Abstract
Russell bodies (RBs) are intracellular inclusions filled with protein aggregates. In diverse lymphoid disorders these occur as immunoglobulin (Ig) deposits, accumulating in abnormal plasma or Mott cells. In heavy-chain deposition disease truncated antibody heavy-chains (HCs) are found, which bear a resemblance to diverse polypeptides produced in Ig light-chain (LC)–deficient (L−/−) mice. In L−/− animals, the known functions of LC, providing part of the antigen-binding site of an antibody and securing progression of B-cell development, may not be required. Here, we show a novel function of LC in preventing antibody aggregation. L−/− mice produce truncated HC naturally, constant region (C)γ and Cα lack CH1, and Cμ is without CH1 or CH1 and CH2. Most plasma cells found in these mice are CD138+ Mott cells, filled with RBs, formed by aggregation of HCs of different isotypes. The importance of LC in preventing HC aggregation is evident in knock-in mice, expressing Cμ without CH1 and CH2, which only develop an abundance of RBs when LC is absent. These results reveal that preventing antibody aggregation is a major function of LC, important for understanding the physiology of heavy-chain deposition disease, and in general recognizing the mechanisms, which initiate protein conformational diseases.
Introduction
In the healthy organism, immunoglobulin (Ig) is present in membrane form, as part of the B-cell receptor (BCR), and in secreted form, as antibody. During B-cell development, VHDJH rearrangement of a heavy chain (HC) precedes VLJL rearrangement of a light chain (LC). Nascent HC polypeptides are associated with the HC-binding protein (BiP, or GRP78) in the endoplasmic reticulum (ER) awaiting LC association, processing (eg, carbohydrate association), and export.1-3 The key function of the BiP chaperone is to provide a scaffold to native or unfolded polypeptides, which prevents aggregation and establishes the appropriate conformation. Abnormal plasma cells called Mott cells, containing Ig aggregates, termed Russell bodies (RBs), have been found in nonsecretory myeloma, inflammatory diseases, and autoimmune disorders.4,5 RB formation appears to indicate cellular indigestion due to a failure to eliminate misfolded or incorrectly assembled proteins.6 An unsolved question is whether the inability to degrade or to export leads to Ig aggregation. It is possible that the production of large quantities of Ig as well as structural alterations may prevent appropriate processing.
In the absence of LC, HC dissociation from chaperones and export is facilitated by deletions in the VH and/or CH1 domain, a feature seen in pathology and also in cultured cell lines.7-9 Shortened HC is produced in various lymphoid malignancies. In heavy-chain diseases (HCDs), HC is not associated with LC, lacks all or part of VH, and frequently shows deletion(s) in its CH domain(s).7 In heavy-chain deposition disease (HCDD), a kidney disease secondary to plasma cell dyscrasia, deletions of CH1 or of both CH1 and CH2 domains are found.10,11 LCs are associated with HCs in HCDD serum antibodies but are missing from the deposits.10,11
Expression studies showed that full-length HC cannot reach the cell surface in the absence of LC or surrogate LC,12,13 although more recent experiments demonstrated that this is not always the case.14-17 At the pre-B cell stage, HC pairing with surrogate LC results in surface expression and oligomerization of the pre-BCR, regarded as important for pre-BCR signaling.18,19 HC-LC pairing permits a high level of surface IgM expression but appears to inhibit basal signaling,20 possibly by preventing auto-aggregation.
We previously found that γ and α HCs lacking CH1, similar to the proteins produced in HCDD, are present in the serum of LC-deficient (L−/−) mice, which appear to be healthy.21,22 HCs are encoded by mRNAs transcribed from somatically truncated HC genes with deletions extending from the switch region. Although membrane μ is required for these class-switch isotypes to be produced, no secreted μHC could be detected.
Here, we report that in L−/− mice, deletions in Cμ result in the production of short HC transcripts and proteins by plasma cells; however, truncated μ proteins are only found in serum from older mice. In addition to this defect of secretion, a large majority of plasma cells, including those expressing γ and α isotypes, give rise to RBs or Mott cells. In knock-in animals expressing truncated IgM, RBs are rare, but when these animals are crossed with L−/− mice, large numbers are found. Our results provide evidence that a major function of LC is to prevent HC aggregation by retaining Ig folding and structure; important for reducing the susceptibility of B cell–derived diseases.
Methods
Mice and hybridoma production
The derivation of LC-deficient (L−/−),23 Cμ truncation (knock-in μNR),24 μNRL−/−,25 and CH deletion (CΔ)26 mice has been described. Animals were housed in the Babraham barrier facility, and procedures were carried out under project license PPL 80/1872, approved by the United Kingdom Home Office. Mice were immunized as previously described,21 and spleen cell fusions were carried out with mouse NSO and rat YO myeloma cells, both negative for Ig production.27
ELISA, Western, and mass spectrometry
For enzyme-linked immunosorbent assay (ELISA), serum dilutions were analyzed as described21 on Falcon plates coated with 10 μg/mL anti–mouse IgM (μ chain specific, Sigma-Aldrich) and detection was performed with biotinylated (BIO) anti–mouse IgM antibodies (Sigma-Aldrich). For Western blot analysis, serum Ig was captured on anti–mouse Ig (μ-, γ-, α-chain specific; Southern Biotech)–coupled Sepharose, separated on Ready-Gels (Bio-Rad) under reducing conditions and transferred to nitrocellulose membranes as described.28 Filters were incubated with BIO anti–mouse IgM followed by incubation with streptavidin BIO horseradish peroxidase (HRP; GE Healthcare) and visualization of bands using SuperSignal West Pico chemiluminescent substrate. Protein molecular weight standards were supplied by Bio-Rad. For hybridoma analysis by mass-spectrometry culture, supernatant was incubated with anti–Ig-coupled Sepharose and separated on Ready-Gels. Coomassie-stained bands were destained, reduced, carbamidomethylated, and digested with 10 ng/μL Sequencing Grade Modified Trypsin (Promega) in 25mM NH4HCO3 at 30°C. The resulting peptide mixtures were separated by reversed-phase liquid chromatography as previously described.21 Proteins/peptides were identified by database searching of the mass spectral data using Mascot software (Matrix Science).
Flow cytometry
Spleen cell suspensions were prepared and stained with BIO anti–mouse IgM (Zymed) or BIO anti-CD138 (syndecan-1; BD Biosciences), followed by phycoerythrin-conjugated (PE) streptavidin (Caltag), and then stained with fluorescein isothiocyanate-conjugated (FITC) anti–mouse B220 (BD Biosciences). Two-color analysis was carried out on a BD FACSCalibur as described, using CellQuest software (BD Biosciences). Sorting was performed on a FACSAria (BD Biosciences). Sorted cells were collected in Eppendorf tubes and resuspended in the appropriate medium for reverse transcriptase (RT) or long DNA polymerase chain reaction (PCR), or dropped onto poly-L-lysine slides. For sorting of intracellular IgM+ plasma cells, spleen cells were surface-stained for CD138, before permeabilization and incubation with FITC-conjugated anti–mouse IgM (Sigma-Aldrich).
RT-PCR analysis
RNA was isolated from spleen cells or sorted cells using Trizol (Gibco-BRL), reverse-transcribed with Omniscript reverse transcriptase (QIAGEN), and PCR reactions performed using KOD Hot Start DNA polymerase (Novagen) as described.21 VH and JH forward primer sequences were as in Zou et al21 . Cμ reverse primer sequences were as follows: Cμ3, 5′GAGACCA-GACAGGTCAGGTTAGCGG3′, and Cμ2, 5′CAAGAAGGTGAGACCCCTGTGATCC3′. Reaction conditions were 94°C for 2 minutes, and 38 cycles of 94°C for 15 seconds, 58°C for 30 seconds, and 72°C for 10 seconds, followed by 72°C for 10 minutes. RT-PCR products were either cleaned up (DNace Quick Clean; Bioline) and sequenced directly, or cloned by adding a 3′ A overhang and using a TA Cloning Kit (Invitrogen).
Genomic DNA analysis
Genomic DNA was prepared as described.21 Long-range PCR was carried out with Platinum PCR Supermix High Fidelity (Invitrogen) with oligonucleotide sequences given in Zou et al21 , except for Cμ3, as above, and Cμ4 (5′TCAGTTGCTCACGAGCTGGTG3′). Reactions were set up with DNA from 100 sorted cells and 100nM of each primer. A first round PCR of 20 cycles from JH4long to Cμ4 was followed by a second PCR of 20 cycles using JH4long or Eμ and Cμ3 or Cμ4 primers. An initial step of 94°C for 1 minute was followed by cycles of 94°C for 15 seconds and 68°C for 15 minutes. Bands obtained were picked from the gel and reamplified, or cloned as in “RT-PCR analysis,” and then sequenced.
Microscopy staining and analysis
Spleen or hybridoma cells were incubated with the following antibodies: FITC goat anti–mouse IgM (Sigma-Aldrich) or goat anti–mouse IgG Alexa-488 (Molecular Probes, Invitrogen); BIO anti–mouse IgA (Sigma-Aldrich) or BIO rat anti–mouse IgM (Zymed), followed by Streptavidin Alexa-647 (Molecular Probes). Cells were then sorted and fixed on poly-L-lysine slides using a fix and perm cell permeabilization kit (Caltag). Alternatively, spleen cells were smeared onto poly-L-lysine slides and fixed in 4% formaldehyde for 30 minutes, air-dried, and stored in the fridge. Staining with 4′-6-diamidino-2-phenylindole (DAPI), hematoxylin, and periodic acid-Schiff (PAS), using a staining kit (Sigma-Aldrich), was carried out according to the manufacturer's instructions. A Leica microscope (40× objective) equipped with a DC300F digital camera was used to capture the bright-field images of the samples stained with hematoxylin and PAS. Immunofluorescence image acquisition was performed with a LSM 510 Meta confocal laser scanning microscope (Carl Zeiss) equipped with a Plan-Apochromat 63×/1.40 differential interference contrast oil-immersion objective. A minimum of 5 fields from each sample were imaged (700 × 700 pixel size). Selected images were assembled and pseudo-colored using Adobe Photoshop CS2.
Results
Age-related build-up of truncated μHC protein
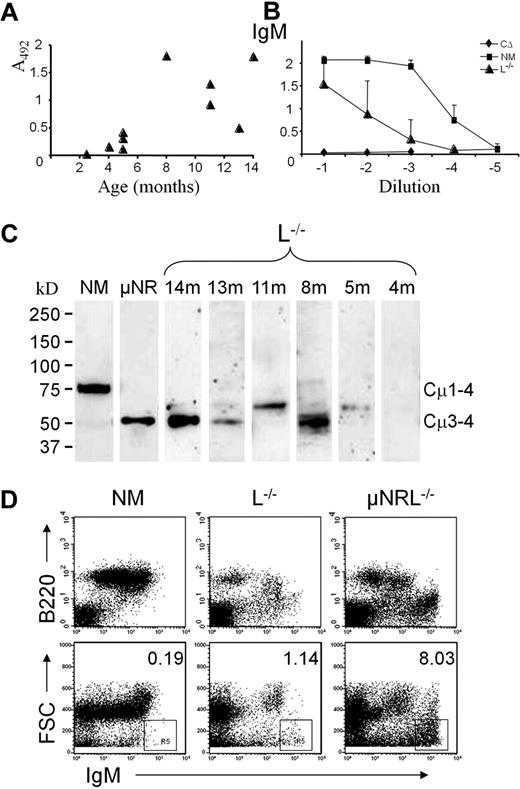
We have recently shown that mice unable to express Igκ and Igλ LC (L−/− mice) produce substantial amounts of HC-only IgG and IgA.21,22 No IgM serum protein could be identified, but the integrity of the μ C gene appeared to be essential as breeding into the μ MT KO background (disrupted Cμ transmembrane exons) prevented HC expression.21 To further analyze the importance of IgM in HC antibody production, we assessed age-related expression.
Significant quantities of μHC were only found in older animals, whereas no serum IgM was present in young mice (Figure 1A-B). This was confirmed by Western blot, which revealed that secreted μHC in L−/− mice is of reduced size similar to the knock-in μNR mouse line in which Cμ1 and Cμ2 are removed23 (Figure 1C). No full-length IgM was found, only products possibly 1 or 2 domains shorter then conventional μ chain, and no truncated IgM in animals younger than 4 months.
Truncated IgM is present in serum of old L−/− mice. (A) Level of μHC in serum of 2- to 14-month-old L−/− mice determined by ELISA at 1/100 dilution. (B) A comparative analysis of IgM serum titers in L−/− (▴), C-region deletion (CΔ26 ) as a negative control (♦) and normal (NM) mice (■). Means and standard deviation were calculated from the 10 L−/− mice in panel A and 7 NM mice. (C) Western analysis of serum antibodies from L−/−, of different age in months (m) as indicated. Sera from L−/−, μNR, and NM mice were purified by incubation with anti–mouse Ig coupled to Sepharose, separated on Ready-Gels under reducing conditions, and visualized with antibodies against mouse IgM. (D) Flow cytometric analysis of spleen cells surface-stained for IgM and B220. A representative presentation of a 1-year-old L−/− mouse compared with NM and μNRL−/− animals. The upper histograms show that, in addition to B220+ IgM+ cells, an IgMbright B220− population can be found in old L−/− mice. This population is more abundant in μNRL−/− mice. Plotting forward scatter (FSC) against IgM shows an IgMbright population of small particles with percentages indicated.
Truncated IgM is present in serum of old L−/− mice. (A) Level of μHC in serum of 2- to 14-month-old L−/− mice determined by ELISA at 1/100 dilution. (B) A comparative analysis of IgM serum titers in L−/− (▴), C-region deletion (CΔ26 ) as a negative control (♦) and normal (NM) mice (■). Means and standard deviation were calculated from the 10 L−/− mice in panel A and 7 NM mice. (C) Western analysis of serum antibodies from L−/−, of different age in months (m) as indicated. Sera from L−/−, μNR, and NM mice were purified by incubation with anti–mouse Ig coupled to Sepharose, separated on Ready-Gels under reducing conditions, and visualized with antibodies against mouse IgM. (D) Flow cytometric analysis of spleen cells surface-stained for IgM and B220. A representative presentation of a 1-year-old L−/− mouse compared with NM and μNRL−/− animals. The upper histograms show that, in addition to B220+ IgM+ cells, an IgMbright B220− population can be found in old L−/− mice. This population is more abundant in μNRL−/− mice. Plotting forward scatter (FSC) against IgM shows an IgMbright population of small particles with percentages indicated.
As μ chains with Cμ1-Cμ2 deletion can be exported to the surface of B cells in the absence of LCs,25 we wondered whether truncated μ-expressing B cells would accumulate in older mice. In flow cytometric analysis, spleens from ≥ 1-year-old L−/− mice showed indeed measurable numbers of B220+ μ+ cells (Figure 1D), but these cells were found to express normal-size μ transcripts (data not shown). Surprisingly, in addition to B220+ cells, IgM staining also occurred on B220− cells. By extending the forward scatter, we identified highly fluorescent particles smaller than cells. Unusual IgM+ staining was even more evident in μNRL−/− mice but absent in normal or μNR mice expressing LC (not shown and Zou et al24 ).
Short μ transcripts are abundant in plasma cells but not surface μ+ B cells
Although L−/− mice less than 4 months old are almost devoid of μ+ cells,21,23 detailed flow cytometric examinations of larger cell numbers revealed a small population of B220high IgM+ cells (0.2%) similar in appearance to those in normal mice (Figure 2A). As truncated μ H-chains can be cell surface–expressed without LC,25 we sought to identify what type of B-lineage cell, characterized by its differentiation markers, would express short μ transcripts in L−/− mice. Staining for B220, IgM, and the plasma cell marker CD138/syndecan-1, followed by sorting and RT-PCR analysis, revealed that the majority of VH to Cμ transcripts in B220high μ+ cells were full length, whereas short transcripts lacking CH1 and CH2 were predominant in CD138+ plasma cells (Figure 2B). Amplification from JH to Cμ2, identified short μ transcripts without CH1 (Figure 2C). Faint bands corresponding to short transcripts could reproducibly be seen in μ+ but not in μ− cell fractions. The results indicate that, although some L−/− B cells might express truncated μ proteins on their surface, selection of cells producing short μ transcripts occurs predominantly at the plasma cell stage.
Short μ transcripts are present in L−/− plasma cells. (A) Spleen cells from a 6-month-old L−/− and a NM mouse were stained either for B220 and IgM or B220 and CD138, and sorted using the indicated gates. IgM+ cells from L−/− spleen represent 0.2% of cells in the lymphocyte gate. (B-C) RT-PCR analysis of the sorted fractions using a degenerate VH (Vgen) or JH (JH3) primer,21 in combination with a Cμ3 or Cμ2 reverse primer, respectively. H2O served as negative control and the white line indicates a repositioned marker lane from the same gel. RNA was prepared and reverse transcribed from the sorted cell fractions (NM and L−/−) as indicated (B220+, B220 int[ermediate], B220 high, μ+, and CD138+) and from total spleen aliquots as control. The cDNA equivalent of 200 cells was used in each assay, except for the μ+ fraction (100 cells) and the L−/− CD138+ fraction (25 cells). (B) A normal μ transcript results in a ∼ 1.1-kb fragment after VH-Cμ3 amplification. The small band of ∼ 0.45 kb, found only in L−/− mice, corresponds to a transcript without CH1 and CH2, and the intermediate band ∼ 0.8 kb corresponds to a transcript lacking CH1. (C) After JH-Cμ2 amplification, a normal band of ∼ 0.7 kb is found, which is reduced to ∼ 0.35 kb, lacking CH1, in L−/− total spleen or CD138+ cells. A weak band is also seen in μ+ cells.
Short μ transcripts are present in L−/− plasma cells. (A) Spleen cells from a 6-month-old L−/− and a NM mouse were stained either for B220 and IgM or B220 and CD138, and sorted using the indicated gates. IgM+ cells from L−/− spleen represent 0.2% of cells in the lymphocyte gate. (B-C) RT-PCR analysis of the sorted fractions using a degenerate VH (Vgen) or JH (JH3) primer,21 in combination with a Cμ3 or Cμ2 reverse primer, respectively. H2O served as negative control and the white line indicates a repositioned marker lane from the same gel. RNA was prepared and reverse transcribed from the sorted cell fractions (NM and L−/−) as indicated (B220+, B220 int[ermediate], B220 high, μ+, and CD138+) and from total spleen aliquots as control. The cDNA equivalent of 200 cells was used in each assay, except for the μ+ fraction (100 cells) and the L−/− CD138+ fraction (25 cells). (B) A normal μ transcript results in a ∼ 1.1-kb fragment after VH-Cμ3 amplification. The small band of ∼ 0.45 kb, found only in L−/− mice, corresponds to a transcript without CH1 and CH2, and the intermediate band ∼ 0.8 kb corresponds to a transcript lacking CH1. (C) After JH-Cμ2 amplification, a normal band of ∼ 0.7 kb is found, which is reduced to ∼ 0.35 kb, lacking CH1, in L−/− total spleen or CD138+ cells. A weak band is also seen in μ+ cells.
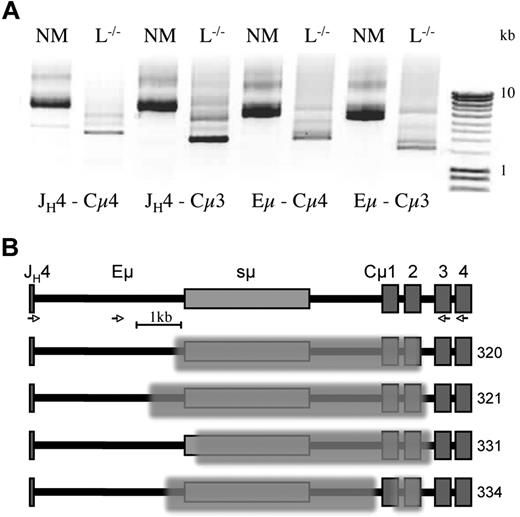
Genomic deletions in Cμ accumulate at the plasma cell stage
RT-PCR analysis established that CD138+ plasma cells generate μHC transcripts in truncated form. To examine the configuration of the IgH locus in these cells, we prepared DNA from sorted CD138+ cells and used a long-range PCR amplification approach, described previously.21 DNA equivalent to 100 cells was used in the first PCR amplification from JH4 to Cμ4. This was followed by separate amplifications using (semi-) nested primers, JH4-Cμ3, Eμ-Cμ4, and Eμ-Cμ3, which significantly enhanced the primary PCR band (Figure 3A). Interestingly, the major amplification products obtained from L−/− CD138+ cells were much shorter than products from normal mice. Using primers outside of the switch sequence, sμ, ensured reproducible amplification. Sequencing of products from normal mice confirmed the integrity of Cμ1and Cμ2 (data not shown).
Acquired genomic deletions in sμ and Cμ. DNA from ∼ 100 sorted CD138+ cells, obtained from the spleen of NM and L−/− mice as in Figure 2, was amplified for 20 cycles using JH4- and Cμ4-specific primers. (A) Separate reamplification for a further 20 cycles was carried out with (nested) primer combination as indicated: JH4-Cμ4, JH4-Cμ3, Eμ-Cμ4, and Eμ-Cμ3. After agarose gel electrophoresis, bands were cut out and reamplified or cloned, before sequencing using the Cμ3 primer. (B) Map of the region encompassing JH4, Eμ, switch (s)μ to Cμ exons 1-4, with examples from 2 different L−/− mice, with deletions indicated by the superimposed shading. PCR fragments and sequences obtained from a normal mouse showed the wild-type configuration (top). Amplification and sequencing primers are indicated by  (see sequences in supplemental Table 1.)
(see sequences in supplemental Table 1.)
Acquired genomic deletions in sμ and Cμ. DNA from ∼ 100 sorted CD138+ cells, obtained from the spleen of NM and L−/− mice as in Figure 2, was amplified for 20 cycles using JH4- and Cμ4-specific primers. (A) Separate reamplification for a further 20 cycles was carried out with (nested) primer combination as indicated: JH4-Cμ4, JH4-Cμ3, Eμ-Cμ4, and Eμ-Cμ3. After agarose gel electrophoresis, bands were cut out and reamplified or cloned, before sequencing using the Cμ3 primer. (B) Map of the region encompassing JH4, Eμ, switch (s)μ to Cμ exons 1-4, with examples from 2 different L−/− mice, with deletions indicated by the superimposed shading. PCR fragments and sequences obtained from a normal mouse showed the wild-type configuration (top). Amplification and sequencing primers are indicated by  (see sequences in supplemental Table 1.)
(see sequences in supplemental Table 1.)
Results from cloned PCR products from 2 L−/− mice revealed a full or partial deletion of Cμ1 and Cμ2, and removal of various amounts of sμ in every case (Figure 3B and supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Surprisingly, one of the sequences contained 2 distinct deletions; one within the switch region and the other comprising most of Cμ1 and Cμ2, disabling both exons. The sole removal of Cμ1 was not found, which may reflect a rare event in agreement with protein and transcript analyses.
Hybridoma production in LC-deficient mice
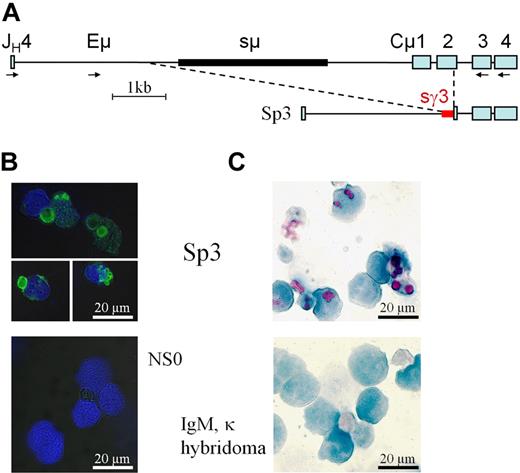
Hybridoma production was inefficient in L−/− mice, linked to the block in B-cell development and a presumed lack of receptive fusion cells in the spleen. Four fusions, performed after immunization with ovalbumin, which generated antigen-specific HC-only IgG in serum,21 gave rise to only 6 clones secreting Ig. In contrast, many antigen-specific clones were obtained from the controls. After expansion and/or cloning, only fusion clone Sp3 produced a few micrograms per milliliter of IgM without LC in the culture supernatant. Mass-spectrometry of purified μHC from Sp3, obtained after protein-A adsorption and separation by SDS-PAGE, identified a VH-gene of the J558 family and μHC sequences from the CH3 and CH4 domain but not CH1 and CH2. RT-PCR from J558-VH to Cμ3 identified a functional VHDJH-Cμ transcript that was 2 domains shorter than conventional μHC. Long-range DNA-PCR and sequencing revealed a product lacking sμ, Cμ1, and all but 24 bp of Cμ2, but containing an insertion of the γ3 switch region (Figure 4A and supplemental Table 1). The alterations in Sp3 are very similar to the genomic deletions identified in L−/− plasma cells and unambiguously link truncated μHC expression to acquired genomic alterations.
Truncated μHC is expressed in a L−/− hybridoma. (A) Genomic alterations in hybridoma Sp3, identified by long-range PCR, cloning, and sequencing (see supplemental Table 1) show that sμ (thick black line), Cμ1, and all but 24 bp of Cμ2 are missing. Immediately adjacent to the remaining sequence of Cμ2 are 220 bp of sγ3 (thick red line). Primers for PCR amplification and nested reamplification are indicated by  . (B) Intracellular staining of Sp3 and NSO, the Ig-negative fusion partner, with FITC-conjugated anti-IgM (green) followed by DAPI (blue). (C) Sp3 smears compared with a control IgM,κ-producing hybridoma-stained with PAS, showing vacuoles in purple-magenta, and hematoxylin, showing the nuclei in blue.
. (B) Intracellular staining of Sp3 and NSO, the Ig-negative fusion partner, with FITC-conjugated anti-IgM (green) followed by DAPI (blue). (C) Sp3 smears compared with a control IgM,κ-producing hybridoma-stained with PAS, showing vacuoles in purple-magenta, and hematoxylin, showing the nuclei in blue.
Truncated μHC is expressed in a L−/− hybridoma. (A) Genomic alterations in hybridoma Sp3, identified by long-range PCR, cloning, and sequencing (see supplemental Table 1) show that sμ (thick black line), Cμ1, and all but 24 bp of Cμ2 are missing. Immediately adjacent to the remaining sequence of Cμ2 are 220 bp of sγ3 (thick red line). Primers for PCR amplification and nested reamplification are indicated by  . (B) Intracellular staining of Sp3 and NSO, the Ig-negative fusion partner, with FITC-conjugated anti-IgM (green) followed by DAPI (blue). (C) Sp3 smears compared with a control IgM,κ-producing hybridoma-stained with PAS, showing vacuoles in purple-magenta, and hematoxylin, showing the nuclei in blue.
. (B) Intracellular staining of Sp3 and NSO, the Ig-negative fusion partner, with FITC-conjugated anti-IgM (green) followed by DAPI (blue). (C) Sp3 smears compared with a control IgM,κ-producing hybridoma-stained with PAS, showing vacuoles in purple-magenta, and hematoxylin, showing the nuclei in blue.
An important finding was the release of short μHC from Sp3 cells, indicating that transport of this incomplete, multi-domain polypeptide through the ER can occur. To test whether circumventing BiP retention permits or retains appropriate processing of HC without LC, cell stainings were carried out (Figure 4B-C). Intracellular staining with anti-IgM revealed the presence of spherical particles or clustered vesicles (Figure 4B), corresponding to the definition of RB. Confirmation of the glycoprotein nature of these vacuoles was obtained by PAS staining, showing a purple-magenta color, which revealed a similar globular structure and distribution as staining with anti-IgM (Figure 4C).
Plasma cells from L−/− mice produce RBs
To follow up the observation that truncated μHC may be prone to form aggregates, we focused on a detailed analysis of splenic plasma cells from L−/− mice to answer whether IgM without LC gives rise to RBs in the animal. For this, sorted CD138+ spleen cells were dropped onto poly-L-lysine coated slides, stained with PAS, and counterstained with hematoxylin (Figure 5A). In normal mice, plasma cells were seen as hematoxylin-stained nuclei, whereas cytoplasm was barely visible. However, in L−/− mice, CD138+ cells similar to Mott cells were seen as (purple-magenta) PAS+ particles, some connected to the nucleus by cytoplasm bridges, others of morula shape. Stainings of whole spleen smears, in particular from older animals, showed much larger RBs of various shapes, some surrounded by rosette forming nuclei (Figure 5B and supplemental Figure 1). The size differences between sorted cells and total smears are due to filtration to remove undesired aggregates interfering with the flow parameters. Cells surrounding RBs are strikingly larger than normal splenocytes, some polymorphonuclear, suggesting that HC aggregates can trigger inflammatory processes.
RB formation in L−/− plasma cells gives rise to Mott cells. (A) Sorted CD138+ spleen cells from 3-month-old normal (NM) and L−/− mice were stained with PAS, showing tightly packed glycoproteins in purple-magenta and hematoxylin-stained cell nuclei in blue. For L−/− cells, PAS staining was found either in connection with the nucleus or in isolation as spherical particles. The arrow indicates a morula cell. (B) PAS stainings of spleen cell smears from a 14-month-old L−/− and a normal aged mouse. RBs in smears, frequently surrounded by nonlymphoid cells, are larger than from sorted cells, prepared as single-cell suspensions devoid of aggregates. (C-E) Intracellular staining after fixation and permeabilization with FITC-labeled (C) anti-IgM (μ+), (D) anti-IgA (α+), and (E) anti-IgG (γ+). The DAPI counterstain shows nucleotides/dsDNA in blue. Frequencies of positive cells were < 1% for all stainings, consistent with the expected low number of plasma cells.
RB formation in L−/− plasma cells gives rise to Mott cells. (A) Sorted CD138+ spleen cells from 3-month-old normal (NM) and L−/− mice were stained with PAS, showing tightly packed glycoproteins in purple-magenta and hematoxylin-stained cell nuclei in blue. For L−/− cells, PAS staining was found either in connection with the nucleus or in isolation as spherical particles. The arrow indicates a morula cell. (B) PAS stainings of spleen cell smears from a 14-month-old L−/− and a normal aged mouse. RBs in smears, frequently surrounded by nonlymphoid cells, are larger than from sorted cells, prepared as single-cell suspensions devoid of aggregates. (C-E) Intracellular staining after fixation and permeabilization with FITC-labeled (C) anti-IgM (μ+), (D) anti-IgA (α+), and (E) anti-IgG (γ+). The DAPI counterstain shows nucleotides/dsDNA in blue. Frequencies of positive cells were < 1% for all stainings, consistent with the expected low number of plasma cells.
As the majority of CD138+ cells stained strongly with PAS, we wondered whether only IgM or also other truncated isoforms without LC could produce similar aggregates. In normal mice, typical plasma cells with large cytoplasmic compartments are found for all Ig classes. In L−/− mice, IgM and IgA staining (Figure 5C-D) revealed large clusters of small fluorescent vesicles representing μ+ and α+ RBs, some connecting the nuclei, whereas typical plasma cells were absent. Small spherical particles may correspond to the IgMbright particles in Figure 1D, and the large RBs seen in whole spleen may be composed of clusters of these particles. With anti-IgG, few cells stained in L−/− mice (Figure 5E) but with larger γ+ RBs compared with those for IgM and IgA. In addition, some L−/− plasma cells retain their normal appearance after anti-IgG staining, whereas other cells, in sorts and smears, looked like conventional large B cells.
Abnormal HC is not sufficient for Russell body formation
To clarify whether truncated HC, alone or associated with LC, leads to predisposition of RB and Mott cell formation, we compared the type and appearance of plasma cells in μNR23 and μNRL−/−25 animals by microscopy. After VHDJH rearrangement, μNR knock-in mice express truncated μHC lacking CH1-2 similar to μHCs generated in older L−/− mice. Monomeric μ chains are produced in both strains; however, RBs were only apparent in μNRL−/− plasma (CD138+) cells (Figure 6A and supplemental Figure 1) and absent in B220high CD138− cells expressing truncated surface μ (data not shown). In CD138+ spleen cells from μNR mice, we found little RB-type aggregation, whereas in μNRL−/− mice, the number of RBs was almost equivalent to the number of plasma cell nuclei. Taking into account that normal class switched isotypes are readily produced in μNR mice, we sorted CD138+ cells that were also stained intracellularly for IgM (Figure 6B). Both strains have considerable numbers of intracellular IgM+ plasma cells, although fewer were present in μNRL−/−. Although the fixation required for intracellular IgM staining resulted in a significant loss of RBs after sorting, numerous RBs were only found in μNRL−/− mice (Figure 6C).
The absence of LC and HC truncation leads to Ig aggregation. (A) Analysis of CD138+ cells from normal (NM), μNR (Cμ1/2 deletion), and μNRL−/− mice, stained after sorting with PAS and hematoxylin as in Figure 5A, showed that RBs are frequently generated when LC is missing. (B) Cells were surface-stained with anti-CD138 followed by intracellular staining with anti-IgM. The ratio of IgM+ CD138+ cells is well maintained in μNRL−/− animals (1.3%) and allows a direct comparison (C) of the RB to nuclei ratio in HC truncation mice with and without LC. ND indicates not determined.
The absence of LC and HC truncation leads to Ig aggregation. (A) Analysis of CD138+ cells from normal (NM), μNR (Cμ1/2 deletion), and μNRL−/− mice, stained after sorting with PAS and hematoxylin as in Figure 5A, showed that RBs are frequently generated when LC is missing. (B) Cells were surface-stained with anti-CD138 followed by intracellular staining with anti-IgM. The ratio of IgM+ CD138+ cells is well maintained in μNRL−/− animals (1.3%) and allows a direct comparison (C) of the RB to nuclei ratio in HC truncation mice with and without LC. ND indicates not determined.
In conclusion, we have provided experimental evidence that LC deficiency is of key importance for the generation of RBs.
Discussion
The Ig LC has an established function in providing part of the antigen-binding site of the BCR and antibody. However, it is thought that it may have wider and more fundamental roles,29 and discussions of its importance have addressed 3 main areas: the phylogenetic pressure retaining LC,30 developmental regulation,23 and preserving structural integrity of antibodies by HC and LC association.31 In early B-cell development, LC pairing with HC displaces the BiP chaperone, which results in cell surface or membrane Ig expression12,13 essential for B-cell survival and differentiation.32,33 LC may also counteract HC toxicity at the plasma cell stage, concluded from the finding that CH domain deletion is a consistent feature of HC-only producing hybridomas.34 Even so, HC without LC is naturally produced in lower vertebrates35,36 and in camelids,37 in which many regular differentiation events are maintained.
For HC secretion without LC, structural alterations in the VH and/or the CH1 domain are necessary.7,21,22 Despite this, B cells can also survive by surface expression of full-length μHC without LC,14-17 although such a protein is not secreted. The deletions we identified in L−/− mice with the loss of Cμ1-2 closely resemble Cμ gene alterations in immortalized cells.8,38,39 All have lost switch μ region, and in one case, part of the γ3 switch sequence has been inserted, which has not been previously described. This suggests that production of truncated μHC, perhaps initiated by prior expression of the same VHDJH bearing full-length μHC, is the consequence of faulty switch attempts and the inherent instability of this region.40 Truncation of Cμ is not strictly required for surface expression at the B-cell stage, as shown by Geraldes et al,17 because we found μ+ cells exhibiting mainly the full-length transcript. In contrast, short μ transcripts are strongly enriched at the plasma cell stage, suggesting that expression of a full-length μ chain is detrimental at this stage in L−/− mice, which is in agreement with the HC toxicity model.34
Although plasma cells with short μ transcripts and genomic CH1-CH2 deletion could be found in the spleens of young LC-deficient mice, no protein was found in the serum. This may suggest either a defect in secretion of the truncated protein or its adequate removal by the unfolded protein response.2,41 It is conceivable that synthesis of aggregating truncated HC exceeds the degradation capacity of plasma cells as antibody factories. A defect in secretion could also lead to HC accumulation within the cell, but this defect might be also a consequence of aggregation. As substantial amounts of HC IgA are secreted in L−/− animals,22 it may well be that the threshold tolerating HC IgA, IgG, or IgM varies and is isotype dependent.
The dramatic increase of RBs in μNRL−/− animals compared with μNR mice indicated that LC deficiency promotes RB formation. The lack of LC was not previously known to cause RB formation, presumably because plasma cells producing normal HC do not survive when LC production ceases. In transfection experiments, truncated μHC produced RBs in the presence of LC,42 although precipitation with anti-μ revealed poor association, which may indicate that transfected LC promotes RB formation in this model not directly but by replacing chaperone binding. More recent studies showed that HC with Cμ1 deletion could form RBs with and without LC, although the latter resulted in smaller, less regular structures.43 In the model developed by Valetti and colleagues, RB formation is related to IgM polymerization.42 This contrasts with the situation in L−/− mice, in which μ chains lacking CH1 and CH2 do not form polymers.24 In L−/− mice, RB formation is observed for all Ig classes with diverse VH regions, and this may account for the differences when in vitro transfection is compared with mouse strains that produce diverse HC in truncated form.
The promotion of RB formation by LC deficiency could be accounted for by its effect on HC aggregation, because in the absence of LC, HCs aggregate in solution44 and RBs are composed of aggregated protein.6 HC aggregation followed by apoptosis has been observed when insufficient LC is produced and upon faulty HC and LC association due to strand charge repulsion.45 Association of LC to HC also inhibits surface μHC signaling, which might be accounted for by inhibition of clustering.20,46 In transgenic mice, human μHCD protein did not associate with LC and spontaneously aggregated on the cell surface.47 It is tempting to speculate that a function of LC is to prevent aggregation by conferring hydrophilicity to the BCR (or antibody). As a result, exposure of hydrophobic amino acid stretches normally involved in LC association would facilitate HC aggregation. We propose a model in which intracellular HCs, when synthesized at high rate, aggregate because of the differences in association without LC. This could simply be the result of incorrect folding or aggregation of correctly folded HCs due to unmatched hydrophobic interfaces. It is interesting to note that homodimerization of the retained CH domains is LC independent, whereas the typical constraints on V regions are LC dependent.22 Thus, the absence of selective pressure for fitting VHVL combinations could retain adverse residues in VH or, in other words, may fail to provide counterbalancing residues to restore charge or shape differences causing aggregation. The capacity of the cell to continue truncated HC production leaves also the possibility that chaperone control is outpaced. This agrees with the finding that full-length HC without LC might be detrimental for the cells (HC toxicity), whereas truncated HC does not impede survival but can lead to RB formation (Figure 7).
HC toxicity model. Lymphocyte development from B cell to plasma cell is normal upon association of full length of H and L chains (top). Development is constrained, as in μNR mice, when Cμ is truncated, and blocked when normal H but no L chains are produced (middle). Truncated H chain, not associated with L chain or the lack of L chain, gives rise to RBs and Mott cells (bottom).
HC toxicity model. Lymphocyte development from B cell to plasma cell is normal upon association of full length of H and L chains (top). Development is constrained, as in μNR mice, when Cμ is truncated, and blocked when normal H but no L chains are produced (middle). Truncated H chain, not associated with L chain or the lack of L chain, gives rise to RBs and Mott cells (bottom).
Summarizing our results in the context of previous disease studies supports the view that HC aggregation with pathogenic consequences can occur at discrete stages in B-cell development: upon surface expression of a signaling BCR variant, as in HCD7 ; by intracellular deposition, resulting in RB formation, as in nonsecreting myelomas4 ; and upon cellular release or secretion in serum, leading to deposits in the kidney, as in HCDD.10 Although nonsecretory myeloma or HCDD is not usually associated with LC deficiency,4,10 a characteristic feature of HCD is the absence of LC association.7 It is also important to note that HCDD aggregates in the tissue deposits do not contain LC. It appears that faulty HC association leads to aggregation, reminiscent of the protein aggregation process seen in a large group of pathologies termed conformational diseases, ranging from amyloidoses to prion encephalopathies.48 To identify the mechanism(s) leading to protein misfolding and aggregation, LC-deficient mice may provide useful insights.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are particularly grateful to Dr David Hardman and Babraham Bioscience Technologies for continued support and encouragement. We thank Dr Mike Taussig for support and inspiration and Dr Elisabetta Babetto for providing anti-IgG detection reagents and advice.
The research was carried out at the Babraham Institute, supported by the Biotechnology and Biological Sciences Research Council (BBSRC) with a Follow-on-Grant to M.B., a studentship to L.S.M., and a European Science Foundation exchange grant (FFG-1233) and Inserm support to D.C.
Authorship
Contribution: D.C. developed the project, performed most of the experiments, and wrote the draft of the manuscript; D.C. and M.B. decided on the experimental strategies and informative presentation of the results, and M.B. coordinated the project; M.J.O. performed the molecular experiments, and L.S.M. and D.O. carried out the protein analysis; F.S. carried out the microscopic analysis, and G.M. helped with the flow cytometry; X.Z. and J.A.S. produced and maintained the mice, and A.H. and M.H. produced hybridomas and were involved in serum analyses. The manuscript was finalized by M.B., M.J.O., and D.C. with the assistance of all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel Corcos, Lymphocyte Development, Team 16, Unit 955, Inserm, Faculté de Médecine-Paris 12, 8 rue du Général Sarrail, 94010 Créteil, France; e-mail: daniel.corcos@inserm.fr; or Marianne Brüggemann, The Babraham Institute, Babraham, Cambridge CB22 3AT, United Kingdom; e-mail: marianne.bruggemann@bbsrc.ac.uk.

![Figure 2. Short μ transcripts are present in L−/− plasma cells. (A) Spleen cells from a 6-month-old L−/− and a NM mouse were stained either for B220 and IgM or B220 and CD138, and sorted using the indicated gates. IgM+ cells from L−/− spleen represent 0.2% of cells in the lymphocyte gate. (B-C) RT-PCR analysis of the sorted fractions using a degenerate VH (Vgen) or JH (JH3) primer,21 in combination with a Cμ3 or Cμ2 reverse primer, respectively. H2O served as negative control and the white line indicates a repositioned marker lane from the same gel. RNA was prepared and reverse transcribed from the sorted cell fractions (NM and L−/−) as indicated (B220+, B220 int[ermediate], B220 high, μ+, and CD138+) and from total spleen aliquots as control. The cDNA equivalent of 200 cells was used in each assay, except for the μ+ fraction (100 cells) and the L−/− CD138+ fraction (25 cells). (B) A normal μ transcript results in a ∼ 1.1-kb fragment after VH-Cμ3 amplification. The small band of ∼ 0.45 kb, found only in L−/− mice, corresponds to a transcript without CH1 and CH2, and the intermediate band ∼ 0.8 kb corresponds to a transcript lacking CH1. (C) After JH-Cμ2 amplification, a normal band of ∼ 0.7 kb is found, which is reduced to ∼ 0.35 kb, lacking CH1, in L−/− total spleen or CD138+ cells. A weak band is also seen in μ+ cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/2/10.1182_blood-2009-07-234864/5/m_zh89990945630002.jpeg?Expires=1767733423&Signature=ULgZUPCM8oPvYpohz8z1jeV17xU~Q~SqxdJRsijzVV2qlyerloNikp-wjsWnF0qu7hhZN3Do50Mpsqm37RlKgO53Kz6SXzOKrGht5e~J1rVbkwbsBf8bPJhslKJp0rfnWpS3Vr8fHv8m7FP33rUl4NDLu70ivcqq2kDhFsTMpWBR9T60Z5WbVBXnYwMpcOCrUT0IT1uA~XFJMEMi-XQJHlf-BUNklSecURW9-J~zg8jufxE1Ln~hsXeAWSA-c2uAjxhmfN2Sx4Hm75AqPtA3~sSBUidh1QgdA2fZzr8Z~vnJfj15~fz0e0BhIyfhcqsd31NHccZFqs5xcrf9IRpl8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal