Abstract
In Budd-Chiari syndrome (BCS), thrombosis develops in the hepatic veins or inferior vena cava. To study the relationship between hypofibrinolysis and BCS, we measured plasma levels of fibrinolysis proteins in 101 BCS patients and 101 healthy controls and performed a plasma-based clot lysis assay. In BCS patients, plasminogen activator inhibitor 1 (PAI-1) levels were significantly higher than in controls (median, 6.3 vs 1.4 IU/mL, P < .001). Thrombin-activatable fibrinolysis inhibitor and plasmin inhibitor levels were lower than in controls (13.8 vs 16.9 μg/mL and 0.91 vs 1.02 U/L, both P < .001). Median plasma clot lysis time (CLT) was 73.9 minutes in cases and 73.0 minutes in controls (P = .329). A subgroup of cases displayed clearly elevated CLTs. A CLT above the 90th or 95th percentile of controls was associated with an increased risk of BCS, with odds ratios of 2.4 (95% confidence interval, 1.1-5.5) and 3.4 (95% confidence interval, 1.2-9.7), respectively. In controls, only PAI-1 activity was significantly associated with CLT. Analysis of single nucleotide polymorphisms of fibrinolysis proteins revealed no significant differences between cases and controls. This case-control study provides the first evidence that an impaired fibrinolytic potential, at least partially caused by elevated PAI-1 levels, is related to the presence of BCS.
Introduction
Budd-Chiari syndrome (BCS) is a rare but life-threatening liver disease that is characterized by an obstruction of venous blood flow from the liver, irrespective of the underlying cause.1 In the Western world, most cases of BCS are caused by thrombosis of one or more hepatic veins, with or without concomitant thrombosis of the inferior vena cava. The etiology of thrombosis in these specific veins is complex, but in the past decades it has become clear that both inherited and acquired factors leading to an increased thrombotic tendency are involved.2-4 To date, an underlying cause can be identified in the majority of patients with BCS, and in a significant number of cases a combination of risk factors is present.5,6
Myeloproliferative disorders are the most common cause of thrombosis in BCS, and either overt or latent forms are found in approximately 50% of all patients.7,8 Another focus of extensive etiologic study has been on disorders of coagulation. The mechanism of clot formation is dependent on the interaction of a large number of procoagulant and anticoagulant factors. The balance between these factors is crucial for normal hemostasis, and disturbances of this balance may lead to a thrombotic tendency. Several disorders of the clotting system that lead to a hypercoagulant state and that are known to be involved in venous thromboembolism have also been associated with BCS, such as factor V Leiden mutation, prothrombin gene mutation, and protein C deficiency.2,3 So far, little attention has been addressed to disorders of the fibrinolytic system as a risk factor for BCS.
Fibrinolysis is the process through which fibrin clots are dissolved by the action of plasmin. Plasmin, an active enzyme that is formed from its inactive precursor plasminogen, degrades fibrin into fibrin degradation products. Two main proteins that are responsible for the activation of plasminogen are tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA).9 The profibrinolytic actions of plasmin, t-PA, and u-PA are all regulated by several specific inhibitors. Plasminogen activator inhibitor-1 (PAI-1) is the main inhibitor of t-PA and u-PA, whereas both plasmin inhibitor (also known as α2-antiplasmin) and thrombin-activatable fibrinolysis inhibitor (TAFI) are important in the inhibition and down-regulation of plasmin.10 The overall fibrinolytic activity is based on the balance between these profibrinolytic enzymes and their inhibitors, together with their affinity for fibrin and the regulatory effects of fibrin itself.
Disturbance of the fibrinolytic balance causing hypofibrinolysis can lead to decreased clot degradation and is a potential risk factor for venous thrombosis. Despite some conflicting results in previous studies,11-15 recent evidence suggests that there is a relationship between reduced fibrinolytic activity and the risk of venous thrombosis.16,17 In the current study, we have investigated the role of hypofibrinolysis as a risk factor for BCS.
Methods
Study population
Patients with BCS were recruited from the European Network for Vascular Disorders of the Liver (EN-Vie) study cohort. The EN-Vie Study, a prospective, multicenter cohort study of patients with BCS from 9 European countries, has been described previously.6 In short, consecutive patients newly diagnosed with BCS were enrolled in different countries. At the time of diagnosis and during follow-up, data concerning clinical condition and etiology and results of radiology, pathology, and laboratory assessments were collected. BCS was defined as a hepatic venous outflow obstruction and its manifestations, regardless of the cause and regardless of the level of obstruction, ranging from the small hepatic veins to the entrance of the inferior vena cava into the right atrium. This definition did not include hepatic outflow obstruction caused by congestive heart failure or sinusoidal obstruction syndrome (previously known as veno-occlusive disease). From October 2003 until October 2005, a total of 163 patients were included in the study.
After patients were enrolled in the study, they were asked to provide their own healthy control. Controls were healthy nonrelatives and had to be of the same gender, ethnic background, and age (within a range of 5 years) as the patient. Furthermore, controls were included only if they did not have a history of previous thrombosis. When patients were unable to provide controls themselves, the study coordinating centers attempted to find equally matched controls from their own resources.
Blood samples were collected from both patients (at time of diagnosis) and controls by venapuncture in tubes containing 0.11M trisodium citrate. Plasma was prepared by centrifugation at 2000g for 10 minutes, and DNA was extracted from whole blood according to local standard methods. Both plasma and DNA samples were transported to the Erasmus University Medical Center in Rotterdam and stored at −70°C until analysis.
The EN-Vie Study was conducted with approval from all national and, if necessary, local ethical committees from all participating centers, in accordance with the nation-specific rules. All patients and controls agreed to participate in the study by a written informed consent, in accordance with the Declaration of Helsinki.
Only patients for whom stored plasma samples and a matched control person were available were considered eligible for the current study. For each patient, Rotterdam prognostic scores were calculated as defined previously.18 All laboratory assessments were performed at the Laboratory of Hematology of the Erasmus University Medical Center Rotterdam.
Measurement of fibrinolytic markers in plasma
In plasma samples of both patients and controls, we determined levels of 4 different fibrinolysis proteins, fibrinogen, and factor XIII (FXIII). Plasma levels of fibrinogen were measured as a function of thrombin clotting time using the Clauss method.19 Levels of t-PA antigen in plasma were assayed by a slightly modified commercially available enzyme-linked immunosorbent assay (t-PA Antigen Elisa Reagent Kit, Technoclone). The activity levels of PAI-1 and TAFI were measured using a chromogenic assay (Chromolize PAI-1, Trinity Biotech; and Actichrome TAFI activity kit, American Diagnostica, respectively). Plasmin inhibitor activity levels were determined by a chromogenic assay using the Coamatic Plasmin Inhibitor kit of Chromogenix (Instrumentation Laboratory). FXIII levels in plasma were also measured, using a spectrometric assay (Berichrom FXIII kit, Dade Behring) adapted to a microtiter plate format, with pooled control plasma (Factor Assay ConTrol plasma, lot 222e1, George King Bio-Medical Inc) as a calibrator.
Plasma fibrinolytic potential
Lysis of a tissue factor–induced plasma clot by exogenous t-PA was studied essentially as described previously with some small modifications.16 Plasma (60 μL) was diluted 1.7-fold in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 137mM NaCl, 3.5mM KCl, 3mM CaCl2, 0.1% bovine serum albumin, pH 7.4). The diluted plasma samples (85 μL) were added to the wells of a microtiter plate containing 15 μL of a reaction mixture. The reaction mixture was composed of tissue factor (Innovin, Dade Behring), CaCl2, t-PA (Actilyse, Boehringer Ingelheim), and phospholipid vesicles (consisting of 40% L-α-dioleoylphosphatidylcholine (PC), 20% L-α-dioleoylphosphatidylserine [PS], and 40% L-α-dioleoylphosphatidylethanolamine [PE], all from Sigma-Aldrich).20,21 Innovin was prepared according to the manufacturer's instructions by reconstituting the supplied lyophilized powder with 10 mL of deionized water, and it went through a freeze/thaw cycle before it was used in the assay.
The final concentrations in the clotted plasma were as follows: tissue factor (1000× diluted), 17mM CaCl2, 25 ng/mL t-PA, and 10μM phospholipid vesicles. After mixing the diluted plasma and the reaction mixture, each clot was covered with paraffin oil (50 μL, Merck no. 107162), and the plate was placed immediately in a prewarmed (37°C) incubation chamber of a microplate reader (PerkinElmer). The optical density at 405 nm was monitored every minute for 400 minutes. The clot lysis time (CLT) was defined as the time from the midpoint of clear to maximum turbid transition, which characterizes clot formation, to the midpoint of the maximum turbid to clear transition, which represents clot lysis. A prolonged CLT compared with the control group CLT is indicative of hypofibrinolysis, whereas a short CLT can indicate hyperfibrinolysis.
Analysis of common polymorphisms
All persons were genotyped for several previously identified single nucleotide polymorphisms of FXIII [100G/T (Val34Leu)], PAI-1 [-675 4G/5G], TAFI [1040C/T (Thr325Ile) and 1538A/T], t-PA [−7351C/T and 27739G/A], and fibrinogen [−148C/T].22-27 The genotype for these 7 single nucleotide polymorphisms was determined by a duplex polymerase chain reaction (PCR) followed by a restriction fragment length analysis. Different PCR mixtures and PCR conditions were used for all polymorphisms. Detailed information regarding the primers and PCR conditions is displayed in Appendix 2 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Statistical analysis
Plasma levels of TAFI, plasmin inhibitor, fibrinogen, and FXIII as well as the CLTs displayed a normal distribution. Because t-PA antigen and PAI-1 activity levels had a skewed distribution, a log-transformation was performed before these variables were analyzed. The results of all parameters were compared between the cases and controls using the Student t test. The association between fibrinolytic parameters in cases and different measures of liver function was assessed using the Spearman rank correlation test. The relative risk of BCS associated with an elevated CLT was estimated as an odds ratio (OR) and corresponding 95% confidence interval (CI) using logistic regression, adjusted for sex and age. Percentiles (70th, 80th, 90th, and 95th) of the CLT measured in the control group were used as cutoff levels. Determinants of the CLT were evaluated in controls with multiple linear regression, including the log-transformed values for PAI-1 activity and t-PA antigen. Differences between cases with and without a CLT above the 90th percentile were assessed using the Mann-Whitney U test. The relationship between the different polymorphisms and BCS was investigated with ORs (and 95% CI) calculated by logistic regression. A P value less than .05 was considered to be statistically significant. All statistical analyses were performed with the Statistical Package for Social Sciences for Windows, Version 14.0 (SPSS).
Results
From the total EN-Vie cohort of 163 patients with BCS, plasma samples for this study were available for 107 patients (66%). Six patients were subsequently excluded because a control person was missing, leaving 101 eligible sex- and age-matched case-control pairs.
Patient characteristics are shown in Table 1. The patient and control groups were comparable with respect to age, sex, and race. Baseline characteristics of the 101 patients in the study group were similar to those of the total EN-Vie cohort (n = 163), indicating that the current study population was a representative patient sample. Median age at diagnosis was 37 years (range, 16-84 years) and 42% of the patients were males. Concurrent portal vein thrombosis was present in 13% of the patients. An underlying myeloproliferative disorder (MPD) was identified in 35 patients (35%).
Baseline characteristics of patients with BCS (n = 101)
| Patient characteristic . | Value . |
|---|---|
| Median age, y (range) | 37 (16-84) |
| Male sex | 42 |
| White race | 74 |
| Location of venous occlusion | |
| HV occlusion | 47 |
| IVC occlusion | 2 |
| Combined HV and IVC occlusion | 52 |
| Concurrent portal vein thrombosis | 13 |
| Rotterdam BCS prognostic index* | |
| Class I | 29 |
| Class II | 51 |
| Class III | 20 |
| Patient characteristic . | Value . |
|---|---|
| Median age, y (range) | 37 (16-84) |
| Male sex | 42 |
| White race | 74 |
| Location of venous occlusion | |
| HV occlusion | 47 |
| IVC occlusion | 2 |
| Combined HV and IVC occlusion | 52 |
| Concurrent portal vein thrombosis | 13 |
| Rotterdam BCS prognostic index* | |
| Class I | 29 |
| Class II | 51 |
| Class III | 20 |
HV indicates hepatic vein; and IVC, inferior vena cava.
As a result of missing values, the Rotterdam BCS prognostic index could not be calculated in one patient.
Plasma fibrinolytic parameters
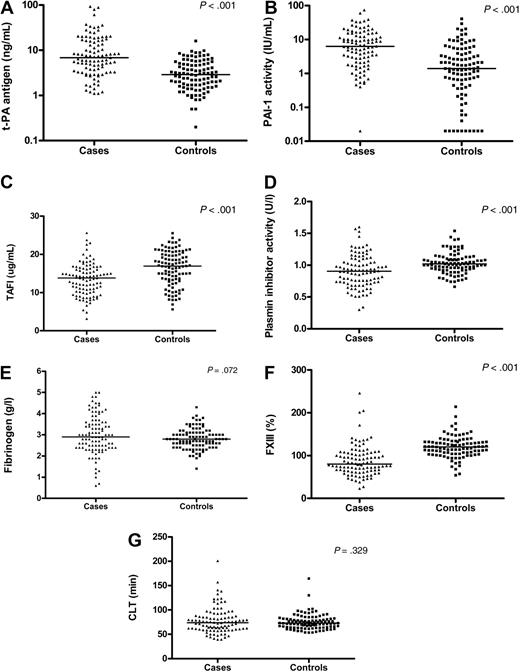
As shown in Figure 1, levels of t-PA antigen (Figure 1A, median 6.8 vs 2.9 ng/mL) and PAI-1 activity (Figure 1B, median 6.3 vs 1.4 IU/mL) were significantly increased in cases versus controls (both P < .001). In contrast, TAFI activity levels were lower in patients compared with the controls (Figure 1C, median 13.8 vs 16.9 μg/mL, P < .001). Plasmin inhibitor activity was also decreased, with a median level of 0.91 U/L in cases and 1.02 U/L in controls (Figure 1D, P < .001).
Fibrinolytic parameters in cases and controls. Comparison of plasma levels of t-PA antigen (A), PAI-1 activity (B), TAFI activity (C), plasmin inhibitor activity (D), fibrinogen (E), and FXIII (F) between patients with BCS (cases, n = 101) and healthy controls (n = 101). For t-PA antigen and PAI-1, the log-transformed values are depicted. (G) CLTs (in minutes) of patients with BCS (cases, n = 100) and healthy controls (n = 101) as measured using a plasma-based in vitro clot lysis assay. Individual data points and median values are given.
Fibrinolytic parameters in cases and controls. Comparison of plasma levels of t-PA antigen (A), PAI-1 activity (B), TAFI activity (C), plasmin inhibitor activity (D), fibrinogen (E), and FXIII (F) between patients with BCS (cases, n = 101) and healthy controls (n = 101). For t-PA antigen and PAI-1, the log-transformed values are depicted. (G) CLTs (in minutes) of patients with BCS (cases, n = 100) and healthy controls (n = 101) as measured using a plasma-based in vitro clot lysis assay. Individual data points and median values are given.
Because the liver synthesizes most coagulation factors and fibrinolytic proteins and patients with BCS may exhibit various degrees of liver dysfunction, we evaluated the association between the fibrinolytic parameters and different liver function tests. PAI-1 activity showed no significant correlation with any liver function test. For t-PA antigen there was a minor correlation with albumin levels and TAFI activity was associated with both albumin and bilirubin levels. Plasmin inhibitor activity correlated with factor V and bilirubin levels. However, all correlation coefficients were less than 0.4, indicating at most only a moderate relationship (Table 2).
Associations of fibrinolytic parameters in BCS patients with different measures of liver function
| . | Spearman ρ correlation coefficient . | |||||
|---|---|---|---|---|---|---|
| PAI-1 . | t-PA . | TAFI . | PI . | FXIII . | Fibrinogen . | |
| Albumin | −0.124 | −0.388* | −0.273* | 0.011 | 0.166 | −0.085 |
| Bilirubin | −0.044 | 0.185 | −0.377* | −0.356* | −0.069 | −0.315* |
| ALT | 0.166 | 0.085 | 0.037 | 0.146 | 0.054 | −0.076 |
| Factor V† | −0.007 | −0.125 | 0.329 | 0.384* | 0.052 | 0.244 |
| . | Spearman ρ correlation coefficient . | |||||
|---|---|---|---|---|---|---|
| PAI-1 . | t-PA . | TAFI . | PI . | FXIII . | Fibrinogen . | |
| Albumin | −0.124 | −0.388* | −0.273* | 0.011 | 0.166 | −0.085 |
| Bilirubin | −0.044 | 0.185 | −0.377* | −0.356* | −0.069 | −0.315* |
| ALT | 0.166 | 0.085 | 0.037 | 0.146 | 0.054 | −0.076 |
| Factor V† | −0.007 | −0.125 | 0.329 | 0.384* | 0.052 | 0.244 |
PI indicates plasmin inhibitor; and ALT, alanine aminotransferase.
P < .05.
Factor V levels were determined in only 29 patients.
Plasma levels of fibrinogen and FXIII were also measured, given their important role in fibrin formation and in the cross-linking of fibrin fibers, respectively. Levels of fibrinogen were higher in cases compared with controls (Figure 1E, median 2.9 vs 2.8 g/L), but this difference failed to reach statistical significance (P = .072). FXIII concentration, however, was significantly lower in the BCS patients (Figure 1F, median 80% vs 120%; P < .001). Association with liver function tests revealed only a minor inverse correlation between fibrinogen and bilirubin levels (Table 2).
To evaluate whether the presence of an underlying MPD had an effect on any of the fibrinolytic parameters, we compared the plasma levels of all fibrinolysis proteins between cases with and without evidence of an MPD. In BCS patients with an MPD (n = 35), median TAFI level was 11.9 μg/mL compared with 14.6 μg/mL in patients without MPD (n = 66, P < .001). Plasmin inhibitor levels were also lower in MPD cases than in non-MPD cases (0.79 vs 0.97 U/L, respectively, P = .009). Levels of PAI-1 activity, t-PA antigen, fibrinogen, and FXIII did not differ significantly between both patient groups.
Plasma fibrinolytic potential
As a measure of overall fibrinolytic potential, we performed an in vitro clot lysis assay. The CLT results for both cases and controls are depicted in Figure 1G. For the total study population, CLTs did not differ between the 2 groups (median 73.9 minutes in cases vs 73.0 minutes in controls, P = .329). In addition, when cases were subdivided into prognostic classes according to the Rotterdam BCS index, there were no significant differences between patient classes and when these classes were compared with their controls (data not shown). Nevertheless, we identified a subgroup of BCS patients with an increased CLT compared with the controls. To investigate the potential contribution of an increased CLT to the presence of BCS, different cutoff levels were set, according to the 70th, 80th, 90th, and 95th percentiles of CLTs in the control population. Using values below the cutoff as a reference, the relative risk of BCS for CLTs above the cutoff points augmented with increasing values of the cutoff (Table 3). With the 90th percentile as a cutoff value (CLT of 93.1 minutes), 21 cases had CLTs above this level compared with 10 controls, corresponding with a more than 2-fold increased relative risk of BCS (OR = 2.4; 95% CI, 1.1-5.5). This risk was even higher when the 95th percentile (CLT of 98.1 minutes) was used as a cutoff value (OR = 3.4; 95% CI, 1.2-9.7).
Risk of BCS according to CLT
| Cutoff percentile . | CLT, minutes . | No. of controls . | No. of cases . | OR (95% CI)* . |
|---|---|---|---|---|
| 70 | 78.5 | 30 | 41 | 1.7 (0.9-3.0) |
| 80 | 84.9 | 20 | 30 | 1.8 (0.9-3.4) |
| 90 | 93.1 | 10 | 21 | 2.4 (1.1-5.5) |
| 95 | 98.0 | 5 | 15 | 3.4 (1.2-9.7) |
| Cutoff percentile . | CLT, minutes . | No. of controls . | No. of cases . | OR (95% CI)* . |
|---|---|---|---|---|
| 70 | 78.5 | 30 | 41 | 1.7 (0.9-3.0) |
| 80 | 84.9 | 20 | 30 | 1.8 (0.9-3.4) |
| 90 | 93.1 | 10 | 21 | 2.4 (1.1-5.5) |
| 95 | 98.0 | 5 | 15 | 3.4 (1.2-9.7) |
ORs adjusted for age and sex.
To determine which factors were associated with the CLT in the control population, we performed a multiple linear regression analysis, including PAI-1, t-PA antigen, TAFI, plasmin inhibitor, fibrinogen, and FXIII. PAI-1 activity levels appeared to be the most important determinant of CLT, with a corresponding regression coefficient of 3.36 (CI, 1.46-5.26) and a P value of .001. Regression coefficients for t-PA antigen, TAFI, plasmin inhibitor, fibrinogen, and FXIII were not statistically significant. Indeed, when we compared BCS patients with CLTs above the 90th percentile to patients with CLTs below the 90th percentile, it became apparent that cases with an increased CLT had significantly higher levels of PAI-1 activity (Table 4). There was no significant difference between CLTs in patients with an underlying MPD compared with cases without MPD (P = .573).
Comparison of BCS patients with prolonged (> 90th percentile) and normal CLT
| Parameter . | Prolonged CLT . | Normal CLT . | P* . |
|---|---|---|---|
| PAI activity, IU/mL | 15.5 (6.5-24.6) | 5.1 (1.0-13.3) | < .001 |
| t-PA antigen, ng/mL | 14.9 (2.5-27.4) | 5.9 (1.1-10.8) | .003 |
| TAFI, μg/mL | 12.6 (9.4-15.8) | 14.0 (10.9-17.0) | .713 |
| Plasmin inhibitor, U/L | 0.97 (0.75-1.19) | 0.90 (0.74-1.07) | .472 |
| Fibrinogen, g/L | 3.1 (2.3-3.9) | 2.8 (2.2-3.4) | .058 |
| Factor XIII | 83 (58-109) | 79 (59-100) | .949 |
| Parameter . | Prolonged CLT . | Normal CLT . | P* . |
|---|---|---|---|
| PAI activity, IU/mL | 15.5 (6.5-24.6) | 5.1 (1.0-13.3) | < .001 |
| t-PA antigen, ng/mL | 14.9 (2.5-27.4) | 5.9 (1.1-10.8) | .003 |
| TAFI, μg/mL | 12.6 (9.4-15.8) | 14.0 (10.9-17.0) | .713 |
| Plasmin inhibitor, U/L | 0.97 (0.75-1.19) | 0.90 (0.74-1.07) | .472 |
| Fibrinogen, g/L | 3.1 (2.3-3.9) | 2.8 (2.2-3.4) | .058 |
| Factor XIII | 83 (58-109) | 79 (59-100) | .949 |
Values are stated as median (interquartile range). Prolonged CLT was defined as a CLT above the 90th percentile of the control population (93.1 minutes); patients with a CLT below the 90th percentile were defined as having a normal CLT.
Mann-Whitney U test.
As the majority of patients with BCS are given anticoagulation immediately after diagnosis, we wanted to estimate the potential effect of anticoagulant treatment on clot lysis times and the fibrinolytic parameters. Therefore, we evaluated the clotting times of control samples as measured in the clot lysis assay. Median clotting time of control samples was 5.28 minutes (interquartile range = 4.14). Considering a clotting time longer than 10 minutes as abnormal, we identified 10 patients in the study population with a clotting time above this cutoff level.
Of these patients, 6 cases also displayed a prolonged CLT (> 90th percentile). Exclusion of these patients and their matched controls from the analysis did not affect the outcome of the plasma measurements (data not shown). Furthermore, although the size of the risk was somewhat attenuated, there remained a clear trend toward an increased risk of BCS in cases with a CLT above the 90th or 95th percentile, with ORs of 1.8 (CI, 0.8-4.4) and 2.7 (CI, 0.8-9.0), respectively.
Analysis of common polymorphisms
Over the past years, different polymorphisms have been described in the genes for PAI-1, t-PA, TAFI, fibrinogen, and FXIII. To investigate the genotype distribution in patients with BCS, the genotypes for 7 common polymorphisms were analyzed and compared between cases and controls. Table 5 displays the results of the genotype analysis. There were no significant differences between cases and controls.
Distribution of single nucleotide polymorphisms between BCS patients and controls
| . | Cases . | Controls . | OR (95% CI) . |
|---|---|---|---|
| FXIII (Val34Leu) | |||
| GG, n (%) | 44 (46) | 40 (40) | 1* |
| GT, n (%) | 48 (50) | 53 (53) | 0.8 (0.5-1.5) |
| TT, n (%) | 4 (4) | 7 (7) | 0.5 (0.1-1.9) |
| T-allele frequency (%) | 29 | 34 | |
| PAI-1 (4G/5G) | |||
| 4G4G, n (%) | 19 (20) | 26 (27) | 1* |
| 4G5G, n (%) | 70 (74) | 62 (65) | 1.5 (0.8-3.1) |
| 5G5G, n (%) | 6 (6) | 7 (7) | 1.2 (0.3-4.1) |
| 5G-allele frequency (%) | 43 | 40 | |
| TAFI (1040) | |||
| CC, n (%) | 43 (44) | 46 (46) | 1* |
| CT, n (%) | 43 (44) | 41 (41) | 1.1 (0.6-2.0) |
| TT, n (%) | 11 (11) | 12 (12) | 1.0 (0.4-2.5) |
| T-allele frequency (%) | 34 | 33 | |
| TAFI (1583) | |||
| AA, n (%) | 51 (51) | 45 (45) | 1* |
| AT, n (%) | 34 (34) | 40 (40) | 0.8 (0.4-1.4) |
| TT, n (%) | 14 (14) | 16 (16) | 0.8 (0.4-1.8) |
| T-allele frequency (%) | 31 | 36 | |
| t-PA (−7351) | |||
| CC, n (%) | 48 (49) | 42 (42) | 1* |
| CT, n (%) | 41 (42) | 45 (45) | 0.8 (0.4-1.4) |
| TT, n (%) | 9 (9) | 13 (13) | 0.6 (0.2-1.6) |
| T-allele frequency (%) | 30 | 36 | |
| TPA (27739) | |||
| AA, n (%) | 77 (84) | 81 (84) | 1* |
| AG or GG, n (%) | 15 (16) | 16 (16) | 1.0 (0.5-2.1) |
| G-allele frequency (%) | 9 | 8 | |
| Fibrinogen (−148) | |||
| CC, n (%) | 49 (57) | 52 (54) | 1* |
| CT, n (%) | 29 (34) | 36 (38) | 0.9 (0.5-1.6) |
| TT, n (%) | 8 (9) | 8 (8) | 1.1 (0.4-3.0) |
| T-allele frequency (%) | 26 | 27 |
| . | Cases . | Controls . | OR (95% CI) . |
|---|---|---|---|
| FXIII (Val34Leu) | |||
| GG, n (%) | 44 (46) | 40 (40) | 1* |
| GT, n (%) | 48 (50) | 53 (53) | 0.8 (0.5-1.5) |
| TT, n (%) | 4 (4) | 7 (7) | 0.5 (0.1-1.9) |
| T-allele frequency (%) | 29 | 34 | |
| PAI-1 (4G/5G) | |||
| 4G4G, n (%) | 19 (20) | 26 (27) | 1* |
| 4G5G, n (%) | 70 (74) | 62 (65) | 1.5 (0.8-3.1) |
| 5G5G, n (%) | 6 (6) | 7 (7) | 1.2 (0.3-4.1) |
| 5G-allele frequency (%) | 43 | 40 | |
| TAFI (1040) | |||
| CC, n (%) | 43 (44) | 46 (46) | 1* |
| CT, n (%) | 43 (44) | 41 (41) | 1.1 (0.6-2.0) |
| TT, n (%) | 11 (11) | 12 (12) | 1.0 (0.4-2.5) |
| T-allele frequency (%) | 34 | 33 | |
| TAFI (1583) | |||
| AA, n (%) | 51 (51) | 45 (45) | 1* |
| AT, n (%) | 34 (34) | 40 (40) | 0.8 (0.4-1.4) |
| TT, n (%) | 14 (14) | 16 (16) | 0.8 (0.4-1.8) |
| T-allele frequency (%) | 31 | 36 | |
| t-PA (−7351) | |||
| CC, n (%) | 48 (49) | 42 (42) | 1* |
| CT, n (%) | 41 (42) | 45 (45) | 0.8 (0.4-1.4) |
| TT, n (%) | 9 (9) | 13 (13) | 0.6 (0.2-1.6) |
| T-allele frequency (%) | 30 | 36 | |
| TPA (27739) | |||
| AA, n (%) | 77 (84) | 81 (84) | 1* |
| AG or GG, n (%) | 15 (16) | 16 (16) | 1.0 (0.5-2.1) |
| G-allele frequency (%) | 9 | 8 | |
| Fibrinogen (−148) | |||
| CC, n (%) | 49 (57) | 52 (54) | 1* |
| CT, n (%) | 29 (34) | 36 (38) | 0.9 (0.5-1.6) |
| TT, n (%) | 8 (9) | 8 (8) | 1.1 (0.4-3.0) |
| T-allele frequency (%) | 26 | 27 |
Reference category.
With respect to the FXIII Val34Leu gene variant (a valine-to-leucine substitution at codon 34), there is evidence suggesting an interaction with plasma fibrinogen levels. Therefore, we determined the genotype distribution of this polymorphism in cases and controls depending on the plasma levels of fibrinogen. High fibrinogen concentration was defined as plasma levels above the 75th percentile in controls (3.1 g/L). Using this definition, 43 cases and 27 controls had high fibrinogen levels, compared with 58 cases and 74 controls with normal fibrinogen levels (levels < 75th percentile). With the FXIII Val/Val genotype as a reference, we calculated ORs for BCS associated with carriership of the Leu allele. In the group with high fibrinogen levels, heterozygous and homozygous carriership of the Leu allele was associated with an OR of 0.5 (CI, 0.2-1.5) and 0.1 (CI, 0.0-1.6), respectively. The presence of either the Val/Leu or the Leu/Leu genotype corresponded with an OR of 0.5 (CI, 0.2-1.2). For the group with normal fibrinogen levels, the ORs for Leu allele heterozygotes, homozygotes, or both heterozygotes and homozygotes were 1.1 (CI, 0.5-2.2), 1.0 (CI, 0.2-5.0), and 1.1 (CI, 0.5-2.2), respectively. Similar results were obtained when we used the 90th percentile of fibrinogen levels in controls (3.5 g/L) as a cutoff level (data not shown). These results suggest that, at high fibrinogen levels, the relative risk of BCS is lower in carriers of the FXIII Leu allele compared with persons with the Val/Val genotype.
Discussion
This case-control study is the first study to show that impaired fibrinolysis is a potential risk factor for BCS. In patients with deep venous thrombosis, it has recently been shown that an impaired plasma fibrinolytic potential is a risk factor for thrombosis.16,17 Whether alterations in the fibrinolytic system can also predispose to thrombosis in patients with BCS has not yet been investigated. In the current study group of BCS patients and healthy controls, we measured the plasma concentration of several factors involved in fibrinolysis (t-PA, PAI-1, TAFI, and plasmin inhibitor) or the final step of fibrin clot formation (fibrinogen and FXIII). As an overall measure of plasma fibrinolytic potential, we performed a plasma-based in vitro clot lysis assay.
Levels of individual components of the fibrinolytic pathway were clearly altered in patients with BCS compared with the group of healthy controls. In patients, plasma concentrations of t-PA antigen and PAI-1 activity were significantly higher. PAI-1 is an important inhibitor of fibrinolysis, and it has been hypothesized that elevated plasma levels are a risk factor for venous thrombosis. Several studies have found a relationship between elevated PAI-1 levels and venous thrombosis.14,28-30 In PAI-1 transgenic mice, it was shown that PAI-1 expression is related to the development of venous thrombosis.12 Still, a few prospective studies have failed to show a relationship between PAI-1 levels and venous thrombosis, suggesting that the predictive power of elevated PAI-1 may be low.13,31,32 In contrast to PAI-1, t-PA promotes fibrin degradation, and the increased concentration of t-PA antigen present in patients with BCS may thus seem to counteract the inhibitory effects of PAI-1 on fibrinolysis. However, t-PA measured in plasma is largely inactive because it rapidly forms a complex with PAI-1.33 The high levels of t-PA antigen we found in this study most likely reflect the presence of these t-PA/PAI-1 complexes and are probably the result of an elevated PAI-1 concentration.34 An alternative explanation for increased circulating t-PA/PAI-1 complexes could be that there is a decreased hepatic clearance of these complexes. In addition to its synthetic function, the liver is also involved in the clearance of many fibrinolytic proteins. Indeed, there was a relationship between t-PA levels and the severity of liver disease, as expressed by the Child-Pugh classification (data not shown). Although this classification is developed for patients with liver cirrhosis, it could indicate that the levels of t-PA antigen were affected by an impaired clearance of complexed t-PA. In contrast, we did not find a correlation between Child-Pugh class and PAI-1 activity, suggesting that an impaired hepatic clearance was not a major causative factor responsible for the increased PAI-1 levels (data not shown). Moreover, the levels of PAI-1 activity we measured do not include the complexed form of PAI-1 and should therefore be interpreted differently. Our findings are in line with data from a study of fibrinolytic parameters in patients with liver cirrhosis, showing increased levels of t-PA antigen with increasing severity of liver disease but no significant change of PAI-1 activity levels compared with healthy controls.35
Only one relatively small study has investigated the status of components of the fibrinolytic system in patients with BCS. Dayal et al36 studied t-PA and PAI-1 levels in 27 BCS patients and compared them with subsets of patients with other liver diseases and with a group of 20 healthy controls. In contrast to our findings, they found no significant differences between the groups. Only 3 of their patients with BCS displayed elevated levels of t-PA and PAI-1. Our study consisted of a considerably larger cohort of patients. Furthermore, cases were included in a prospective manner, which may partially account for these differences in results. The substantially increased PAI-1 activity we found in patients with BCS provides the first evidence that fibrinolysis may be impaired in these patients. As yet, it is unclear what factors could account for these elevated PAI-1 levels. There is evidence that fibrinolysis may be impaired in patients with an underlying MPD, such as polycythemia vera and essential thrombocytosis.37,38 In this study, CLTs and PAI-1 activity levels did not differ significantly between cases with and without an underlying MPD. However, this does not exclude an effect of an MPD on fibrinolysis as there may be an interplay of different mechanisms leading to elevated PAI-1 levels. Further studies will have to be performed to establish the causal role of PAI-1 levels in the pathogenesis of thrombosis in BCS patients.
To further characterize the status of the fibrinolytic system in BCS, the plasma fibrinolytic potential was determined with an in vitro plasma clot lysis assay. We found that median CLTs were not significantly different between BCS patients and controls. However, a subset of patients had a clearly prolonged CLT. Calculation of cutoff levels in the control group revealed that a CLT above the 90th or 95th percentile was associated with an increased risk of BCS (OR of 2.4 and 3.4, respectively). Further characterization of patients who had signs of hypofibrinolysis showed that they had significantly higher plasma levels of PAI-1. The concentration of TAFI, fibrinogen, and FXIII did not differ between patients with and without hypofibrinolysis, suggesting that PAI-1 might be the most important determinant of hypofibrinolysis in these patients. This was also supported by the finding that PAI-1 was significantly associated with CLTs in the control population.
TAFI did not seem to contribute to the observed hypofibrinolysis as TAFI levels in plasma of patients with BCS were actually lower than in controls. Activity levels of plasmin inhibitor were also decreased in the cases with BCS. Interestingly, we found that both TAFI and plasmin inhibitor levels were significantly lower in BCS patients with an underlying MPD, compared with cases without signs of an MPD. It is well known that, in MPD patients, both hemorrhagic and thrombotic complications can occur.39 Whether changes in the fibrinolytic system contribute to bleeding or thrombosis in these patients has not yet been elucidated. Although evidence is scarce, one other study has also shown that levels of plasmin inhibitor were significantly decreased in patients with chronic MPD.40 Our findings suggest that the levels of TAFI and plasmin inhibitor were, in part, influenced by the presence of an underlying MPD.
FXIII levels were significantly lower in cases compared with controls. FXIII is responsible for the cross-linking of fibrin fibers during clot formation. A severe deficiency of FXIII is associated with a bleeding tendency, but slightly decreased levels of FXIII do not seem to impair its function significantly.41 Moreover, it has been suggested that increased levels of FXIII have a protective effect on the development of venous thrombosis.42 Our findings are in line with that report and may even indicate a pathogenetic role of decreased FXIII levels in BCS. However, this needs to be further investigated.
An important confounder when studying the hemostatic system in patients with liver disease is the potential effect of impaired liver function itself. Most clotting factors are exclusively synthesized by the liver, and it is well known that in patients with acute or chronic liver failure major changes in both procoagulant and anticoagulant pathways can occur.43 Nevertheless, deterioration of synthetic function predominantly occurs along the course of parenchymal liver diseases, whereas in vascular liver disorders protein synthesis is mostly well preserved. In the patient group we studied, we found no major correlations between several measures of liver function and the levels of PAI-1, t-PA antigen, TAFI, plasmin inhibitor, fibrinogen, and FXIII. Therefore, we think that the observed differences in plasma fibrinolytic parameters between BCS patients and controls cannot be explained by disturbances in liver synthetic function alone. Moreover, PAI-1 is not only secreted by hepatocytes but can also be synthesized by endothelial cells, smooth muscle cells, and adipocytes.44 These alternative sites of PAI-1 synthesis may partly compensate for a potential decrease of PAI-1 synthesis resulting from liver dysfunction.
Increased levels of PAI-1 activity can be difficult to interpret, considering that PAI-1 is found to be an acute-phase reactant.45 To evaluate the possible influence of an acute-phase response on PAI-1 activity in BCS patients, we studied the association between PAI-1 levels and the plasma concentration of fibrinogen, which is also considered an acute-phase protein. There was no correlation between PAI-1 activity and fibrinogen levels in the cases (Spearman ρ correlation coefficient = −0.037, P = .713). These findings illustrate that PAI-1 activity levels were probably not elevated as a result of an acute-phase response.
Another factor that could have influenced our findings is the fact that many patients were already treated with anticoagulants when plasma samples were taken. Anticoagulation affects not only the clotting cascade but may also alter the fibrinolytic system. In our study population, we identified 10 patients with a clearly prolonged clotting time, for whom a significant effect of anticoagulant treatment on the CLTs could not be excluded. However, subsequent exclusion of these patients did not substantially change the results of our analyses, suggesting that the effect of anticoagulation on fibrinolytic parameters in our study was probably negligible.
Despite previous studies showing that certain single nucleotide polymorphisms of PAI-1, t-PA, FXIII, and TAFI influence the risk of venous thrombosis,42,46-50 our results did not show an association between any of the studied polymorphisms and thrombosis in BCS patients. With respect to the 2 TAFI-gene polymorphisms, this is in accordance with a recent study from our group.49 In patients with splanchnic vein thrombosis (ie, BCS or portal vein thrombosis), there was no association between TAFI C1040T and TAFI T1583A polymorphisms and the risk of splanchnic vein thrombosis. For the FXIII Val34Leu polymorphism, it has been suggested that there is an interaction with fibrinogen levels.51 At high fibrinogen concentrations, the 34Leu variant has been associated with a protective effect against venous thrombosis and coronary artery disease.52,53 In line with these findings, we also observed a trend toward a decreased risk of hepatic vein thrombosis in FXIII Leu allele carriers with high fibrinogen levels. Carriership of the Leu allele was associated with an OR for BCS of 0.5 (CI, 0.2-1.2) in persons with high fibrinogen compared with an OR of 1.1 (0.5-2.2) in persons with normal fibrinogen. However, our results failed to reach statistical significance, which may be the result of the relatively small number of patients and controls.
In conclusion, this study provides the first evidence that an impaired fibrinolysis may play a role in the pathogenesis of BCS. Although there was no significant overall difference in plasma fibrinolytic potential between patients and controls, a subgroup of patients with a prolonged CLT could be identified in whom the risk of BCS was clearly increased. These patients were characterized by increased plasma levels of PAI-1 but not by high TAFI-levels. Hypofibrinolysis may thus prove to be a previously unknown risk factor for the development of BCS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participating physicians for the recruitment of patients and their cooperation with this study.
The EN-Vie project was supported by the Fifth Framework Program of the European Commission (contract number QLG1-CT-2002-01 686). S.D.M. is a member of the Mosaic Program of the Netherlands Organisation for Scientific Research. Ciberehd is supported by Instituto de Salud Carlos III.
Authorship
Contribution: J.H. analyzed the data and wrote the paper; A.H.C.G. performed research and analyzed data; F.W.G.L. assisted with analysis of results and writing of the paper; J.J.M.C.M. performed the plasma measurements and the analysis of common polymorphisms; S.D.M., A.P., and M.H.-G. collected patient data and samples from patients and controls and coordinated the data organization; P.L., E.E., J.T., and M.P. collected patient data and samples from patients and controls; J.-C.G.-P. and D.C.V. designed the study and coordinated the multicenter collaboration; D.C.R. designed the study and assisted with the analysis of results and writing of the paper; and H.L.A.J. designed the study, coordinated the multicenter collaboration, and assisted with writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of EN-Vie appears in the online supplemental Appendix.
Correspondence: Harry L. A. Janssen, Erasmus University Medical Center Rotterdam, Department of Gastroenterology and Hepatology, Gravendijkwal 230, Room Ha 206, 3015 CE Rotterdam, The Netherlands; e-mail: h.janssen@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal