Abstract
We investigated the cause of mild mucocutaneous bleeding in a 14-year-old male patient (P1). Platelet aggregation and ATP secretion induced by arachidonic acid and the thromboxane A2 receptor (TxA2R) agonist U46619 were reduced in P1 compared with controls, whereas the responses to other platelet agonists were retained. P1 was heterozygous for a transversion within the TBXA2R gene predictive of a D304N substitution in the TxA2R. In Chinese hamster ovary-K1 cells expressing the variant D304N TxA2R, U46619 did not increase cytosolic free Ca2+ concentration, indicating loss of receptor function. The TxA2R antagonist [3H]-SQ29548 showed an approximate 50% decrease in binding to platelets from P1 but absent binding to Chinese hamster ovary-K1 cells expressing variant D304N TxA2R. This is the second naturally occurring TxA2R variant to be associated with platelet dysfunction and the first in which loss of receptor function is associated with reduced ligand binding. D304 lies within a conserved NPXXY motif in transmembrane domain 7 of the TxA2R that is a key structural element in family A G protein-coupled receptors. Our demonstration that the D304N substitution causes clinically significant platelet dysfunction by reducing ligand binding establishes the importance of the NPXXY motif for TxA2R function in vivo.
Introduction
The thromboxane A2 receptor (TxA2R) is the G protein-coupled receptor (GPCR) for the eicosanoid thromboxane A2 (TxA2) and the isoprostane 8-iso-prostaglandin F2α1 and is encoded by the TBXA2R gene, which is located at 19p13.3. Alternative splicing of TBXA2R creates different α or β isoforms of the TxA2R that differ only at the carboxy-terminus.2 Both isoforms are expressed in vascular tissues and mediate activities that include smooth muscle contraction, activation and migration of endothelial cells, and angiogenesis.3,4
The TxA2Rα isoform is also expressed in platelets5 and plays a critical feedback role in mediating platelet activation during primary hemostasis. This pathway is initiated by liberation of arachidonic acid (AA) by the action of cytosolic phospholipase A2 followed by metabolism of AA to the intermediary prostaglandin H2 and then to TxA2. Binding of TxA2 to TxA2R then initiates proactivation signals through Gq family G proteins, leading to phospholipase C β-mediated Ca2+ release and protein kinase C activation.6 TxA2R also signals through G13 family G proteins to activate Rho-mediated pathways.7 The TxA2R is therefore a critical mediator of platelet function in primary hemostasis and is an attractive target for antithrombotic drugs for use as alternatives or adjuncts to existing antiplatelet agents.8,9
The development of the TxA2R as a drug target has required identification of the functional domains within the receptor that mediate ligand binding and signal transduction. Accordingly, the TxA2R has been studied in detail ex vivo using site directed mutagenesis and by direct imaging techniques.4 These analyses indicate that conserved residues in extracellular loop 2 mediate ligand binding10,11 and that intracellular loops 1 and 3 mediate coupling to Gq.12,13
A single naturally occurring variant TxA2R has been reported previously that contained an R60L substitution in intracellular loop 1.14,15 The R60L substitution caused loss of TxA2R function by abrogating TxA2R coupling to Gq and was associated with abnormal platelet functional responses ex vivo and a mild clinical bleeding phenotype.14,15 Characterization of the variant R60L TxA2R has thereby provided valuable independent evidence of the significance of intracellular loop 1 for TxA2R function. We now extend the repertoire of informative human TxA2R variants by reporting the clinical and laboratory phenotype of a second kindred with a naturally occurring variant TxA2R containing a D304N substitution in transmembrane domain 7. We also present evidence that the D304N substitution causes TxA2R dysfunction through prevention of ligand binding.
Methods
The study kindred was identified in a systematic analysis of subjects with mild heritable platelet function disorders registered at hemophilia centers in the United Kingdom. This study has received UK Multicentre Research Ethics Committee approval, and informed consent was obtained in accordance with the Declaration of Helsinki.
Platelet function studies
Platelet aggregation and ATP secretion in response to a panel of agonists were measured in platelet rich plasma (PRP) using a dual Chronolog lumi-aggregometer as described previously.16 The aggregation and secretion responses were compared with responses in control platelets collected from healthy volunteer donors at the same time as the patient samples. The maximum amplitude of the aggregation responses and the total ATP secretion were also compared with normal reference ranges generated in our laboratory using platelets from a panel of 20 healthy volunteer donors.
Analysis of TBXA2R
The TBXA2R coding sequence and splice donor and acceptor sites were amplified by polymerase chain reaction (PCR) from genomic DNA purified from venous blood (oligonucleotide primers and PCR conditions available on request). PCR amplicons were sequenced with a model 3700 DNA analyzer using an ABI PRISM Big Dye V2 reaction kit (Applied Biosystems), and sequence variations were identified by comparison with the TBXA2R cDNA reference sequences NM_001060 and NM_201636. The presence of the TBXA2R c.910G>C transversion was confirmed by restriction analysis using AvaII.
Ligand binding studies and thromboxane B2 synthesis in platelets
For ligand binding studies and measurement of thromboxane B2 (TxB2) formation, platelets were isolated from PRP by centrifugation in the presence of 0.02 U/mL of apyrase and prostaglandin E1 (140nM). The pellet was resuspended in a modified Tyrode-HEPES buffer (145mM NaCl, 2.9mM KCl, 10mM HEPES, 1mM MgCl2, and 5mM glucose, pH 7.3).
For ligand binding studies, the platelets were fixed with 4% formaldehyde before resuspending in binding buffer (20mM HEPES and 1mM MgCl2) and incubated with [3H]-SQ29548 (3 Ci/mmol, 0.01-0.1μM) either in the presence or absence of unlabeled ligand (10μM). After incubation for 20 minutes at room temperature, reactions were terminated with ice-cold binding buffer and rapid filtration through Whatman GF/C glass fiber filters under vacuum. Radioactivity bound to the filters was measured by scintillation counting.17
TxB2 levels were measured in washed platelets after incubation for 5 minutes in the presence or absence of 1μM of AA using an enzyme-linked immunosorbent assay (ELISA; GE Healthcare UK) in accordance with the manufacturer's instructions.
TxA2R expression experiments
The wild-type TxA2R expression construct comprised a DNA3.1 hygromycin vector containing the TxA2Rα cDNA fused at the N-terminus with the FLAG epitope tag. The variant p.D304N expression construct was then generated from this template by site-directed mutagenesis using a QuikChange Site-directed Mutagenesis Kit (Stratagene) in accordance with the manufacturer's instructions. The wild-type and D304N TxA2Rα expression constructs were stably transfected into Chinese hamster ovary-K1 (CHO-K1) cells as described previously.18
Expression of wild-type and D304N TxA2R in CHO-K1 cells
Expression of the FLAG-tagged receptors in transfected cells was assessed by ELISA and immunofluorescence microscopy using the FLAG M2 monoclonal antibody (Sigma-Aldrich) as described previously.19
Measurement of cytosolic [Ca2+]i and ligand binding studies in CHO-K1 cells
Transfected CHO-K1 cells were cultured on poly(L-lysine)–coated glass coverslips and used at approximately 60% confluence. Cells were washed twice with Locke solution (154mM NaCl, 5.6mM KCl, 1.2mM MgCl2, 2.2mM CaCl2, 5mM HEPES, and 10mM glucose, pH 7.4) and incubated with the fluorescent Ca2+ indicator fura-2/AM (3μM) at 37°C for 60 minutes. Glass coverslips were mounted into a quartz cuvette and placed into a thermostatically controlled cell holder at 37°C. Cells were continuously perfused with Locke solution. Fluorescence was measured at 340 and 380nM excitation and 510nM emission. U46619 (0.001-10μM) was perfused onto cell monolayers, and [Ca2+]i was determined from ratiometric data as described previously.20 For ligand binding studies, cells expressing receptor constructs were harvested and incubated with [3H]-SQ29548 (3 Ci/mmol, 0.01-0.1μM) for 20 minutes at room temperature. Ligand binding was then determined in the presence of unlabeled ligand (10μM) as described previously.17
Results
Clinical description and platelet phenotype of the study proband
The proband (P1) was a 14-year-old British, white male patient with nonconsanguinous parents who presented for investigation with easy bruising and prolonged epistaxes since infancy that could not be attributed to any local abnormality in the upper airway. There were no other pathologic bleeding symptoms, although P1 had not experienced any significant traumatic or surgical hemostatic challenges. Investigations for coagulation factor deficiencies and von Willebrand disease were normal (not shown).
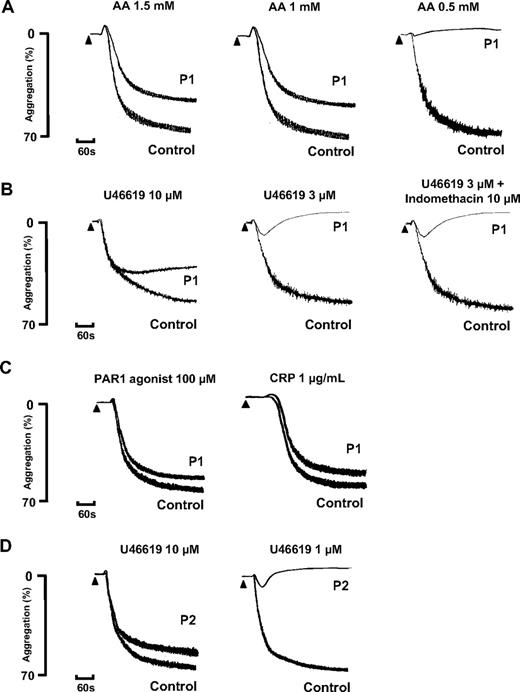
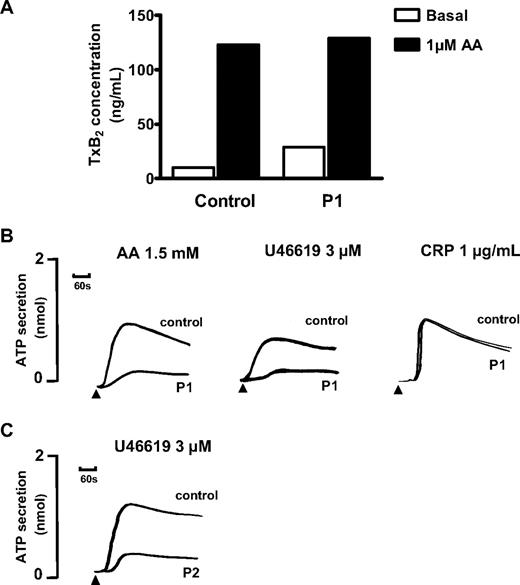
Platelet aggregation in response to a low concentration of 0.5mM AA was absent in P1, whereas this induced full aggregation in control platelets that were collected on the same day. There was also a reduced level of aggregation to 1mM and 1.5mM AA compared with the control platelets, although the level of the response fell within the normal reference range for maximal aggregation determined in cohort of 20 healthy donors16 (Figure 1A). The aggregation response to a high concentration of the TxA2R agonist U46619 (10μM) was also reduced in platelets from P1 compared with control platelets and had begun to return to baseline by 5 minutes. The response to 3μM U46619 was transient and showed a complete return to baseline within the observation time (Figure 1B). In comparison, we did not observe a transient response to 3μM U46619 in the control platelets collected on the same day or in more than 20 healthy volunteer donors used to generate laboratory reference ranges. There was also no diminution of the aggregation response to 3μM U46619 in the presence of indomethacin in platelets from P1 or from controls, thereby demonstrating that the impairment in platelet aggregation responses was not due to a defect in TxA2 synthesis (Figure 1B). Consistent with this, thromboxane B2 (TxB2) synthesis induced by 1μM AA using washed platelets from P1 was similar to that in control platelets (Figure 2A). Importantly, platelets from P1 showed similar aggregation responses to controls with high concentrations of a protease-activated receptor (PAR1) peptide agonist (100μM) and a GPVI-specific collagen related peptide (CRP; 1 μg/mL; Figure 1C), thereby indicating a defect in the TxA2 proactivation pathway at the level of theTxA2R rather than a generalized aggregation defect.
Agonist-induced platelet activation in subjects P1 and P2. (A-C) Platelet aggregation in response to the indicated concentrations of arachidonic acid (AA), U46619, PAR 1 agonist, and CRP in citrated PRP from subject P1 and healthy control platelets collected on the same day. Platelet aggregation to 3μM of U46619 was also tested in the presence of indomethacin (10μM). (D) Aggregation responses to U46619 are also shown in PRP from subject P2 who had the same variant D304N TxA2R as P1. The results are representative of 2 experiments.
Agonist-induced platelet activation in subjects P1 and P2. (A-C) Platelet aggregation in response to the indicated concentrations of arachidonic acid (AA), U46619, PAR 1 agonist, and CRP in citrated PRP from subject P1 and healthy control platelets collected on the same day. Platelet aggregation to 3μM of U46619 was also tested in the presence of indomethacin (10μM). (D) Aggregation responses to U46619 are also shown in PRP from subject P2 who had the same variant D304N TxA2R as P1. The results are representative of 2 experiments.
Agonist-induced platelet ATP secretion and thromboxane B2 synthesis. (A) TxB2 was measured by ELISA in buffer containing washed platelets from subject P1 and from a healthy experimental control at baseline and after incubation with 1μM AA. The results are from 1 experiment performed in triplicate. (B) ATP secretion in response to the indicated concentrations of AA, U46619, and CRP was determined by measuring luminescence signal from PRP in the presence of luciferase in a Chronolog lumiaggregometer. (C) ATP secretion in response to 3μM U46619 in washed platelets from subject P2, who had the same variant D304N TxA2R as P1. Data are expressed as concentration of ATP in PRP. The results are representative of 2 experiments.
Agonist-induced platelet ATP secretion and thromboxane B2 synthesis. (A) TxB2 was measured by ELISA in buffer containing washed platelets from subject P1 and from a healthy experimental control at baseline and after incubation with 1μM AA. The results are from 1 experiment performed in triplicate. (B) ATP secretion in response to the indicated concentrations of AA, U46619, and CRP was determined by measuring luminescence signal from PRP in the presence of luciferase in a Chronolog lumiaggregometer. (C) ATP secretion in response to 3μM U46619 in washed platelets from subject P2, who had the same variant D304N TxA2R as P1. Data are expressed as concentration of ATP in PRP. The results are representative of 2 experiments.
The ATP secretion in platelets from P1 in response to 3μM U46619 or 1.5mM AA was also markedly reduced compared with control platelets (Figure 2B), and the total ATP secretion was less than the lower limits of our laboratory reference ranges for these agonists.16 In contrast, ATP secretion responses in platelets from P1 induced by 1-μg/mL CRP (Figure 2B) and 100μM PAR1 agonist (not shown) were similar to control platelets and were within our laboratory reference ranges.
Clinical and platelet phenotype in family members
The father (P2) and mother (P3) of the proband had no significant history of abnormal bleeding. Platelets from P2 showed abnormal aggregation and ATP secretion responses to AA and U46619 that were similar to the abnormal responses in P1 (Figures 1D and 2C) but showed normal responses to PAR1 agonist and CRP (not shown). In contrast, platelets from P3 showed normal aggregation and ATP secretion responses to all agonists (data not shown).
Identification of the TBXA2R D304N mutation
Subjects P1 and P2, who showed the same abnormal platelet aggregation and secretion responses, were heterozygous for a c.910G>C transversion in TBXA2R that is predictive of an aspartate to asparagine substitution at position 304 in transmembrane domain 7 of the TxA2R (p.D304N). The TBXA2R c.910G>C transversion was not identified as polymorphic in either the National Center for Biotechnology Information (http://www.ncbi.nim.nih.gov/) or Ensemble (http://www.ensembl.org/index.html) databases. Subject P3, who showed normal platelet aggregation and secretion responses, had wild-type TBXA2R sequence.
Heterologous expression of variant D304N TxA2R
To investigate further the effect of the D304N substitution on TxA2R expression and function, we generated CHO-K1 cell clones that stably expressed either wild-type or variant D304N TxA2R as the α receptor isoform, which is the predominant isoform expressed in platelets.5 The wild-type and variant D304N TxA2R showed similar levels of surface expression as determined by ELISA and immunofluorescence microscopy (Figure 3A-B).
Expression and functional analysis of wild-type and variant D304N TxA2R expressed in CHO-K1 cells. (A) CHO-K1 cells were transfected with expression constructs containing wild-type (WT TxA2R) or variant D304N TxA2R (D304N TxA2R). Expression levels of TxA2R in CHO cell lysates were determined by ELISA using anti-FLAG M2 monoclonal antibody. Data represent means ± SEM of 3 independent experiments and are reported as arbitrary units (AU) relative to expression level of the wild-type TxA2R (designated as 1AU). (B) Transfected CHO-K1 expressing wild-type and variant D304N TxA2R were also studied by immunofluorescence microscopy using an anti-FLAG M2 monoclonal antibody. The images are representative of > 20 high power fields. (C) Changes in cytoplasmic calcium concentration [Ca2+]i were measured by preincubating washed transfected CHO-K1 cells with fura-2/AM (3μM) before addition of U46619 (0.001-10μM). Data are expressed as peak calcium response quantified as described previously20 and represent the means ± SEM of 3 independent experiments.
Expression and functional analysis of wild-type and variant D304N TxA2R expressed in CHO-K1 cells. (A) CHO-K1 cells were transfected with expression constructs containing wild-type (WT TxA2R) or variant D304N TxA2R (D304N TxA2R). Expression levels of TxA2R in CHO cell lysates were determined by ELISA using anti-FLAG M2 monoclonal antibody. Data represent means ± SEM of 3 independent experiments and are reported as arbitrary units (AU) relative to expression level of the wild-type TxA2R (designated as 1AU). (B) Transfected CHO-K1 expressing wild-type and variant D304N TxA2R were also studied by immunofluorescence microscopy using an anti-FLAG M2 monoclonal antibody. The images are representative of > 20 high power fields. (C) Changes in cytoplasmic calcium concentration [Ca2+]i were measured by preincubating washed transfected CHO-K1 cells with fura-2/AM (3μM) before addition of U46619 (0.001-10μM). Data are expressed as peak calcium response quantified as described previously20 and represent the means ± SEM of 3 independent experiments.
Variant D304N and wild-type TxA2R function was then examined in CHO-K1 cells by measuring changes in cytosolic Ca2+ concentration ([Ca2+]i) induced by U46619. CHO-K1 cells expressing wild-type TxA2R showed a near maximal rise in [Ca2+]i at 10μM U46619 (Figure 3C). However, at this ligand concentration, the maximal rise in [Ca2+]i in CHO-K1 cells expressing variant D304N TxA2R was reduced by more than 85% and was similar to that in nontransfected control CHO-K1 cells (Figure 2C). This indicates complete loss of function of variant D304N TxA2R expressed in CHO-K1 cells.
Binding of [3H]-SQ29548 to platelets and transfected CHO-K1 cells
To study to how the D304N substitution caused loss of TxA2R function, we measured binding of the TxA2R antagonist [3H]-SQ29548 to platelets from the study subjects and to CHO-K1 cells expressing the wild-type and variant D304N TxA2R. Binding of [3H]-SQ29548 to platelets and to the transfected CHO-K1 cells was saturable and maximal at a ligand concentration of 0.1μM (Figure 4A-B). The binding affinity of [3H]-SQ29548 to platelets from P1 was similar to that observed with platelets from an unrelated control donor and from family member P3, who did not show platelet dysfunction (Kd P1 34 ± 21nM; P3 22 ± 10nM; unrelated control 23 ± 10nM). This was also comparable with the binding affinity of [3H]-SQ29548 to CHO-K1 cells expressing wild-type TxA2R (Kd 16 ± 5nM).
Binding of [3H]-SQ29548 to platelets from P1 and a healthy control and to CHO-K1 cells expressing either wild-type or variant D304N TxA2R. (A) Washed fixed platelets from P1 from a related control (P3) who did not have the D304N TxA2R substitution and from a healthy unrelated donor were incubated for 20 minutes with [3H]-SQ29548 (0.1μM) in the presence of unlabeled ligand (10μM) to determine specific receptor-dependent binding. Binding was then determined by measuring bound labeled ligand after a 20-minute incubation. Data are expressed as disintegrations per minute per 4 × 106 platelets and represent the means ± SEM of 3 independent experiments. (B) Specific receptor-dependent [3H]-SQ29548 binding to either wild-type (WT TxA2R) or variant D304N TxA2R (D304N TxA2R) expressed in CHO-K1 cells was determined as described in “Methods.” Data are expressed as disintegrations per minute per milligram of protein and represent the means ± SEM of 3 independent experiments.
Binding of [3H]-SQ29548 to platelets from P1 and a healthy control and to CHO-K1 cells expressing either wild-type or variant D304N TxA2R. (A) Washed fixed platelets from P1 from a related control (P3) who did not have the D304N TxA2R substitution and from a healthy unrelated donor were incubated for 20 minutes with [3H]-SQ29548 (0.1μM) in the presence of unlabeled ligand (10μM) to determine specific receptor-dependent binding. Binding was then determined by measuring bound labeled ligand after a 20-minute incubation. Data are expressed as disintegrations per minute per 4 × 106 platelets and represent the means ± SEM of 3 independent experiments. (B) Specific receptor-dependent [3H]-SQ29548 binding to either wild-type (WT TxA2R) or variant D304N TxA2R (D304N TxA2R) expressed in CHO-K1 cells was determined as described in “Methods.” Data are expressed as disintegrations per minute per milligram of protein and represent the means ± SEM of 3 independent experiments.
The maximum binding of [3H]-SQ29548 to platelets from P1 was reduced by just over 50% compared with control platelets (Bmax P1 167 ± 40 dpm/4 × 106 platelets; P3 403 ± 61 dpm/4 × 106 platelets; unrelated control 383 ± 70 dpm/4 × 106 platelets; Figure 4A). The maximum binding of [3H]-SQ29548 to CHO-K1 cells expressing variant D304N TxA2R was reduced by more than 85% compared with those expressing wild-type TxA2R (Bmax D304N TxA2R 79 ± 27 dpm/mg protein; wild-type TxA2R 520 ± 33 dpm/mg protein; Figure 4B). Maximum binding to CHO-K1 cells expressing variant D304N TxA2R was similar to that observed in nontransfected CHO-K1 cells, indicating that the D304N substitution abolishes [3H]-SQ29548 binding.
Discussion
We have demonstrated that platelets from 2 subjects from the same kindred showed greatly reduced aggregation and ATP secretion in response to AA, which is the metabolic precursor of TxA2 and to the TxA2R agonist U46619. In contrast, the responses to high concentrations of other agonists were either normal or showed minimal abnormality. There was no further diminution in the aggregation response to U46619 in the presence of the indomethacin, which inhibits TxA2 synthesis consistent with the fact that the response to U46619 is not reinforced by endogenous synthesis of TxA2.16 Moreover, the synthesis of thromboxane B2, which is generated in platelets from TxA2 by nonenzymatic hydrolysis, was normal in response to AA. This laboratory phenotype indicated a functional defect in the TxA2 proactivation pathway that was unrelated to TxA2 synthesis but occurred at the level of the TxA2R. We have demonstrated that this phenotype segregated within the study kindred with a heterozygous D304N substitution in the TxA2R.
It is significant that the aggregation responses to AA in PRP from P1 were outside our laboratory normal reference ranges only at the 0.5mM concentration, which is lower than the minimum AA concentration suggested recently for clinical diagnostic laboratories.21 We also demonstrated an abnormal aggregation response to 3μM U46619 and abnormal ATP secretion to 1.5mM AA and 3μM U46619. U46619 is not used widely in clinical diagnostic laboratories, and assays to measures platelet ATP secretion are not used widely,22 even though a lumi-aggregometer has available commercially for more than 25 years. This means that the platelet defect that we identified in P1 is unlikely to have been detected in a nonspecialized diagnostic laboratory. Our findings therefore support the inclusion of a wider range of AA concentrations and the introduction of U46619 as an agonist in diagnostic platelet aggregation assays to improve sensitivity for mild TxA2R defects.
We expressed variant D304N TxA2R at a similar level to wild-type TxA2R on the surface of CHO-K1 cells. However, D304R TxA2R failed to mediate increased [Ca2+]i in response to U46619, indicating that the D304N substitution confers complete loss of receptor function. This is consistent with the partial loss of TxA2R function observed in platelets from the study subjects, as these subjects were heterozygous for the D304N substitution and were therefore predicted to express both wild-type and variant TxA2R. The results of the CHO-K1 expression experiments therefore confirm that the partial loss of TxA2R function observed in platelets from the study subjects is caused by the D304N substitution. This report is only the second description of a naturally occurring TxA2R variant in which there is loss of receptor function.14
Heterozygosity for the D304N substitution was associated with abnormal mucocutaneous bleeding in subject P1. At first sight, this suggests that expression of variant TxA2R from the D304N allele is sufficient to reduce platelet hemostatic function even though normal TxA2R is expressed from the remaining wild-type allele. However, this conclusion is not supported by our observation that subject P2 had no abnormal bleeding symptoms despite also being heterozygous for the D304N TxA2R substitution.
In the previously reported naturally occurring variant R60L TxA2R, bleeding symptoms were reported in subjects who were homozygous for this substitution.14,23,24 However, heterozygotes for the R60L TxA2R substitution did not show abnormal bleeding even though platelet aggregation responses to AA and U46619 were abnormal and similar to those observed in our study subjects.15 The R60L substitution prevents coupling of the TxA2R to Gq and abolishes function of the variant receptor expressed in heterologous cells.14 Therefore, heterozygotes for the variant R60L TxA2R are predicted to express both functional and nonfunctional TxA2R similar to the heterozygotes for the D304N substitution in our study. The consensus conclusion from the previous reports of the R60L variant and this report of the D304N variant is therefore that heterozygosity for loss of function mutations of TxA2R is sufficient to cause abnormal platelet functional responses ex vivo. However, heterozygosity is insufficient to cause platelet dysfunction that is clinically significant in vivo. Abnormal mucocutaneous bleeding is a common presentation that is multifactorial and frequently has no identifiable cause despite laboratory testing.21,25 We therefore speculate that the clinical bleeding phenotype displayed by subject P1 in our study represented the effect of the heterozygous D304N substitution combined with an additional, unidentified hemostatic defect.
We demonstrated that platelets from subject P1 showed approximately 50% maximum binding of the TxA2R antagonist [3H]-SQ29548 compared with control platelets but that the binding affinities were similar. These results indicate either a partial reduction in total TxA2R expression on the platelet surface or expression of a subset of TxA2R with absent [3H]-SQ29548 binding. In CHO-K1 cells, the variant D304N TxA2R expressed normally on the cell surface but failed to bind [3H]-SQ29548. The partial ligand binding defect observed in platelets is therefore consistent with expression of both wild-type TxA2R with normal [3H]-SQ29548 binding and variant D304N TxA2R with absent binding. The failure of variant D304N to bind [3H]-SQ29548, which is a highly specific TxA2R antagonist, suggests that the ligand binding site of the variant receptor is disrupted.11 This is consistent with the loss of functional responses to the TxA2R agonist U46619 and to AA, which induces synthesis of the native receptor agonist TxA2. This mechanism of loss of receptor function is therefore different to the previously reported variant R60L TxA2R in which ligand binding was normal.14
The D304N substitution observed in our study subjects lies within transmembrane domain 7 of the TxA2R that is distinct from extracellular loop 2, which has previously been identified as the TxA2R ligand binding site.10,11 However, D304 is a key polar residue that lies at position 1 of the NPXXY motif within transmembrane domain 7 of the TxA2R, which corresponds to residue 7.49, using the Ballesteros-Weinstein generic nomenclature for GPCRs.26 Recent crystal structures of other group A GPCRs have revealed a network of ordered water molecules within transmembrane domain 7 that interact with a series of residues, including 7.49 in the NPXXY motif, to form a hydrogen-bond network extending from the ligand binding crevice toward the cytoplasmic face of the receptor.26,27 This network is critical for the maintenance of interhelical associations within GPCRs in both inactive (R) and active (R*) states and is therefore expected to maintain the integrity of the ligand binding site in the R state receptor (reviewed in28 ). Residue 7.49 also interacts directly with a conserved D residue at 2.50 in transmembrane domain 2 during transition between R and R* states.26,27
The critical 7.49 residue is an Asn (N) in 75% of family A GPCRs and is an Asp (D) in 21%, including the TxA2R.29 However, the D and N residues at this position are not interchangeable within individual GPCRs, and an N substitution at 7.49 cannot support GPCR function where there is a D residue in the wild-type receptor.30 This corresponds to the D304N substitution observed in our study kindred in the TxA2R and provides a highly plausible explanation for the loss of TxA2R function. Our results indicate that at least part of this loss of function occurs because of absent ligand binding.
This report of a novel variant D304N TxA2R establishes the clinical and laboratory phenotype associated with reduced expression of functional TxA2R on platelets in vivo. Although the NPXXY motif has proven function in other group A GPCRs, we provide the first direct evidence that this motif is critical for functionality of the TxA2R.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the British Heart Foundation (BHF PG/06/038). S.J.M. holds a BHF Senior Lectureship. S.P.W. holds a BHF Chair.
Authorship
Contribution: S.P.W. coordinated the study that was designed and initiated by A.D.M., M.E.D., S.J.M., and S.P.W; M.D.W., S.J.M., and B.B.D. generated the clinical description and platelet phenotype data for the study; B.B.D., M.E.D., S.L.M., M.B.P., J.C.S., and S.J.M. designed and performed the genetic analysis of TBXA2R and the heterologous expression experiments; and A.D.M., M.W., and S.P.W. contributed to preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Andrew Mumford, Bristol Heart Institute, University of Bristol, Bristol Royal Infirmary, Bristol, BS2 8HW, United Kingdom; e-mail a.mumford@bristol.ac.uk.

![Figure 3. Expression and functional analysis of wild-type and variant D304N TxA2R expressed in CHO-K1 cells. (A) CHO-K1 cells were transfected with expression constructs containing wild-type (WT TxA2R) or variant D304N TxA2R (D304N TxA2R). Expression levels of TxA2R in CHO cell lysates were determined by ELISA using anti-FLAG M2 monoclonal antibody. Data represent means ± SEM of 3 independent experiments and are reported as arbitrary units (AU) relative to expression level of the wild-type TxA2R (designated as 1AU). (B) Transfected CHO-K1 expressing wild-type and variant D304N TxA2R were also studied by immunofluorescence microscopy using an anti-FLAG M2 monoclonal antibody. The images are representative of > 20 high power fields. (C) Changes in cytoplasmic calcium concentration [Ca2+]i were measured by preincubating washed transfected CHO-K1 cells with fura-2/AM (3μM) before addition of U46619 (0.001-10μM). Data are expressed as peak calcium response quantified as described previously20 and represent the means ± SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/2/10.1182_blood-2009-08-236976/5/m_zh89990945640003.jpeg?Expires=1769082573&Signature=2Oh8h~mYD9tWg7vyvhKtnR7CEppvr19Rg7fEZOuV~W1xSsAPV3Pj0CqzQZH1vv9F7JaLjOzRsZqrk~ZakOmDi1XOXLJyXysqK2PoT~9zejE944U-iO~fG5YFMl2KoGmupeuKWXS~SX8GBaLaPYeZW0ZdbhnSUTSGOC6SErZqkeeZH1SRr-Rtgn0UIK2GqY7t4IX-XCmrWJaxu654BV12rtE4R8JEzWa0UPOfdPorrdFLsVoM5nrpmx3SfgqZyIHA5KGksSqZVYiQr6lVTbwWGcxy4zoKmngjbatZ7zO2m9~9cGqN~DUxS6nQ9sigNp4uiyoholHh1Ia1bOs8jVNucA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Binding of [3H]-SQ29548 to platelets from P1 and a healthy control and to CHO-K1 cells expressing either wild-type or variant D304N TxA2R. (A) Washed fixed platelets from P1 from a related control (P3) who did not have the D304N TxA2R substitution and from a healthy unrelated donor were incubated for 20 minutes with [3H]-SQ29548 (0.1μM) in the presence of unlabeled ligand (10μM) to determine specific receptor-dependent binding. Binding was then determined by measuring bound labeled ligand after a 20-minute incubation. Data are expressed as disintegrations per minute per 4 × 106 platelets and represent the means ± SEM of 3 independent experiments. (B) Specific receptor-dependent [3H]-SQ29548 binding to either wild-type (WT TxA2R) or variant D304N TxA2R (D304N TxA2R) expressed in CHO-K1 cells was determined as described in “Methods.” Data are expressed as disintegrations per minute per milligram of protein and represent the means ± SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/2/10.1182_blood-2009-08-236976/5/m_zh89990945640004.jpeg?Expires=1769082573&Signature=Nc~BT2y0mW8HhVC2I1AI8UCqOWoy9YdSiIJCurtfw0TuvaFAi3pgZzuzpWD~5i4GVWg1a5ErZH67~iCDrihkrhzASjb4yXp-6-j64bgdNvIR0TEwj~SZ6d3iOxH2sZxqMWHo~OrG47pf6IloAWkCIW5Jk0r2Pfndwo9HpmVPqk10377iA6Fvww6QliH5j-jZd0CGaFEynRBMh0Zr8vYVzb9kt80OjdEqMvriZUT391V4nYYJfT1Bf1CliVfLH0ufUcbCNLKvlMWXhK7VkS5jt5UlSdeNInDq6cxdGlfi0lHZrx2hxcsw1BQrst~R4QoJ1l0Hh8appLrUp9qRobEjNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal