their major effector functions are phagocytosis and release of antibacterial peptides, proteases, and reactive oxygen species.1 Patients with congenital or acquired neutropenias have increased susceptibility to life-threatening infections.2 These patients often are treated with recombinant granulocyte colony-stimulating factor (G-CSF), which promotes myeloid progenitor cell proliferation and neutrophil differentiation, as well as neutrophil mobilization from the bone marrow reserve into circulating blood. G-CSF is commonly used in conjunction with chemotherapy, which can induce neutropenia. However, some patients do not respond well to G-CSF for reasons that are unclear. In fact, the mechanisms of G-CSF action are not well understood, thus prompting further investigation into its molecular function.
G-CSF binds the homodimeric G-CSF receptor (G-CSFR), which activates the associated Jak1 and Jak2 protein tyrosine kinases, and in turn stimulates the transcription factors signal transducers and activators of transcription 1 (STAT1), STAT3, and STAT5.3 STATs form dimers and accumulate in the nucleus, where they activate transcription of cytokine-responsive genes. Experiments in vitro have demonstrated that G-CSF weakly activates STAT1 and STAT5 but strongly activates STAT3.4 Deletion of STAT3 in the bone marrow results in neutrophilia, indicating that STAT3 restrains granulopoiesis under steady-state conditions.5-8 STAT3 is required to activate suppressor of cytokine signaling 3 (SOCS3), which is important in terminating signals from the G-CSFR.9 Therefore, these data collectively suggest that STAT3-SOCS3 signaling is required to suppress steady-state granulopoiesis.
Additional research, however, suggested a more complex role for STAT3 in neutrophil regulation. The STAT3 recruitment site in the G-CSFR was found to be critical for controlling G-CSF–responsive neutrophil production and mobilization.10 Moreover, we showed that STAT3 regulates G-CSF–dependent accumulation of immature bone marrow granulocytes and acute G-CSF–induced neutrophil mobilization, indicating important roles in neutrophil production and function under demand conditions.7 STAT3-deficient neutrophils have a cell-autonomous defect in migration toward ligands for CXCR2,7 the major chemokine receptor expressed on murine neutrophils.11
Here, we investigated the mechanisms by which STAT3 regulates neutrophil mobilization in response to G-CSF or the CXCR2 ligand, macrophage-inflammatory protein-2 (MIP-2). Our studies revealed that STAT3 controls MIP-2–responsive neutrophil mobilization from the bone marrow. STAT3 regulates the amplitude of MIP-2–induced Raf, mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK), and extracellular signal-regulated kinase (ERK) signaling, which is necessary for CXCR2-mediated neutrophil chemotaxis. Moreover, G-CSF directly induces CXCR2 expression on immature neutrophils in a STAT3-dependent manner and enhances their responsiveness to MIP-2. G-CSF treatment stimulates MIP-2 and represses stromal cell–derived factor-1 (SDF-1) expression in the bone marrow microenvironment via a STAT3-dependent pathway. Therefore, we demonstrate that STAT3 regulates CXCR2 expression and function as well as the production of chemokines in the bone marrow microenvironment, thus controlling several stages of the neutrophil mobilization response.
Methods
Bone marrow STAT3-deficient mice, cell isolation
STAT3-deficient (Tg[Tek-cre]12Flv, Stat3f/Δ) and control mice were bred as described,7 maintained in a specific pathogen-free facility, and used with the approval of the Institutional Animal Care and Use Committee guidelines at The University of Texas M. D. Anderson Cancer Center (UT MDACC).
G-CSF and MIP-2 administration in vivo
After dilution in sterile phosphate-buffered saline (PBS) containing endotoxin-free bovine serum albumin (BSA/PBS), recombinant human G-CSF (Amgen) was administered by subcutaneous injection (250 μg/kg, 24 hours before) and murine MIP-2 (PeproTech) by intraperitoneal injection (50 μg/kg, 30 minutes before). Control mice received equivalent volumes of BSA/PBS or remained untreated.
Ex vivo MIP-2 stimulation, immunoblot analysis
Bone marrow neutrophils were treated with MIP-2 (100 ng/mL) plus or minus pretreatment with U0126 inhibitor (Promega). Whole-cell lysates were analyzed by immunoblotting with antibodies against phospho-c-Raf (Ser338), phospho-MEK1/2 (Ser217/Ser221), phospho-ERK1/2 (Thr202/Tyr204), total c-Raf, total MEK1/2 (Cell Signaling Technology), or total ERK2 (Santa Cruz Biotechnology Inc). Densitometric analyses were performed with National Institutes of Health ImageJ software Version 1.42q (http://rsbweb.nih.gov/ij/).
Chemotaxis assays
Bone marrow neutrophils were resuspended in Dulbecco modified Eagle medium/BSA and plated in 3-μm Transwells (Fisher Scientific) plus or minus SDF-1α (250 ng/mL; PeproTech) or MIP-2, as described.7 Cells were pretreated in U0126 for 30 minutes as indicated.
Flow cytometry, fluorescence-activated cell sorting
Cells used for CXCR2 staining were resuspended in PBS containing 2% fetal calf serum (FCS) and labeled with fluorescein isothiocyanate (FITC)–conjugated Gr-1 (Pharmingen) and phycoerythrin-conjugated CXCR2 (R&D Systems) antibodies and analyzed by flow cytometry on a BD FACSCalibur or BD LSR II. Bone marrow samples were labeled with FITC-conjugated Gr-1; Gr-1lo and Gr-1hi populations were sorted by the use of a BD FACSAria.
RNA isolation, real-time polymerase chain reaction
Total RNA was isolated with Tri-Reagent (Molecular Research Center Inc) and reverse transcribed by the use of iScript cDNA Synthesis kit (Bio-Rad). Real-time polymerase chain reaction (PCR) was performed with iQ SYBR Green Supermix, detected on the iQ5 Real-Time PCR Detection System, and analyzed with iQ5 Optical System Software (Bio-Rad). Calculations were performed as previously described.12 Primers used were Il8rb: forward 5′-AGCAAACACCTCTACTACCCTCTA-3′, reverse 5′-GGGCTGCATCAATTCAAATACCA-3′13 ; Rpl13a forward 5′-GAGGTCGGGTGGAAGTACCA-3′, reverse 5′-TGCATCTTGGCCTTTTCCTT-3′14 ; Cxcl1 forward 5′-CCGAAGTCATAGCCACACTCAA-3′, reverse 5′-GCAGTCTGTCTTCTTTCTCCGTTAC-3′15 ; Cxcl2 forward 5′-AGACAGAAGTCATAGCCACTCTCAAG-3′, reverse 5′-CCTCCTTTCCAGGTCAGTTAGC-3′15 ; and Cxcl12 forward 5′-GAGAGCCACATCGCCAGAG-3′, reverse 5′-TTTCGGGTCAATGCACACTTG-3′.16
32D cell culture, retroviral transduction
32D cells expressing human G-CSFR (32D.G-CSFR) were generated by retroviral transduction with transfer vector pMX-G-CSFR-IRES-GFP. GFP-G-CSFR+ cells were enriched by fluorescence-activated cell sorting. Cells were maintained in RPMI/10% FCS/10% WEHI-conditioned media (source of interleukin-3 [IL-3]).
Identification of the Il8rb promoter, reporter assays
The putative proximal promoter for Il8rb (ENSMUSG00000026180) was identified with the use of Ensembl, and the putative STATx site was found with TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html). The Il8rb promoter was amplified with AccuPrime Pfx DNA Polymerase (Invitrogen) and the following primers, forward 5′-GGAGGTACCGCACAGCAAGCTGAGAGG-3′ and reverse 5′-GGAAGATCTGACCTGGGCTACCGATGGGGA-3′, and cloned into the pGL3-Basic plasmid (Promega). The mutation in the STATx site was induced by PCR-based mutagenesis. Sequences were confirmed by the UT MDACC DNA Core Analysis Facility.
32D.G-CSFR cells were electroporated with Cell Line Nucleofector Kit V (Lonza) and The Nucleofector Device (Lonza) and treated with 25 ng/mL Recombinant human G–CSF (Amgen). Luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega) and a Sirius luminometer (Berthold Detection Systems).
Electrophoretic mobility shift assays, chromatin immunoprecipitations
Electrophoretic mobility shift assays (EMSAs) were performed as described.17 EMSA oligonucleotide sequences are as follows: wild-type (WT) Il8rb sense 5′-ACAGTTTCAGGGAAAGAGACT-3′, antisense 5′-AGTCTCTTTCCCTGAAACTGT-3′; mutant Il8rb sense 5′-ACAGTCCAAGGGAAAGAGACT-3′, antisense 5′-AGTCTCTTTCCCTTGGACTGT-3′ (underline indicates site of mutation). Anti-STAT3 c-20X antibody (Santa Cruz Biotechnology Inc) was used for supershift assays. The chromatin immunoprecipitation (ChIP) assay kit (Millipore) was used following the manufacturer's instructions with anti-STAT3 c-20 antibody or normal rabbit-immunoglobulin G control (Santa Cruz Biotechnology Inc).
Analysis of SDF-1 binding
Recombinant SDF-1α containing a human Fc tag was a generous gift from Dr Qing Ma (UT MDACC). SDF-1α binding was determined for the Gr-1+ population by the use of bone marrow cells that were freshly isolated or cultured for 6 hours at 37°C in Dulbecco modified Eagle medium/FCS + G-CSF (100 ng/mL). Fc-conjugated SDF-1α was detected with FITC-conjugated mouse adsorbed anti–human Fc (Caltag) and analyzed with a Beckman Coulter Epics XL-MCL machine.
Infection with Listeria monocytogenes
Experiments were performed with L monocytogenes strain 1043S, generously provided by Dr Chen Dong (UT MDACC) and Dr Hao Shen (University of Pennsylvania). A volume of 200 μL of bacteria (2 × 104 cells) was injected intravenously. Peripheral blood and tissue samples were collected 12 hours after infection; tissues were homogenized, serially diluted, and plated on BHI agar to determine the number of colony-forming units (CFUs).
Statistical analysis
Shown are mean values plus or minus SEM. P values were determined by unpaired 2-tailed Student t tests with the use of GraphPad Prism Version 5 for Mac OS X (http://www.graphpad.com). P values less than .05 were considered statistically significant.
Results
STAT3 controls MIP-2–dependent neutrophil migration from the bone marrow
STAT3 is required for neutrophil chemotaxis toward ligands for CXCR2,7 and yet its role in CXCR2-mediated neutrophil migration in vivo remains unclear. To examine this role, we administered MIP-2, a CXCR2 ligand, to bone marrow STAT3-deficient and WT mice by intraperitoneal injection and measured circulating, bone marrow, and splenic neutrophil levels after 30 minutes. MIP-2 elicited a 2- to 3-fold increase in circulating neutrophil amounts in WT mice (−MIP-2, 1060.0 ± 238.2 neutrophils/μL; +MIP-2, 2834.9 ± 835.6 neutrophils/μL; Figure 1A-B), which is consistent with previous studies.18 By contrast, STAT3-deficient mice, which have basal neutrophilia as well as increased splenic neutrophil numbers,7 failed to up-regulate the number of peripheral blood neutrophils in response to MIP-2 (−MIP-2, 4363.7 ± 1020.6 neutrophils/μL; +MIP-2, 3920.7 ± 1030.5 neutrophils/μL; Figure 1A-B). Moreover, spleen neutrophil counts did not increase in either genotype after MIP-2 treatment (Figure 1B). Bone marrow neutrophil numbers were reduced by approximately 50% in WT mice upon MIP-2 treatment, whereas STAT3-deficient animals failed to show a significant decline (Figure 1B). STAT3-deficient and WT neutrophils expressed equivalent levels of CXCR2 and showed a similar extent of ligand-mediated receptor down-regulation (Figure 1C). These results demonstrate that MIP-2–stimulated neutrophil migration from the bone marrow to the blood is impaired in STAT3-deficient mice without effects on basal CXCR2 expression, suggesting STAT3 may regulate other aspects of the MIP-2 mobilization response, such as CXCR2 signaling.
STAT3-dependent regulation of neutrophil migration and CXCR2-mediated Raf/MEK/ERK signaling. (A) Peripheral blood neutrophil numbers were determined for WT (□) and STAT3-deficient (knockout [KO], ■) mice treated with MIP-2 (50 μg/kg) or BSA carrier for 30 minutes. Neutrophil amounts in MIP-2–treated mice were normalized to BSA-treated controls of the appropriate genotype and relative levels are shown (n = at least 4 for WT and KO). (B) Absolute neutrophil numbers in total spleen, blood, and bone marrow of BSA- or MIP-2–treated mice are shown (n = at least 3 for WT and KO for each condition). (C) CXCR2 expression within the Gr-1+ bone marrow population of BSA- and MIP-2–treated WT (black line) and STAT3-deficient (KO, gray line) mice is shown, relative to isotype controls (dashed black line). Results are representative of 3 independent experiments. (D) Activation of c-Raf, MEK1/2, and ERK1/2 in MIP-2–treated (100 ng/mL) or unstimulated Gr-1+ cells was analyzed by immunoblotting and quantified by densitometry. Results are representative of at least 3 independent experiments. (E) MIP-2–responsive chemotaxis of bone marrow neutrophils from WT mice was examined in the absence of or after, 30 minutes pretreatment with the MEK1/2 inhibitor U0126 (10μM or 50μM as indicated, n = 5 for each condition). Average values from 3 independent experiments are shown. (F) WT bone marrow neutrophils were stimulated with MIP-2 (100 ng/mL for 5 minutes) in the presence or absence of U0126 (10μM or 50μM as indicated). ERK1/2 activation was assessed by immunoblotting with whole-cell lysates; results are representative of 3 independent experiments. Error bars represent SEM; *P < .05, **P < .01, ***P < .001.
STAT3-dependent regulation of neutrophil migration and CXCR2-mediated Raf/MEK/ERK signaling. (A) Peripheral blood neutrophil numbers were determined for WT (□) and STAT3-deficient (knockout [KO], ■) mice treated with MIP-2 (50 μg/kg) or BSA carrier for 30 minutes. Neutrophil amounts in MIP-2–treated mice were normalized to BSA-treated controls of the appropriate genotype and relative levels are shown (n = at least 4 for WT and KO). (B) Absolute neutrophil numbers in total spleen, blood, and bone marrow of BSA- or MIP-2–treated mice are shown (n = at least 3 for WT and KO for each condition). (C) CXCR2 expression within the Gr-1+ bone marrow population of BSA- and MIP-2–treated WT (black line) and STAT3-deficient (KO, gray line) mice is shown, relative to isotype controls (dashed black line). Results are representative of 3 independent experiments. (D) Activation of c-Raf, MEK1/2, and ERK1/2 in MIP-2–treated (100 ng/mL) or unstimulated Gr-1+ cells was analyzed by immunoblotting and quantified by densitometry. Results are representative of at least 3 independent experiments. (E) MIP-2–responsive chemotaxis of bone marrow neutrophils from WT mice was examined in the absence of or after, 30 minutes pretreatment with the MEK1/2 inhibitor U0126 (10μM or 50μM as indicated, n = 5 for each condition). Average values from 3 independent experiments are shown. (F) WT bone marrow neutrophils were stimulated with MIP-2 (100 ng/mL for 5 minutes) in the presence or absence of U0126 (10μM or 50μM as indicated). ERK1/2 activation was assessed by immunoblotting with whole-cell lysates; results are representative of 3 independent experiments. Error bars represent SEM; *P < .05, **P < .01, ***P < .001.
CXCR2-dependent ERK1/2 signaling is regulated by STAT3 and is required for neutrophil chemotaxis
To examine the possibility that STAT3 controls intracellular signal transduction pathways downstream of CXCR2, we compared signaling responses between STAT3-deficient and WT neutrophils after stimulation with MIP-2. Activation of ERK1/2 was rapid and sustained in WT neutrophils across all time points examined, whereas it appeared relatively nonresponsive in STAT3-deficient neutrophils (Figure 1D). Similarly, activation of MEK1/2 and Raf was suppressed in STAT3-deficient neutrophils compared with WT cells (Figure 1D). By contrast, we were unable to detect differences in p38 or Akt phosphorylation levels between WT and STAT3-deficient neutrophils, and neutrophils from both genotypes elicited similar amounts of intracellular Ca2+ flux after MIP-2 treatment (data not shown). Therefore, STAT3 appears to control the amplitude of signaling through the Raf/MEK/ERK module downstream of the CXCR2 receptor, without global regulation of CXCR2 signal transduction or steady state receptor cell-surface expression.
To determine the role of the Raf/MEK/ERK cascade in CXCR2-mediated neutrophil chemotaxis, we treated WT bone marrow neutrophils with the MEK inhibitor U0126 and determined their ability to migrate toward MIP-2 in Transwell assays. U0126 inhibited MIP-2–responsive migration in a dose-dependent manner (Figure 1E). Analysis of MIP-2–responsive ERK1/2 phosphorylation confirmed that U0126 blocked MEK/ERK signal transduction (Figure 1F). These results indicate that MEK/ERK signaling is required for efficient neutrophil migration toward MIP-2 and suggest that STAT3-dependent control of the Raf/MEK/ERK module is an important component of CXCR2-mediated chemotaxis and potentially MIP-2–elicited mobilization in vivo.
G-CSF treatment in vivo stimulates STAT3-dependent induction of CXCR2 expression in neutrophils
Like MIP-2–dependent neutrophil mobilization, G-CSF–responsive neutrophil mobilization requires STAT3,7 but the mechanisms involved are not clearly defined. G-CSF itself is not directly chemotactic in murine models19 ; however, G-CSF is reported to regulate the expression of CXCR2.20 We therefore examined whether STAT3 stimulated CXCR2 expression during neutrophil development or in response to G-CSF treatment in vivo. The total Gr-1+ bone marrow neutrophil population can be subdivided into immature Gr-1lo granulocytes and mature Gr-1hi neutrophils,7,21 thus permitting analysis of CXCR2 expression at discrete developmental stages by flow cytometry. We found that the majority of mature Gr-1hi bone marrow neutrophils expressed cell surface CXCR2: no significant differences were detected between WT and STAT3-deficient cells in steady-state conditions (Figure 2A top right; Figure 2B). Immature Gr-1lo granulocytes showed heterogeneous expression of cell surface CXCR2 in the basal state and expressed reduced CXCR2 levels relative to mature Gr-1hi neutrophils, as judged by differences in mean fluorescence intensity (MFI; ie, MFI = 1007 ± 126 for WT Gr-1lo vs MFI = 2877 ± 295 for WT Gr-1hi neutrophils, P < .001; Figure 2A top; Figure 2B; data not shown).
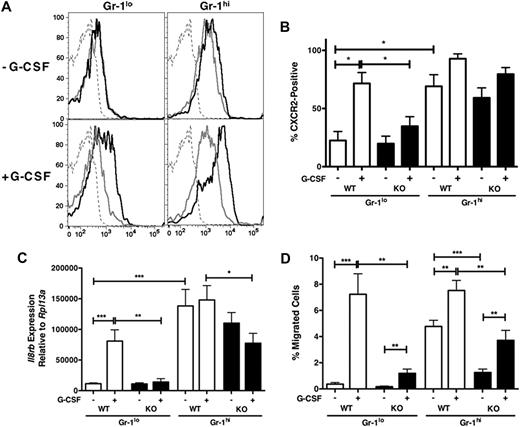
Role for STAT3 in G-CSF–inducible CXCR2 expression. (A) WT and STAT3-deficient (KO) mice were treated with G-CSF (250 μg/kg; +G-CSF, bottom) or left untreated (−G-CSF, top) and bone marrow samples were isolated after 24 hours. CXCR2 expression in the Gr-1+ population is shown: WT (black line), KO (gray line). Isotype controls are included (dashed gray line). Data shown are from a representative experiment (n = at least 3 for each group). (B) The average percentage of CXCR2-positive cells within the bone marrow Gr-1hi and Gr-1lo subsets from untreated (−G-CSF) or G-CSF–treated (+G-CSF) WT and STAT3-deficient (KO) mice is shown (n = at least 3 for each group). (C) WT and STAT3-deficient (KO) mice were untreated or treated with G-CSF as indicated in panel A. Relative Il8rb mRNA levels were determined in purified Gr-1lo and Gr-1hi subsets, by comparison with the housekeeping gene Rpl13a. Shown are mean relative expression levels (n = at least 4). (D) WT and STAT3-deficient (KO) mice were untreated or treated with G-CSF as indicated in panel A. MIP-2–responsive chemotactic activity of purified immature Gr-1lo and mature Gr-1hi neutrophils was determined by Transwell assays. The average percentage of migrated cells/total cells is shown for each condition (n = 5 for each group). Error bars represent SEM; *P < .05, **P < .01, ***P < .001.
Role for STAT3 in G-CSF–inducible CXCR2 expression. (A) WT and STAT3-deficient (KO) mice were treated with G-CSF (250 μg/kg; +G-CSF, bottom) or left untreated (−G-CSF, top) and bone marrow samples were isolated after 24 hours. CXCR2 expression in the Gr-1+ population is shown: WT (black line), KO (gray line). Isotype controls are included (dashed gray line). Data shown are from a representative experiment (n = at least 3 for each group). (B) The average percentage of CXCR2-positive cells within the bone marrow Gr-1hi and Gr-1lo subsets from untreated (−G-CSF) or G-CSF–treated (+G-CSF) WT and STAT3-deficient (KO) mice is shown (n = at least 3 for each group). (C) WT and STAT3-deficient (KO) mice were untreated or treated with G-CSF as indicated in panel A. Relative Il8rb mRNA levels were determined in purified Gr-1lo and Gr-1hi subsets, by comparison with the housekeeping gene Rpl13a. Shown are mean relative expression levels (n = at least 4). (D) WT and STAT3-deficient (KO) mice were untreated or treated with G-CSF as indicated in panel A. MIP-2–responsive chemotactic activity of purified immature Gr-1lo and mature Gr-1hi neutrophils was determined by Transwell assays. The average percentage of migrated cells/total cells is shown for each condition (n = 5 for each group). Error bars represent SEM; *P < .05, **P < .01, ***P < .001.
In addition, WT and STAT3-deficient immature Gr-1lo granulocytes expressed similar levels of CXCR2 on the cell surface under steady-state conditions (Figure 2A top left; Figure 2B). In agreement, we found that basal mRNA levels of Il8rb, the gene encoding CXCR2, were comparable in WT and STAT3-deficient immature Gr-1lo granulocytes (Figure 2C). Il8rb mRNA levels were significantly elevated in mature Gr-1hi neutrophils, relative to immature Gr-1lo granulocytes, and again no obvious differences were found between WT and STAT3-deficient cells in the basal state (Figure 2C). These data indicate that Il8rb mRNA and cell-surface CXCR2 expression are induced during neutrophil differentiation in vivo, yet the pathways that control developmental expression are independent of functional STAT3.
Examination of CXCR2 in G-CSF–treated mice showed that cell-surface CXCR2 expression was significantly induced in WT immature Gr-1lo granulocytes versus cells from untreated animals (Figure 2A left; Figure 2B). Moreover, G-CSF up-regulated cell-surface CXCR2 expression on WT mature Gr-1hi neutro-phils compared with untreated control animals (−G-CSF, MFI = 2877 ± 295; +G-CSF, 5212 ± 675 for Gr-1hi; Figure 2A, right panels; data not shown). By contrast, G-CSF–dependent induction of CXCR2 was not detected on STAT3-deficient immature Gr-1lo granulocytes or mature Gr-1hi neutrophils (Figure 2A bottom panels; Figure 2B). Furthermore, STAT3 was required for G-CSF–dependent up-regulation of Il8rb mRNA in immature Gr-1lo granulocytes (Figure 2C). Because the bone marrow Gr-1lo population may also contain CD115+ monocytes, we evaluated CXCR2 expression in WT and STAT3-deficient Gr-1lo CD115− cells, which comprise more than 80% of Gr-1lo cells, and found similar patterns of CXCR2 cell surface and mRNA expression relative to the total Gr-1lo subset (data not shown). Thus, STAT3 is necessary to enhance CXCR2 expression in the immature Gr-1lo granulocyte subset during G-CSF administration in vivo, at least in part via induction of Il8rb mRNA expression.
To test the function of G-CSF–induced CXCR2 expression, we isolated immature Gr-1lo and mature Gr-1hi granulocytes from G-CSF– or untreated WT and STAT3-deficient mice and assayed chemotaxis in response to MIP-2. G-CSF treatment in vivo enhanced CXCR2-mediated chemotaxis of immature and mature neutrophil subsets isolated from WT and STAT3-deficient mice, although the migratory response was significantly reduced in STAT3-deficient cells relative to WT (Figure 2D). WT immature Gr-1lo granulocytes from G-CSF–treated animals showed significantly more chemotactic activity toward MIP-2 compared with Gr-1lo cells from untreated animals, whereas G-CSF–induced chemotactic activity was attenuated in STAT-deficient Gr-1lo granulocytes (Figure 2D). These results are consistent with our previous observation of effective mobilization of immature Gr-1lo neutrophils in WT but not STAT3-deficient mice upon G-CSF treatment.7 Thus, our data collectively demonstrate that STAT3 controls the induction of Il8rb mRNA and CXCR2 protein in immature Gr-1lo granulocytes during systemic G-CSF administration, correlating with their enhanced migratory potential.
STAT3 directly controls CXCR2 transcription by interaction with a STAT consensus site in the Il8rb promoter
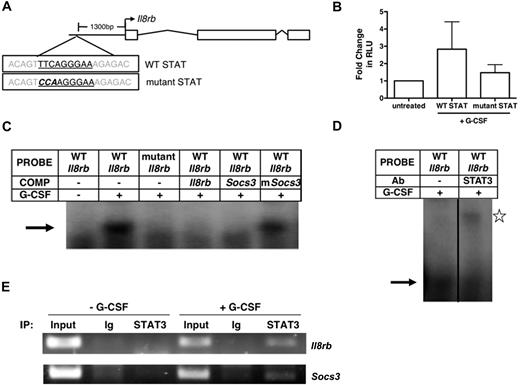
We examined the murine Il8rb proximal promoter and identified a putative STAT-binding site approximately 1300 bp upstream of the transcriptional start site (Figure 3A). Comparison with the human IL8RB promoter revealed a putative STAT-binding site (TTCCAGGAA) in a similar relative position (data not shown). The predicted transcriptional start site of Il8rb (Ensembl) was verified by 5′-rapid amplification of cDNA ends (data not shown). Luciferase reporter constructs containing the proximal promoter region encompassing the STAT site or a mutant STAT element (Figure 3A) were generated. G-CSF treatment induced Il8rb reporter activity approximately 3-fold, relative to nonstimulation conditions (Figure 3B), indicating G-CSF activates transcription from the Il8rb promoter. G-CSF–responsive Il8rb reporter activity was suppressed upon mutation of the STAT site (Figure 3B), demonstrating a role for this element in G-CSF–dependent transcription. EMSAs with an oligonucleotide containing the Il8rb STAT element revealed induction of a specific protein:oligonucleotide complex after G-CSF stimulation (Figure 3C). Complex formation was abrogated by mutation of the STAT3 site, competition with the nonmutagenized Il8rb promoter probe, or competition with an oligonucleotide containing the Socs3 promoter STAT3 site (Figure 3C). Moreover, incubation with a STAT3 antibody supershifted the Il8rb protein:oligonucleotide complex (Figure 3D). These results collectively demonstrate that G-CSF–activated STAT3 interacts with the Il8rb STAT element in vitro. To examine whether STAT3 binds the Il8rb promoter in vivo, we performed ChIPs in 32D.G-CSFR cells. These assays showed that STAT3 is recruited to the Il8rb promoter upon G-CSF stimulation, similar to its inducible interaction with the Socs3 promoter (Figure 3E). Taken together, our data indicate that G-CSF–stimulated Il8rb transcription occurs by direct binding of STAT3 to the Il8rb promoter.
Regulation of Il8rb transcription by G-CSF–responsive STAT3 signaling. (A) A schematic of the Il8rb gene showing the location of the STATx site (top) and sequence of the mutant STAT element (bottom). (B) 32D.G-CSFR cells were electroporated with WT (WT STAT) or mutant pGL3-Il8rb (mutant STAT) and pTK-Renilla reporter plasmids, treated with or without G-CSF for 6 hours, and assayed for luciferase activity. The ratio of firefly:renilla relative light units (RLU) in G-CSF–treated cells relative to unstimulated cells (−G-CSF) was averaged from 3 independent experiments. (C) EMSAs were performed with nuclear extracts from 32D.G-CSFR cells, stimulated with or without G-CSF for 30 minutes by the use of radiolabeled oligonucleotide probes corresponding to the Il8rb promoter region containing the STATx site (WT Il8rb) or an oligonucleotide containing a mutation in the STATx site of the Il8rb promoter (mutant Il8rb), as indicated. An excess of unlabeled oligonucleotide, corresponding to the WT Il8rb probe (Il8rb), an oligonucleotide encompassing the STAT3 binding site in Socs3 (Socs3), or an oligonucleotide containing a mutation in the STAT3 site from Socs3 (mSocs3) was used as competitor, as indicated. Results are representative of 3 independent experiments. (D) EMSAs were performed as in panel C with the WT Il8rb probe in the absence or presence of anti-STAT3 antibody. Results are representative of 3 independent experiments. (E) ChIPs were performed on 32D.G-CSFR cells ± G-CSF treatment for 30 minutes with anti-STAT3 or Ig control antibody, as indicated. Purified DNA samples were subjected to PCR to detect Il8rb (top) or Socs3 (bottom) promoter sequences. Data are representative of 3 independent experiments. Error bars represent SEM.
Regulation of Il8rb transcription by G-CSF–responsive STAT3 signaling. (A) A schematic of the Il8rb gene showing the location of the STATx site (top) and sequence of the mutant STAT element (bottom). (B) 32D.G-CSFR cells were electroporated with WT (WT STAT) or mutant pGL3-Il8rb (mutant STAT) and pTK-Renilla reporter plasmids, treated with or without G-CSF for 6 hours, and assayed for luciferase activity. The ratio of firefly:renilla relative light units (RLU) in G-CSF–treated cells relative to unstimulated cells (−G-CSF) was averaged from 3 independent experiments. (C) EMSAs were performed with nuclear extracts from 32D.G-CSFR cells, stimulated with or without G-CSF for 30 minutes by the use of radiolabeled oligonucleotide probes corresponding to the Il8rb promoter region containing the STATx site (WT Il8rb) or an oligonucleotide containing a mutation in the STATx site of the Il8rb promoter (mutant Il8rb), as indicated. An excess of unlabeled oligonucleotide, corresponding to the WT Il8rb probe (Il8rb), an oligonucleotide encompassing the STAT3 binding site in Socs3 (Socs3), or an oligonucleotide containing a mutation in the STAT3 site from Socs3 (mSocs3) was used as competitor, as indicated. Results are representative of 3 independent experiments. (D) EMSAs were performed as in panel C with the WT Il8rb probe in the absence or presence of anti-STAT3 antibody. Results are representative of 3 independent experiments. (E) ChIPs were performed on 32D.G-CSFR cells ± G-CSF treatment for 30 minutes with anti-STAT3 or Ig control antibody, as indicated. Purified DNA samples were subjected to PCR to detect Il8rb (top) or Socs3 (bottom) promoter sequences. Data are representative of 3 independent experiments. Error bars represent SEM.
STAT3 regulates changes in the bone marrow microenvironment
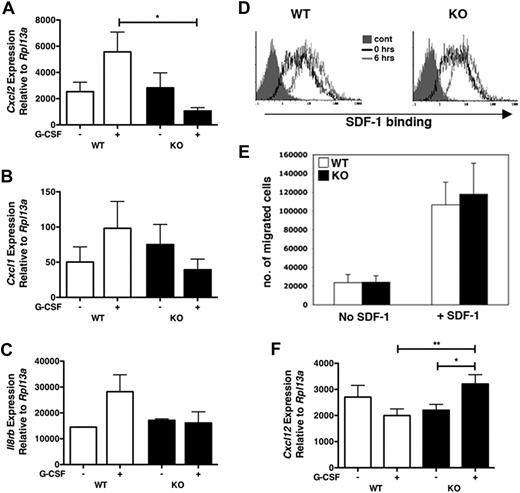
G-CSF administration in humans is reported to induce IL-8, a potent neutrophil chemoattractant.22 This prompted us to test the expression of chemokines in bone marrow samples from WT or STAT3-deficient mice in response to G-CSF. We found G-CSF–dependent induction of Cxcl2 mRNA, which encodes MIP-2 (CXCL2), the murine homologue of human IL-8, in bone marrow of WT but not STAT3-deficient mice (Figure 4A). We observed a similar trend for Cxcl1, which encodes keratinocyte-derived chemokine (KC; CXCL1), a distinct CXCR2 chemokine (Figure 4B). Furthermore, G-CSF administration up-regulated Il8rb in WT but not STAT3-deficient bone marrow (Figure 4C), similar to its induction in neutrophils (Figure 2C). Collectively, these results suggest that G-CSF stimulates mRNA expression of the neutrophil chemotactic molecules MIP-2 and KC within the bone marrow microenvironment, a response that is abrogated by hematopoietic STAT3 deletion.
G-CSF treatment results in STAT3-dependent alterations in the chemokine profile of the bone marrow microenvironment. WT or STAT3-deficient (KO) mice were injected with G-CSF or left untreated. Total bone marrow cells were isolated after 24 hours, and purified RNA samples were analyzed by real-time PCR for (A) MIP-2 (Cxcl2), (B) KC (Cxcl1), or (C) CXCR2 (Il8rb) expression. Gene expression was determined relative to Rpl13a (n = at least 4). (D) SDF-1α binding assays were performed with purified bone marrow neutrophils from WT or STAT-deficient (KO) mice either by the use of freshly isolated neutrophils (0 hrs, open black histograms) or neutrophils cultured ex vivo in G-CSF for 6 hours (6 hours, open gray histograms). Staining with human-Fc (solid gray histograms) was used as a control. Results are representative of 3 independent experiments. (E) Neutrophils isolated from the bone marrow of WT (□) or KO (■) mice were tested for SDF-1–dependent chemotaxis (n = 4 for each genotype). Average values from 3 independent experiments are shown. (F) WT or STAT-deficient (KO) mice were treated with G-CSF or left untreated. SDF-1 (Cxcl12) mRNA expression was determined in total bone marrow 24 hours after treatment by the use of real-time PCR as in panels A to C (n = at least 4). Error bars represent SEM; *P < .05, **P < .01.
G-CSF treatment results in STAT3-dependent alterations in the chemokine profile of the bone marrow microenvironment. WT or STAT3-deficient (KO) mice were injected with G-CSF or left untreated. Total bone marrow cells were isolated after 24 hours, and purified RNA samples were analyzed by real-time PCR for (A) MIP-2 (Cxcl2), (B) KC (Cxcl1), or (C) CXCR2 (Il8rb) expression. Gene expression was determined relative to Rpl13a (n = at least 4). (D) SDF-1α binding assays were performed with purified bone marrow neutrophils from WT or STAT-deficient (KO) mice either by the use of freshly isolated neutrophils (0 hrs, open black histograms) or neutrophils cultured ex vivo in G-CSF for 6 hours (6 hours, open gray histograms). Staining with human-Fc (solid gray histograms) was used as a control. Results are representative of 3 independent experiments. (E) Neutrophils isolated from the bone marrow of WT (□) or KO (■) mice were tested for SDF-1–dependent chemotaxis (n = 4 for each genotype). Average values from 3 independent experiments are shown. (F) WT or STAT-deficient (KO) mice were treated with G-CSF or left untreated. SDF-1 (Cxcl12) mRNA expression was determined in total bone marrow 24 hours after treatment by the use of real-time PCR as in panels A to C (n = at least 4). Error bars represent SEM; *P < .05, **P < .01.
In contrast to the function of MIP-2 and KC, SDF-1 (CXCL12) mediates the retention of hematopoietic stem and granulocytic progenitor cells in the bone marrow via interaction with its receptor CXCR4.23 Correspondingly, SDF-1 and CXCR4 are down-regulated upon G-CSF treatment, and this response has been hypothesized to promote mobilization.24,25 To determine whether STAT3 plays a role in the SDF-1/CXCR4 axis, we analyzed CXCR4 expression and function in STAT3-deficient neutrophils. We found that STAT3 was dispensable for CXCR4 expression under basal conditions, as well as in aged neutrophils, which have been shown to up-regulate this receptor in vitro (Figure 4D).26 Moreover, CXCR4-mediated neutrophil chemotaxis was not STAT3 dependent (Figure 4E). In agreement with previous studies,24,25 we found that G-CSF suppressed bone marrow Cxcl12 mRNA expression in WT mice. By contrast, G-CSF stimulated a significant increase in Cxcl12 mRNA expression in STAT3-deficient bone marrow (Figure 4F). These data suggest that STAT3 regulates the amount of bone marrow SDF-1 expression after G-CSF administration but does not control basal CXCR4 expression or CXCR4-mediated chemotaxis. Altogether, our results indicate that G-CSF stimulates MIP-2 and KC expression while concomitantly down-regulating SDF-1 in the bone marrow of WT animals, which is consistent with the proposed role for these chemokines in the neutrophil mobilization response.18 By contrast, STAT3-deficient mice failed to up-regulate CXCR2 and its ligands and furthermore have increased Cxcl12 mRNA amounts relative to WT mice after G-CSF exposure. These data suggest that aberrant regulation of chemokine expression in the bone marrow may contribute to the diminished neutrophil mobilization response of STAT3-deficient animals.
STAT3-deficient mice fail to mobilize neutrophils in response to L monocytogenes infection and exhibit prolonged infection
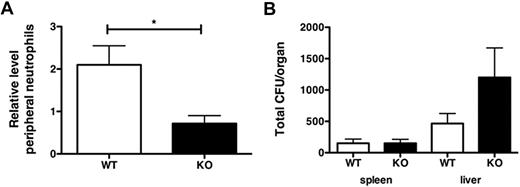
To determine whether the role for STAT3 in G-CSF– and MIP-2–responsive neutrophil mobilization reflected STAT3 function during bacterial infection, we assessed neutrophil mobilization in WT and STAT3-deficient mice after infection with L monocytogenes, a bacterial pathogen that is restrained by the actions of G-CSF in vivo.27,28 WT mice rapidly induced circulating neutrophil numbers upon infection (uninfected, 579.2 ± 84.1 neutrophils/μL; L monocytogene 12 hours, 1332.2 ± 329.6 neutrophils/μL; Figure 5A). By contrast, blood neutrophil amounts were not up-regulated in STAT3-deficient mice (uninfected 5101.0 ± 1760.5 neutrophils/μL; L monocytogene 12 hours, 2800.1 ± 792.2 neutrophils/μL; Figure 5A), suggesting a refractory mobilization response to L monocytogenes in the absence of functional STAT3. Bacterial load was significantly increased in the livers of STAT3-deficient mice relative to WT animals at 12 hours after infection, whereas no apparent differences were detected in spleen (Figure 5B).
Impaired mobilization of STAT3-deficient neutrophils during Listeria monocytogenes infection. WT or STAT3-deficient (KO) mice were infected with L monocytogenes by intravenous injection, as described in “Methods.” (A) At 12 hours after infection, circulating neutrophil numbers in peripheral blood were determined by automated counting. Data shown are average fold change in peripheral neutrophil numbers in infected versus uninfected animals (n = 5 for WT, n = 4 for KO). (B) Spleens and livers were isolated 12 hours after infection with L monocytogenes, homogenized, and cultured. CFUs were enumerated 24 hours after culture. Shown are mean CFU/organ (n = 6 for WT, n = 4 for KO). Error bars represent SEM *P < .05.
Impaired mobilization of STAT3-deficient neutrophils during Listeria monocytogenes infection. WT or STAT3-deficient (KO) mice were infected with L monocytogenes by intravenous injection, as described in “Methods.” (A) At 12 hours after infection, circulating neutrophil numbers in peripheral blood were determined by automated counting. Data shown are average fold change in peripheral neutrophil numbers in infected versus uninfected animals (n = 5 for WT, n = 4 for KO). (B) Spleens and livers were isolated 12 hours after infection with L monocytogenes, homogenized, and cultured. CFUs were enumerated 24 hours after culture. Shown are mean CFU/organ (n = 6 for WT, n = 4 for KO). Error bars represent SEM *P < .05.
These results are consistent with a study29 in which the authors used a granulocyte-depleting antibody during L monocytogenes infection, demonstrating that neutrophils are important for bacterial clearance from liver at early stages. Furthermore, 8 days after infection, WT mice were able to resolve the infection, whereas STAT3-deficient mice retained significant bacterial burden, as assayed by CFU determinations (data not shown). These results indicate that STAT3 is required for neutrophil mobilization in response to L monocytogenes and suggest that the aberrant neutrophil response contributes to an increased bacterial load in infected STAT3-deficient animals.
Discussion
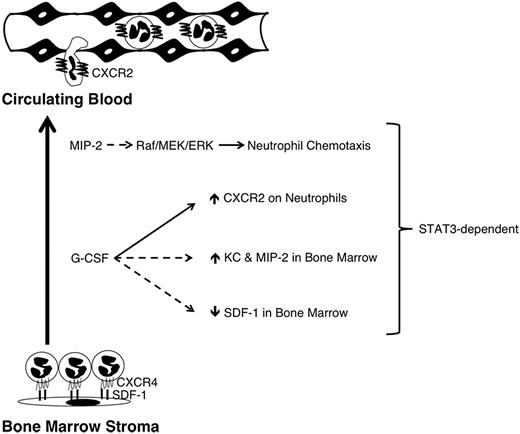
Here, we demonstrate that STAT3 controls several aspects of the bone marrow response to the mobilizing agents MIP-2 or G-CSF. These include ligand-inducible neutrophil mobilization, CXCR2 signal transduction, G-CSF–responsive CXCR2 expression in immature granulocytes, and bone marrow chemokine mRNA expression after G-CSF administration (Figure 6). Circulating neutrophils have a relatively short half-life (t1/2 ∼ 6-8 hours). Thus, continued replenishment from the bone marrow neutrophil reserve is necessary to sustain blood neutrophil levels, and these can be further enhanced during infection30 or clinical use of G-CSF. Although the use of G-CSF therapy is common, little is understood regarding the mechanisms by which it elicits neutrophil mobilization. Because G-CSF is itself not chemotactic,19 we and others have hypothesized that CXCR2 and its ligands, for example, MIP-2 and KC, participate in G-CSF–responsive neutrophil mobilization.7,18,19 This concept is supported by the observation that G-CSF fails to induce circulating neutrophil amounts in CXCR2-knockout mice.18 Our data demonstrate a role for STAT3 in neutrophil responses to G-CSF and MIP-2, further suggesting a functional relationship between these mobilization pathways.
A model for STAT3 function in neutrophil mobilization. Neutrophils are retained in the bone marrow in part through their expression of CXCR4, which interacts with SDF-1 expressed by stromal cells. G-CSF treatment directly or indirectly down-regulates CXCR4 and SDF-1; repression of SDF-1 requires STAT3 (dashed line). G-CSF treatment also induces the neutrophil chemoattractants KC and MIP-2 in the bone marrow (dashed line), as well as concurrent up-regulation of their shared receptor CXCR2 on the surface of neutrophils (solid line); induction of KC, MIP-2 and CXCR2 each require STAT3. STAT3 also controls the amplitude of MIP-2-induced Raf/MEK/ERK signaling (dashed line), which is crucial for neutrophil chemotaxis. Solid line denotes direct molecular regulation by STAT3; dashed lines indicate STAT3 regulation may be direct or indirect.
A model for STAT3 function in neutrophil mobilization. Neutrophils are retained in the bone marrow in part through their expression of CXCR4, which interacts with SDF-1 expressed by stromal cells. G-CSF treatment directly or indirectly down-regulates CXCR4 and SDF-1; repression of SDF-1 requires STAT3 (dashed line). G-CSF treatment also induces the neutrophil chemoattractants KC and MIP-2 in the bone marrow (dashed line), as well as concurrent up-regulation of their shared receptor CXCR2 on the surface of neutrophils (solid line); induction of KC, MIP-2 and CXCR2 each require STAT3. STAT3 also controls the amplitude of MIP-2-induced Raf/MEK/ERK signaling (dashed line), which is crucial for neutrophil chemotaxis. Solid line denotes direct molecular regulation by STAT3; dashed lines indicate STAT3 regulation may be direct or indirect.
The inability of STAT3-deficient mice to enhance peripheral blood neutrophil numbers upon MIP-2 treatment suggested a defective mobilization response. However, STAT3-deficient mice are neutrophilic at steady state, which complicates an analysis of neutrophil responses. Previously, we showed that sustained G-CSF treatment induces peripheral blood neutrophils approximately 10-fold beyond the steady-state amount in both WT and STAT3-deficient mice,7 ruling out the idea that circulating amounts are saturated in STAT3-deficient animals under basal conditions. Moreover, we found that bone marrow neutrophils were significantly decreased in WT mice by MIP-2 treatment yet unaffected in STAT3-deficient animals. Because the reduction in WT bone marrow neutrophil numbers could not be accounted for by accumulation in other hematopoietic organs (peripheral blood or spleen), the results suggest MIP-2 elicits neutrophil mobilization as well as margination to nonhematopoietic tissues. STAT3-deficient mice were nonresponsive to MIP-2, indicating deficient neutrophil mobilization and potentially margination, which would be consistent with impaired CXCR2-mediated chemotaxis of STAT3-deficient neutrophils.7
The Raf/MEK/ERK signaling axis has well-documented functions in cell migration,31 and its role in IL-8–mediated chemotaxis has been demonstrated in human neutrophils.32 Our experiments revealed that STAT3 regulates the amplitude of Raf/MEK/ERK signaling triggered by MIP-2 in murine neutrophils. This appears to be a specific effect on MIP-2-dependent Raf/MEK/ERK signaling because other downstream responses, eg, Akt activation and Ca2+ flux, were similar in WT and STAT3-deficient neutrophils (data not shown). Furthermore, use of the MEK inhibitor U0216 demonstrated that Raf/MEK/ERK signaling was critical for MIP-2–mediated neutrophil chemotaxis. Our results therefore suggest that STAT3-mediated control of Raf/MEK/ERK signaling is a central component of the MIP-2 chemotactic response in neutrophils. The mechanism(s) by which STAT3 functions to regulate the amplitude of MIP-2–dependent Raf/MEK/ERK signaling remain unknown. MIP-2 treatment does not induce STAT3 phosphorylation in neutrophils (data not shown), indicating that STAT3 is not directly activated by CXCR2. This finding suggests STAT3 indirectly regulates the Raf/MEK/Erk cascade downstream of CXCR2, perhaps via control of genes encoding scaffolding proteins and/or regulatory proteins for Rho GTPase family members, which have been implicated in ERK signaling.33,34
We previously found that STAT3-deficient neutrophils have aberrant MIP-2–dependent actin rearrangement,7 which may also involve Rho GTPase family members. Furthermore, a recent report demonstrated that STAT3 can regulate Rac1 activity in mouse embryonic fibroblasts by interaction with βPIX, a Rac1 activator.35 It will be important to assess the function of βPIX and Rac1 in WT and STAT3-deficient neutrophils to determine whether this signaling cascade operates in hematopoietic cells and to understand the potential involvement of STAT3. The precise mechanisms by which STAT3 acts are important to elucidate because they have a critical impact on neutrophil function and may also reveal how STAT proteins operate in other cell migration responses, including developmentally regulated cell movement and/or cancer metastasis.36,37
Little is known about the control of chemokine receptor expression; however, deregulated CXCR2-mediated neutrophil migration has been linked to inflammatory diseases such as rheumatoid arthritis 38 or chronic obstructive pulmonary disorder,39 indicating that further understanding of CXCR2 regulatory mechanisms may be important in clinical conditions. Although developmental expression of CXCR2 is STAT3 independent, we found that CXCR2 cell-surface expression was induced by systemic administration of G-CSF via a STAT3-responsive pathway. Examination of Il8rb molecular regulation showed that the gene encoding CXCR2 is a direct STAT3 target in immature Gr1lo granulocytes, and its induction correlates with enhanced neutrophil migratory activity. In light of the role for STAT3 in G-CSF–responsive mobilization, these results suggest that neutrophil release from the bone marrow may be augmented by G-CSF–STAT3-dependent enhancement of CXCR2 expression. This pathway could be a potential response in G-CSF–driven rheumatoid arthritis,40 serving to enhance the migratory capabilities of joint-infiltrating granulocytes, a possibility that requires further investigation. Overall, our results highlight an additional mechanism used by G-CSF to influence neutrophil migratory activity.
A previous study41 with human neutrophils produced conflicting results as to the chemokinetic or chemotactic properties of G-CSF, whereas in another,19 G-CSF did not show chemokinetic or chemotactic activity upon murine neutrophils. Trans-acting factors are, however, known to be important in the neutrophil mobilization response to G-CSF,42 suggesting that regulated chemokine expression may participate in neutrophil release under G-CSF–driven demand conditions. We found that G-CSF induces MIP-2 and KC expression in the bone marrow of WT mice, which is consistent with induction of serum IL-8 in humans receiving G-CSF therapy.22 G-CSF–responsive induction of MIP-2 and KC was not observed in STAT3-deficient bone marrow, indicating a role for STAT3 in regulation of these chemokines. Because the G-CSFR is expressed mainly on myeloid cells43 and because myeloid cells, including neutrophils, produce MIP-2, we speculate that the myeloid subset is a primary G-CSF target population that is responsible for MIP-2 production. Moreover, CXCR2 knockout mice were reported to have impaired neutrophil mobilization in response to G-CSF, underscoring the importance of CXCR2 in inducible neutrophil release.18 Thus, collectively, data from our laboratory and others suggests that G-CSF–dependent neutrophil mobilization may use several distinct mechanisms related to CXCR2 function, including regulated synthesis of MIP-2 and KC in bone marrow as well as inducible CXCR2 receptor expression and control of CXCR2 signal transduction.18,19
SDF-1 is constitutively expressed in the bone marrow, contributing to the retention of cells that express CXCR4.23 Inhibiting the SDF-1/CXCR4 interaction is sufficient to enable neutrophil release, as shown by use of the CXCR4 antagonist AMD3100.44 We found that G-CSF treatment decreases bone marrow SDF-1 expression in WT mice, in agreement with previous reports.24,25 SDF-1 down-regulation was not observed in STAT3-deficient mice; in fact, G-CSF appeared to stimulate bone marrow SDF-1 levels, which may contribute to the impaired neutrophil mobilization response of STAT3-deficient animals. In agreement, STAT3-deficient neutrophils do not appear to be mobilized as effectively as WT cells in response to AMD3100 (data not shown). Moreover, CXCR4 expression and CXCR4-mediated chemotaxis are similar between WT and STAT3-deficient neutrophils, suggesting G-CSF and STAT3 do not control CXCR4 synthesis or function. Therefore, the data collectively indicate that G-CSF and STAT3 regulate the bone marrow microenvironment, including SDF-1 expression, potentially influencing responses to mobilizing agents. SDF-1 expression may be controlled at a transcriptional level by G-CSF and STAT3. Alternatively, SDF-1 may be regulated indirectly, for example by control of SDF-1–expressing cells (eg, osteoblasts and endothelial cells24,45 ) in the bone marrow, because STAT3 has been implicated in regulation of osteoblast number.46
Mutations in the DNA binding or transactivation domain of STAT3 are linked to the primary immunodeficiency Hyper IgE syndrome (HIES), also known as Job syndrome.47,48 In addition to elevated levels of serum IgE, these patients are highly susceptible to recurrent skin abscesses and pneumonia cysts primarily caused by Staphylococcus aureus and Candida species. This aspect of the HIES phenotype is consistent with defective neutrophil function and, in fact, neutrophils isolated from HIES patients are reported to have impaired chemotaxis.49 We found that STAT3-deficient mice are susceptible to L monocytogenes, failing to show early (12 hours) clearance of bacteria in liver or resolution of infection after 8 days, unlike their WT counterparts (data not shown). The innate immune response, mediated by neutrophils and macrophages, is particularly critical for suppressing early phases of infection with L monocytogenes. We did not observe a significant difference in circulating monocyte levels 12 hours after infection in WT and STAT3-deficient mice (data not shown), which is consistent with a dominant role for neutrophils at this time point postinfection.29 Collectively, therefore, impaired neutrophil migration and reduced bacterial clearance in infected STAT3-deficient mice are indicative of the importance of neutrophil recruitment to suppress L monocytogenes at early stages. These results furthermore suggest that defective STAT3 activity in HIES patients may contribute to impaired neutrophil function and systemic immunodeficiency. STAT3-regulated pathways in granulocytes require additional investigation to understand their potential contribution to HIES as well as other diseases with aberrant neutrophil activity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Qing Ma for Fc-conjugated SDF-1α; Drs Chen Dong and Hao Shen for Listeria monocytogenes strain 1043S; Dr Judy Layton for human G-CSFR cDNA; Ling Zhang for technical support; Valeria Facchinetti for helpful discussions; and Karen Ramirez, Z. David He, and Amy Cortez for assistance with cell sorting.
The flow core facility at UT MDACC is supported by NCI#P30CA16672. This study was supported by grants from the National Institutes of Health (T32-CA-09598-16, to H.N.-J, and A.D.P.; AI073587, to S.S.W) and an investigator-initiated Preclinical Research Agreement with Amgen Inc (to S.S.W.).
H.N.-J. is a PhD candidate at The University of Texas Health Science Center at Houston, and this work is submitted in partial fulfillment of the requirement for the PhD.
National Institutes of Health
Authorship
Contribution: H.N.-J. designed and performed experiments, analyzed data, and wrote the paper; A.D.P. designed and performed experiments, analyzed data, and contributed to writing the paper; H.Z. designed and performed experiments; H.S.L. provided STAT3-deficient mice; and S.S.W. supervised the project, analyzed data and wrote the paper.
Conflict-of-interest disclosure: This study was funded in part through an investigator-initiated preclinical Research Agreement with Amgen Inc.
The current address for A.D.P. is Gene Expression Laboratory, The Salk Institute for Biological Studies, La Jolla, CA.
Correspondence: Stephanie S. Watowich, PhD, Department of Immunology, UT M. D. Anderson Cancer Center, PO Box 301402, Unit 902, Houston, TX 77030-1903; e-mail: swatowic@mdanderson.org.

![Figure 1. STAT3-dependent regulation of neutrophil migration and CXCR2-mediated Raf/MEK/ERK signaling. (A) Peripheral blood neutrophil numbers were determined for WT (□) and STAT3-deficient (knockout [KO], ■) mice treated with MIP-2 (50 μg/kg) or BSA carrier for 30 minutes. Neutrophil amounts in MIP-2–treated mice were normalized to BSA-treated controls of the appropriate genotype and relative levels are shown (n = at least 4 for WT and KO). (B) Absolute neutrophil numbers in total spleen, blood, and bone marrow of BSA- or MIP-2–treated mice are shown (n = at least 3 for WT and KO for each condition). (C) CXCR2 expression within the Gr-1+ bone marrow population of BSA- and MIP-2–treated WT (black line) and STAT3-deficient (KO, gray line) mice is shown, relative to isotype controls (dashed black line). Results are representative of 3 independent experiments. (D) Activation of c-Raf, MEK1/2, and ERK1/2 in MIP-2–treated (100 ng/mL) or unstimulated Gr-1+ cells was analyzed by immunoblotting and quantified by densitometry. Results are representative of at least 3 independent experiments. (E) MIP-2–responsive chemotaxis of bone marrow neutrophils from WT mice was examined in the absence of or after, 30 minutes pretreatment with the MEK1/2 inhibitor U0126 (10μM or 50μM as indicated, n = 5 for each condition). Average values from 3 independent experiments are shown. (F) WT bone marrow neutrophils were stimulated with MIP-2 (100 ng/mL for 5 minutes) in the presence or absence of U0126 (10μM or 50μM as indicated). ERK1/2 activation was assessed by immunoblotting with whole-cell lysates; results are representative of 3 independent experiments. Error bars represent SEM; *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/16/10.1182_blood-2009-08-240317/4/m_zh89991051570001.jpeg?Expires=1767719382&Signature=43emeKfrGKunKcJguaU5p5x7plBr7lpd9Gh~2U4dp-XdPBA57uVQbacafwZyimS84nG6OygfBFpA~x0nrsM6A6lUrWx4SNW643AdTpxyV8ErLPVHXHoIQoRbITFS5-CLYkTAklZwhw0prCqDonwx4KnIO5AuTxsRHRUObJAUIV4KAt-SWM1uDfI96A4nnu1oDIfHRcvzrE~ieBEdvaWXxNVqjqDtlnX0cGnxnovmVFynggfN8Xkg8ljSHhGteNNcu8-Cp0t78lD-J6macMbnpafMDorFiJUIpuCGXVzyjvnF75m1IKEPQpz6CSxEmql1IEgfBtK1qhHsYxhHCkYn6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal