Abstract
The main interface of the 2 subunits of platelet integrin αIIbβ3 comprises the β-propeller domain of αIIb and the βA domain of β3. In the center of the β-propeller, several aromatic residues interact by cation-π and hydrophobic bonds with Arg261 of βA. In this study, we substituted αIIb-Trp110 or β3-Arg261 by residues abundant in other α or β subunits at corresponding locations and expressed them in baby hamster kidney cells along with normal β3 or αIIb, respectively. These mutant cells displayed normal surface expression and fibrinogen binding but grossly impaired outside-in signaling–related functions: adhesion to immobilized fibrinogen, cell spreading, focal adhesion kinase phosphorylation, clot retraction, and reduced αIIbβ3 stability in EDTA (ethylenediaminetetraacetic acid). Expression of mutants with substitutions of Arg261 in β3 by alanine or lysine with normal αv yielded normal surface expression of αvβ3 and soluble fibrinogen binding as well as normal outside-in signaling–related functions, contrasting findings for αIIbβ3. Structural analysis of αIIbβ3 and αvβ3 revealed that αvβ3 has several strong interactions between αv and β3 subunits that are missing in αIIbβ3. Together, these findings indicate that the interaction between Trp110 of αIIb and Arg261 of β3 is critical for αIIbβ3 integrity and outside-in signaling–related functions.
Introduction
Integrins are a family of cell surface receptors that mediate cell-cell and cell-matrix interactions.1 All integrins consist of α and β subunits that are noncovalently associated.2 αIIbβ3 is the most abundant integrin of platelets whose main ligand is fibrinogen. Other ligands include von Willebrand factor (VWF), fibronectin, and vitronectin. Deficiency or dysfunction of αIIbβ3 causes a rare disorder, Glanzmann thrombasthenia (GT), which is characterized by a severe bleeding tendency and by a lack of platelet aggregation in response to all physiologic agonists.3 αIIbβ3 is a calcium-dependent heterodimer that is expressed in megakaryocytes, platelets,4 and probably mast cells.5 On the surface of resting platelets, αIIbβ3 is exhibited in a low-affinity conformation in which the ligand binding site is not exposed. After activation of platelets by agonists such as adenosine diphosphate, thrombin, or collagen, inside-out signaling occurs, giving rise to exposure of the ligand binding site of αIIbβ3. The ligand binding leads to outside-in signaling, which results in cytoskeletal changes, shape change, and adherence to extracellular matrix.6 After platelet spreading and clot formation, tyrosine dephosphorylation at the β3 cytoplasmic tail causes cleavage of its C-terminus by calpain leading to clot retraction.7
During their synthesis, αIIb and β3 subunits are introduced into the endoplasmic reticulum where they form a complex and undergo N-linked glycosylation and intrasubunit disulfide bonding. The αIIbβ3 complex is then transported to the Golgi apparatus, where additional oligosaccharide processing takes place and αIIb is cleaved into a heavy and a light chain, after which the αIIbβ3 complex is transported to the plasma membrane and α granules.8 Uncomplexed αIIb degrades,9 whereas uncomplexed β3 can form a complex with αv subunit forming the vitronectin receptor, αvβ3, that is abundant in many tissues. In contrast to αIIb that binds exclusively to β3, αv can form a complex with 5 different β subunits: β1, β3, β5, β6, and β8.
Each of the integrin subunits, αIIb, αv, and β3, consists of a large extracellular region, a transmembrane domain, and a short cytoplasmic tail. The extracellular region comprises an ovoid “head” from which 2 nearly parallel “legs” emerge in the direction of the cell membrane.2 The head region of αIIbβ3 and αvβ3 includes the main interface between the α and β subunits and the ligand binding site. It consists of a 7-blade β-propeller domain of the α subunit and a βA domain of the β3 subunit. The crystal structures of αvβ3 and αIIbβ3 exhibit a unique interaction in the center of the propeller.10,11 A fingerlike loop of β3 consisting of a positively charged amino acid at its end, Arg261, protrudes into the central cavity of the β-propeller of αIIb or αv, where it is caged by cation-π and hydrophobic interactions with predominantly aromatic residues. The importance of the positively charged residue at this position of the β subunit was demonstrated in αvβ3 and αMβ2 integrins.12 Replacing Arg261 in β3 or Lys252, the corresponding residue in β2, by a negatively charged residue significantly reduced surface expression of αvβ3 or αMβ2, respectively. The importance of aromatic residues in the center of the αIIb propeller was previously demonstrated by us in patients with GT who harbored a mutation that disrupted Phe171 and in patients in whom Trp110 along with other residues were deleted.13-15
Characterization of the defect in GT is usually defined by expressing the identified alteration in αIIbβ3 proteins in cultured cells such as baby hamster kidney (BHK) cells or Chinese hamster ovary cells. Generally, such experiments have been used to discern the effect of mutations on protein maturation and function. In most instances, there has been an excellent correlation between the phenotypic expression in platelets from patients with GT and transfected cells.16-18
The aim of this study was to analyze the interaction between Phe171 or Trp110 residues of the αIIb β-propeller and Arg261 of β3. It will be shown that disruption of the interaction of Trp110 and Arg261 in transfected BHK cells is associated with impaired functions related to outside-in signaling albeit normal ligand binding.
Methods
Plasmids and monoclonal antibodies
cDNAs of αIIb and β3 in pcDNA3 vectors were generously provided by Dr Peter Newman (The Blood Center of Southeastern Wisconsin) and subcloned to pCEP4 (Invitrogen) as previously described.15 cDNA of αv in pcDNAINEO vector was generously provided by Dr David Cheresh (University of California, San Diego) and subcloned to pcDNA3. The following antibodies were used: P2 fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody to the αIIbβ3 complex (Immunotech); CA3 monoclonal antibody to αIIb (Chemicon) used with FITC-conjugated anti–mouse immunoglobulin G (IgG; Jackson ImmunoResearch); SZ22 monoclonal antibody to αIIb (Immunotech); anti–mouse IgG horseradish peroxidase (Jackson ImmunoResearch); FITC-conjugated anti–human fibrinogen antibody (Dako); αIIbβ3 activating antibody PT25-2 (Takara); αvβ3 and αIIbβ3 activating antibody and anti–ligand-induced binding site 6 (anti-LIBS6) antibody, kindly provided by Dr Mark Ginsberg (University of California, San Diego); FITC-conjugated anti–mouse actin AC-40 (Abcam); LM609 and 23C6 FITC-conjugated monoclonal antibodies to αvβ3 (Chemicon); focal adhesion kinase (FAK) polyclonal antibody to focal adhesion kinase (Santa Cruz Biotechnology); FAK-PY polyclonal antibody to phosphorylated FAK at Tyr397 (Biosource); and rabbit polyclonal anti-calnexin antibody (StressGen Biotechnologies).
Mutagenesis of expression vectors
All substitutions were made using the QuikChange site-directed mutagenesis kit (Stratagene). The following substitutions were carried out on pcDNA3/αIIb template: Trp110Ala, Trp110Arg, Trp110Leu or Phe171Ala, Phe171Leu, and Phe171Ile. Arg261Ala or Arg261Lys substitutions were performed in pcDNA3/β3 or pCEP/β3. The list of primers that were used to generate these substitutions is available in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cotransfection of αIIb, αv, and β3 cDNAs
Baby hamster kidney (BHK) cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 2 mg/mL l-glutamine and 5% fetal calf serum (Biological Industries). For αIIbβ3 expression, cells were cotransfected with 1 μg of wild-type (WT) or the mutated form of pcDNA3/αIIb and 1 μg of WT pCEP4/β3, or 1 μg of WT or pCEP43/αIIb and 1 μg of WT or the mutated form pcDNA3/β3 using lipofectamine reagent (Gibco BRL). For αvβ3 expression, 1 μg of WT pcDNA3/αv and 1 μg of WT or the mutated pCEP4/β3 were also cotransfected by lipofectamine reagent (Gibco BRL). Mock cells were produced by transfecting BHK cells with 1 μg of pCEP4 and 1 μg of pcDNA3. All cell lines were subjected to selection in medium containing 0.7 mg/mL G418 (Gibco BRL) and 0.5 mg/mL hygromycin (Boehringer Gmbh) for at least 3 weeks before use.
Analysis of surface expression of αIIbβ3 and fibrinogen binding by flow cytometry
Transfected BHK cells were harvested with phosphate-buffered saline (PBS)/1mM EDTA (ethylenediaminetetraacetic acid), pelleted, and incubated in DMEM for 30 minutes at room temperature. Cells were then pelleted again, resuspended in PBS, 1mM CaCl2, and 1mM MgCl2 (106 cells/100μL), and incubated for 30 minutes at room temperature with an FITC-conjugated monoclonal antibody to the αIIbβ3 complex (P2). Subsequently, cells were diluted to 106 cells/mL and analyzed for surface fluorescence by flow cytometry (EPICS; Coulter).
For fibrinogen binding, 106 cells were resuspended in 100 μL of Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS), 0.1% bovine serum albumin (Sigma-Aldrich), and 0.25mM MnCl2 (a known activator of αIIbβ3) and incubated for 1 hour at room temperature with 10 μg/100 μL human fibrinogen (Sigma-Aldrich) and 1 μL of anti-LIBS6 or 0.5 μL of PT25-2. Subsequently, cells were pelleted and resuspended in the same buffer and incubated for 20 minutes at room temperature with 10 μL of FITC-conjugated rabbit anti–human fibrinogen, diluted to 106 cells/mL and analyzed by flow cytometry. Background fluorescence was measured using the same antibodies in mock BHK cells.
The flow cytometric results are shown as mean of 3 different transfections and calculated as percentage of WT mean fluorescent intensity (MFI).
Analysis of integrin stability in the presence of EDTA
Transfected BHK cells were harvested with PBS/1mM EDTA, pelleted, and incubated in DMEM or PBS/5mM EDTA for 30 minutes at room temperature. Cells were pelleted again, resuspended in PBS/1mM CaCl2/1mM MgCl2 or only PBS, respectively, at a concentration of 5 × 105 cells/100 μL, and incubated for 30 minutes at room temperature with an FITC-conjugated monoclonal antibody to αIIbβ3 complex (P2) or αvβ3 complex (LM609). Subsequently, cells were diluted to 5 × 105cells/mL and analyzed for surface fluorescence by flow cytometry (Coulter).
Immunoblot analysis of BHK cells
Transfected BHK cells were lysed with lysis buffer and the lysates were electrophoresed on NuPAGE Novex 3% to 8% Tris-acetate gels (Invitrogen) with 1mM dithiothreitol. Separated proteins were then transferred to a polyvinyldifluoride membrane (Millipore). The membrane was immersed in Tris-buffered saline/0.05% Tween (TBS-T) containing a 1:1000 dilution of a monoclonal antibody to αIIb (SZ22) or antibody to calnexin (as a marker for loading), washed with TBS-T, and then immersed in TBS-T containing 1:2000 horseradish peroxidase–conjugated anti–mouse IgG. Immunoreactive bands in the membrane were detected using SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology) and x-ray film exposure. Band densitometry was carried out using EZQuant densitometry program (EZQuant Version 2.1).
Immunoblot analysis of focal adhesion kinase (FAK) phosphorylation was preformed on lysates of cells that were allowed to adhere to fibrinogen-coated wells for 30 minutes. The blot was first stained with anti–FAK-PY, and after stripping it was stained with anti-FAK. An index of phosphorylation was calculated as the ratio between the band intensities of anti–FAK-PY and anti-FAK.
Cell adhesion and spreading assay
Impact R wells (DiaMed) were coated with fibrinogen (0.25 mg/mL) or VWF (0.25 units/mL) for 2 hours at room temperature. After washing with PBS, the wells were blocked with 1% bovine serum albumin in PBS for 0.5 hour. Cells expressing αIIbβ3 were harvested with PBS/1mM EDTA, pelleted, and incubated in DMEM for 30 minutes at room temperature. Cells (5 × 105) in DMEM were allowed to adhere to the surface of fibrinogen- or VWF-coated wells for 30 minutes or 45 minutes, respectively. To estimate the amount of cells that adhered to the wells, the wells were washed twice with PBS and cells were fixed and stained with May-Grünwald eosin–methylene blue solution (Merck). Surface coverage was measured using Impact R analyzer (DiaMed). Spreading was examined by staining the cells after PBS washing and fixation with acetone-methanol (1:1) solution, with FITC-conjugated anti-actin antibody (0.04 mg/mL). The actin-stained cells in 10 fields were counted, and the percentage of fully spread cells of total adhered cells was calculated.
Clot retraction
Transfected BHK cells were harvested with PBS/1mM EDTA, pelleted, and incubated in DMEM for 30 minutes at room temperature. Cells (250 μL; 3 × 106) were placed in glass aggregometry cuvettes and mixed with 25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM capronic acid (Sigma-Aldrich), 15mM CaCl2, 0.5 units of bovine thrombin (Dade Behring), and 100 μg of fibrinogen (Sigma-Aldrich). The clots were allowed to retract at 37°C for 16 hours and then photographed.
Structural analysis
Residues located in the interface between αIIb (PDB code 1TXV)10,19 and αv (PDB code 1JV2)11,19 with β3 were identified using the SURFV program (developed by Honig's group, Columbia University and the Howard Hughes Medical Institute) with a probe sphere of radius 1.4 Å and default parameters. The structures of the αIIbβ3 and αvβ3 were superimposed using the C-alpha match program (Haim Wolfson and Ruth Nussinov, Tel Aviv University, Tel Aviv, Israel).20
The residues of αIIb and αV that interact with β3 were compared for their contribution to the predicted stability of the interface. The equivalent residues between αIIb and αV were matched using structural alignment and multiple sequence alignment (MSA) of the α-integrins described in the next paragraph. The evolutionary conservation analyses of the α and β subunits were calculated using the ConSurf server,21,22 based on MSAs of the α or β subunit homologous sequences.
The β-propeller domain of αIIb was used to search homologous sequences in the UNIPROT database23,24 using BLAST.25,26 The identified homologous sequences were aligned using MUSCLE (Robert C. Edgar).27 A resultant alignment of 37 homologues was used to generate a hidden Markov model,28 which was subsequently used to collect and align 86 homologous sequences from National Center for Biotechnology Information's nonredundant protein sequence database.29 From this alignment, we collected 67 mammalian α-integrins that were used for analysis of the evolutionary conservation analysis of the α-integrins. The same procedure was applied to generate an MSA of the β subunit homologues using β3 as the query sequences. A final alignment of 46 mammalian homologous sequences was used to calculate the evolutionary conservation analysis of the β-integrins.
Results
Effect of αIIb-Trp110Ala and -Phe171Ala substitutions on αIIbβ3 formation, surface expression, and ligand binding
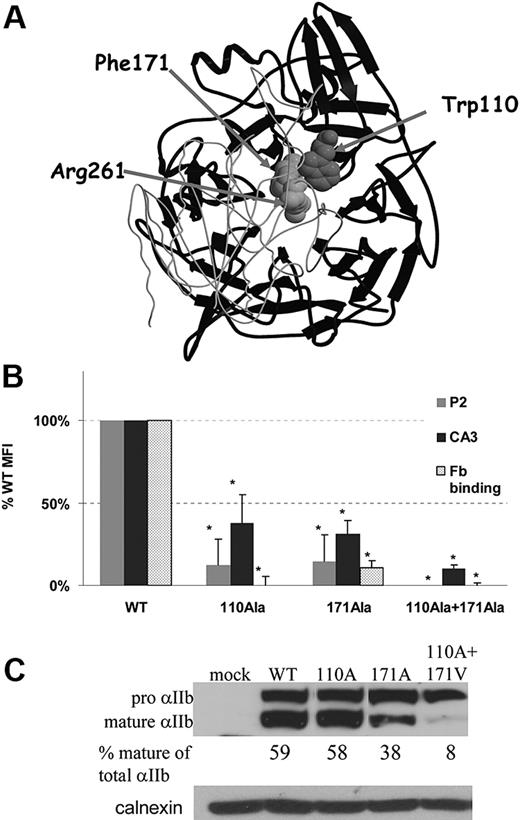
To determine the role of the interaction of aromatic residues located in the center of αIIb β-propeller with β3-Arg261, we focused on Trp110 and Phe171 located in blades 2 and 3, respectively, for the following reasons: (1) Recombinant forms of αIIb containing only the first 3 blades of the propeller have already been shown to form a complex with β3.30 This finding implies that blades 4, 5, 6, and 7 are not essential for the interaction of αIIb and β3. (2) Blades 1, 2, and 3 lack cation binding domains. (3) Inserts from blades 2 and 3 are involved in the ligand binding site of αIIbβ3.31 (4) Mutations in the aromatic residues of blades 2 and 3 cause GT.13-15 (5) Trp110 and Phe171 are the only aromatic residues in these blades that face the center of the propeller in the cage motif (Figure 1A).
Interactions in the head domain of αIIbβ3 between aromatic residues from blades 2 and 3 of the β-propeller of αIIb (Trp110 and Phe171) and Arg261 of β3. (A) The β-propeller domain of αIIb is presented in black ribbons and the βA domain of β3 is presented in light gray coil derived from PDB 1TXV. β3-Arg261, αIIb-Trp110, and αIIb-Phe171 are depicted by space-filled atoms. (B) Effect of αIIb-Trp110Ala and -Phe171Ala substitutions on αIIbβ3 surface expression and ligand binding. Flow cytometric analysis of αIIbβ3 surface expression in BHK cells expressing WT β3 and mutated or WT αIIb was preformed using monoclonal antibody against αIIbβ3 (P2) and monoclonal antibody against αIIb (CA3). Fibrinogen (Fb) binding was measured with anti–human fibrinogen antibody after activation by PT25-2 activating antibody. All antibody bindings are presented as mean percentage of WT MFI ± SD (at least 3 different transfections). *P < .05. (C) Intracellular maturation of αIIb was measured by immunoblotting of reduced cell lysates in Tris-acetate 3%-8% gels with monoclonal antibody specific to αIIb (SZ22). The proportion of mature αIIb of total αIIb (mature and pro-αIIb) in percentage was calculated by measuring band intensity using EZQuant densitometry program. Total protein loading was measured by polyclonal anti-calnexin antibody.
Interactions in the head domain of αIIbβ3 between aromatic residues from blades 2 and 3 of the β-propeller of αIIb (Trp110 and Phe171) and Arg261 of β3. (A) The β-propeller domain of αIIb is presented in black ribbons and the βA domain of β3 is presented in light gray coil derived from PDB 1TXV. β3-Arg261, αIIb-Trp110, and αIIb-Phe171 are depicted by space-filled atoms. (B) Effect of αIIb-Trp110Ala and -Phe171Ala substitutions on αIIbβ3 surface expression and ligand binding. Flow cytometric analysis of αIIbβ3 surface expression in BHK cells expressing WT β3 and mutated or WT αIIb was preformed using monoclonal antibody against αIIbβ3 (P2) and monoclonal antibody against αIIb (CA3). Fibrinogen (Fb) binding was measured with anti–human fibrinogen antibody after activation by PT25-2 activating antibody. All antibody bindings are presented as mean percentage of WT MFI ± SD (at least 3 different transfections). *P < .05. (C) Intracellular maturation of αIIb was measured by immunoblotting of reduced cell lysates in Tris-acetate 3%-8% gels with monoclonal antibody specific to αIIb (SZ22). The proportion of mature αIIb of total αIIb (mature and pro-αIIb) in percentage was calculated by measuring band intensity using EZQuant densitometry program. Total protein loading was measured by polyclonal anti-calnexin antibody.
To investigate the effect of Trp110 and Phe171 residues on surface expression and function of αIIbβ3, we substituted each or both residues by alanine and expressed their cDNAs in BHK cells together with WT β3 cDNA. Flow cytometric analysis with an anti-αIIbβ3 monoclonal antibody P2 demonstrated a reduction of surface expression to less than 15% of WT for each Trp110Ala and Phe171Ala mutants, and completely impaired surface expression for the double mutant (Figure 1B). Because the epitope of P2 antibody31 is located in the head domain of αIIbβ3, minor conformational changes in this domain might have impaired binding of this antibody. Hence, we repeated the flow cytometric analysis with another monoclonal antibody (CA3) that is specific for αIIb, although the exact epitope is unknown.32 The results showed that Trp110Ala- or Phe171Ala-transfected cells bound 30% to 35% of CA3 compared with WT cells, whereas cells with the combined mutant bound only 10% of CA3 compared with WT cells.
Fibrinogen binding to αIIbβ3 was then measured after exposure of cells harboring WT or the mutants to PT25 activating antibody. Figure 1B shows that fibrinogen binding to Trp110Ala and to the combined Trp110Ala+Phe171Ala–transfected mutant cells was totally abolished, whereas in the Phe171Ala mutant, fibrinogen binding was substantially reduced to the same extent as P2 binding. Immunoblotting of reduced cell lysates showed that in both Trp110Ala and Phe171Ala mutants, the percentage of mature αIIb was reduced compared with WT, and in Trp110Ala+Phe171Ala double mutant it was very low (Figure 1C). Taken together, these findings imply that substitution of Trp110 by alanine probably distorts the structure of the head domain of αIIbβ3, reduces the surface expression of αIIbβ3, and abrogates fibrinogen binding. Substitution of Phe171 by alanine impairs complex maturation and surface expression but preserves ligand binding proportionally to surface expression of αIIbβ3. In cells containing the double mutant, αIIb failed to mature.
Effect of αIIb Trp110 or Phe171 substitutions by corresponding residues of other α subunits on αIIbβ3 formation, surface expression, and ligand binding
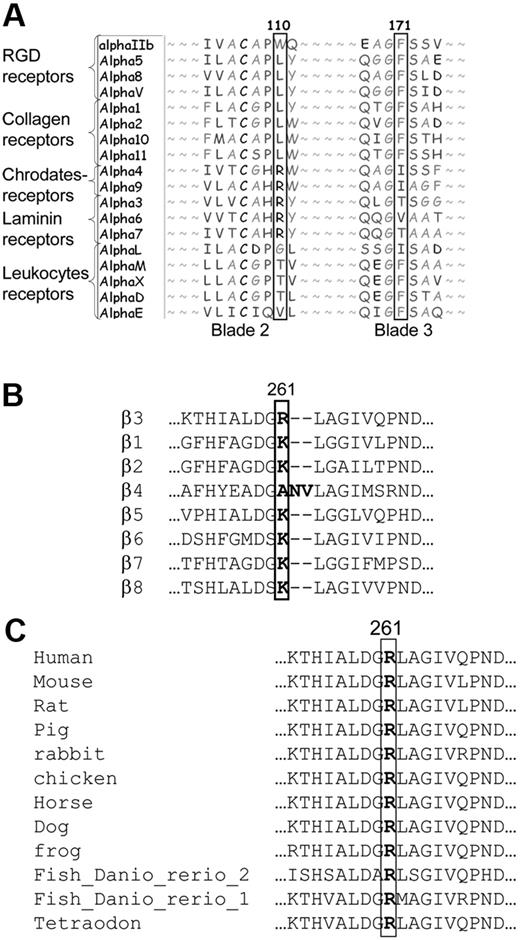
Sequence alignment of αIIb with other human α subunits revealed that Trp110 in αIIb is unique among α subunits. Other α subunits harbor at the corresponding location nonaromatic hydrophobic residues (eg, leucine and arginine; Figure 2A). In contrast, Phe171 is conserved in other α subunits such as RGD, collagen, and several leukocyte receptors, but not in other α subunits; in α6 there is valine and in α4, α9, and αL there is isoleucine. Consequently, we substituted Trp110 by leucine or arginine, and Phe171 by valine or isoleucine. Flow cytometric analysis of cells containing Trp110Leu or Trp110Arg mutants revealed almost normal expression of αIIbβ3 as measured by P2 and CA3 antibodies, and almost normal fibrinogen binding after activation by PT25 (Figure 3). In contrast, cells harboring substitutions of Phe171 by isoleucine or valine yielded a reduction in P2 antibody binding to 31% and 1% of WT cells, respectively, and reduced CA3 antibody binding to 70% and 25% of WT, respectively (Figure 3A). Irrespective of these variable reductions of αIIbβ3 surface expression, fibrinogen binding to cells with Phe171 mutants was completely absent after PT25 activation.
Sequence alignments of different α and β subunits and β3 of different species. (A) Human α subunits at the region of Trp110 and Phe171 of αIIb. (B) Human β subunits flanking Arg261 position in β3 and (C) β3 from different species at the region of Arg261 in human β3.
Sequence alignments of different α and β subunits and β3 of different species. (A) Human α subunits at the region of Trp110 and Phe171 of αIIb. (B) Human β subunits flanking Arg261 position in β3 and (C) β3 from different species at the region of Arg261 in human β3.
Analysis of BHK cells harboring WT β3 with WT αIIb or αIIb with substitutions of Trp110 or Phe171 by hydrophobic residues. (A) Flow cytometry of cells harboring Trp110Leu or Trp110Arg exhibited surface expression of αIIbβ3 and fibrinogen binding that was not significantly different from WT cells, whereas Phe171Ile and Phe171Val mutants exhibited reduced αIIbβ3 expression and fibrinogen binding. Binding is shown as mean percentage of WT MFI ± SD (mean of at least 3 different transfections). *P < .05. (B) Immunoblotting of cell lysates on Tris-acetate 3% to 8% gels developed with monoclonal antibody against αIIb (SZ22). Percentage mature αIIb of total αIIb was calculated by measuring band intensity for each cell line using the EZQuant densitometry program.
Analysis of BHK cells harboring WT β3 with WT αIIb or αIIb with substitutions of Trp110 or Phe171 by hydrophobic residues. (A) Flow cytometry of cells harboring Trp110Leu or Trp110Arg exhibited surface expression of αIIbβ3 and fibrinogen binding that was not significantly different from WT cells, whereas Phe171Ile and Phe171Val mutants exhibited reduced αIIbβ3 expression and fibrinogen binding. Binding is shown as mean percentage of WT MFI ± SD (mean of at least 3 different transfections). *P < .05. (B) Immunoblotting of cell lysates on Tris-acetate 3% to 8% gels developed with monoclonal antibody against αIIb (SZ22). Percentage mature αIIb of total αIIb was calculated by measuring band intensity for each cell line using the EZQuant densitometry program.
Immunoblot analysis of reduced cell lysates using SZ22 antibody supported the flow cytometric results; mutants that exhibited reduced surface expression (Phe171Ile and Phe171Val) also displayed relatively reduced mature αIIb (Figure 3B). In contrast, Trp110Leu and Trp110Arg, which exhibited normal surface expression and fibrinogen binding, also displayed normal αIIb maturation.
These results indicate that the nonconserved residue Trp110 can be replaced by corresponding residues from other α subunits in the formation of αIIbβ3 and ligand binding, whereas substitution of the more conserved residue Phe171 by corresponding residues of other α subunits abrogates the αIIbβ3 surface expression and fibrinogen binding.
Effect of αIIb-Trp110Leu or -Trp110Arg substitutions on αIIbβ3 adhesion to immobilized fibrinogen or VWF and on clot retraction
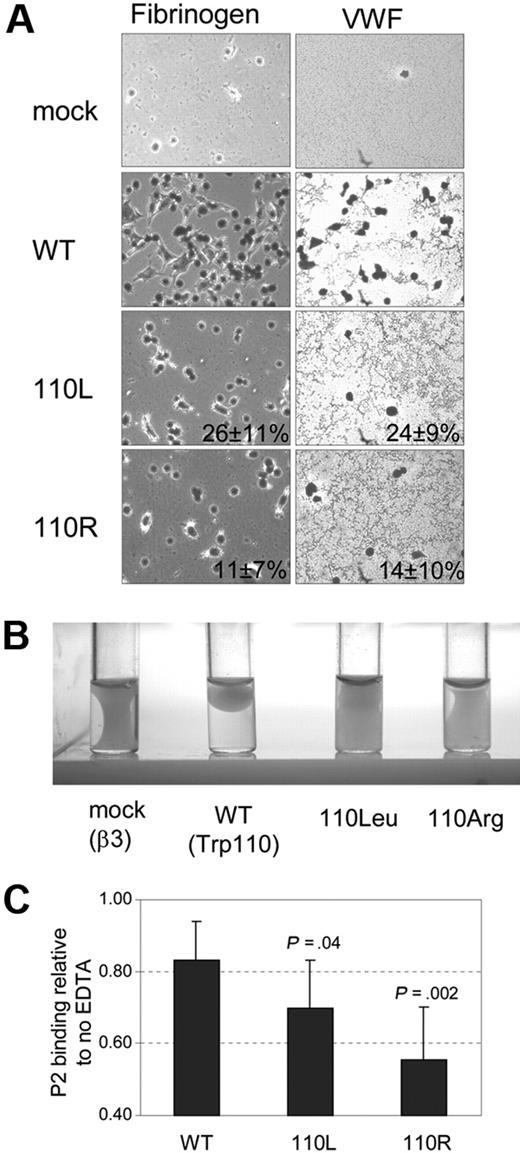
Because αIIb-Trp110Leu or -Trp110Arg substitutions yielded normal surface expression and normal fibrinogen binding, we examined whether these substitutions influence post–ligand binding functions such as adhesion of cells to immobilized ligand as well as clot retraction.7 Although fibrinogen is the main ligand of αIIbβ3, fibrinogen-independent platelet adhesion and aggregation clearly exist.33,34 Consequently, we examined adhesion to immobilized fibrinogen and VWF. Adhesion to immobilized fibrinogen was examined after 30-minute incubation and adhesion to immobilized VWF after 45 minutes. Both Trp110Leu and Trp110Arg substitutions impaired cell adhesion to both ligands (Figure 4A) and clot retraction (Figure 4B). Thus, replacing Trp110 by residues located in the same position of other α subunits impairs αIIbβ3 outside-in signaling–related functions.
Effect of αIIb-Trp110Leu or -Trp110Arg substitutions on αIIbβ3 outside-in signaling–related functions and stability. (A) BHK cell adhesion to immobilized fibrinogen and to immobilized VWF-coated wells. Mean percentage of WT surface coverage ± SD is shown (mean of 3 different transfections). (B) Clot retraction of cells resuspended in DMEM in the presence of fibrinogen and thrombin and after incubation for 18 hours at 37°C. (C) αIIbβ3 complex stability measured by flow cytometry with P2 antibody in the presence and absence of 5mM EDTA. The results for WT and the 2 mutants are expressed as mean proportion ± SD of MFI P2 binding in the absence of EDTA.
Effect of αIIb-Trp110Leu or -Trp110Arg substitutions on αIIbβ3 outside-in signaling–related functions and stability. (A) BHK cell adhesion to immobilized fibrinogen and to immobilized VWF-coated wells. Mean percentage of WT surface coverage ± SD is shown (mean of 3 different transfections). (B) Clot retraction of cells resuspended in DMEM in the presence of fibrinogen and thrombin and after incubation for 18 hours at 37°C. (C) αIIbβ3 complex stability measured by flow cytometry with P2 antibody in the presence and absence of 5mM EDTA. The results for WT and the 2 mutants are expressed as mean proportion ± SD of MFI P2 binding in the absence of EDTA.
Effect of αIIb-Trp110Leu or -Trp110Arg substitutions on αIIbβ3 stability
The integrity of αIIbβ3 depends on the presence of calcium cations, and therefore its stability is impaired in the presence of EDTA.35 After incubation of WT cells in a 5mM solution of EDTA for 30 minutes at room temperature, there was a 17% decline in binding of P2 antibody to αIIbβ3 in WT cells, whereas in cells harboring Trp110Leu and Trp110Arg, there was a decline of 30% and 44% of P2 antibody binding, respectively (Figure 4C). The differences in the stability of αIIbβ3 between mutants and WT were statistically significant. Thus, replacing αIIb-Trp110 by hydrophobic residues of other α subunits not only impairs functions related to outside-in signaling but also affects αIIbβ3 stability.
Effect of β3-Arg261Ala or Arg261Lys substitutions on αIIbβ3 and αvβ3 surface expression and ligand binding
Sequence alignment of β3 and other β subunits revealed that lysine occupies the 261 position in most integrins except for β3 and β4 in which this position is occupied by arginine and alanine, respectively (Figure 2B). Notably, all known β3 subunits in different species harbor arginine at the 261 position (Figure 2C). Arginine and lysine are both positively charged residues and have been considered to contribute similarly to the hydrophobic and cation-π contacts between β3 and the central cavity of the propeller.12
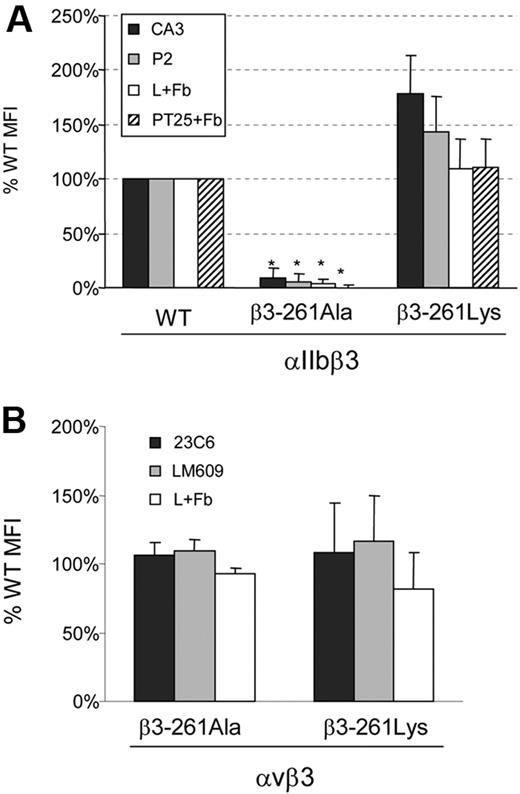
To define the importance of arginine at the 261 position, we created Arg261Ala and Arg261Lys substitutions, expressed the mutants and WT β3 with normal human-αIIb or normal human-αv in BHK cells, and examined αIIbβ3 and αvβ3 surface expression and soluble-fibrinogen binding after activation by anti-LIBS6 or PT25. Figure 5A displays striking differences between cells bearing the 2 αIIbβ3 substitutions at position 261. Whereas Arg261Ala substitution hardly exhibited any surface expression of αIIbβ3 and no fibrinogen binding, cells harboring Arg261Lys substitution displayed increased surface expression of αIIbβ3 associated with normal fibrinogen binding after activation by anti-LIBS6 or PT25 antibodies. In contrast, in αvβ3, both αvβ3-Arg261Ala and αvβ3-Arg261Lys exhibited normal surface expression and normal fibrinogen binding after anti-LIBS6 activation (Figure 5B). These findings imply that a positively charged residue at position 261 (arginine or lysine) is essential for the integrity of αIIbβ3 but not for αvβ3.
Flow cytometry of BHK cells harboring mutants at position Arg261 of β3. (A) Surface expression of αIIb (CA3 antibody) and αIIbβ3 (P2 antibody) was measured in cells expressing WT αIIbβ3, αIIbβ3-261Ala, or αIIbβ3-261Lys. Fibrinogen binding was determined after artificial activation by activating antibodies PT25-2 (PT25+Fb) or anti-LIBS6 (L+Fb). Cells harboring only the vectors pCEP and pcDNA3 were used to evaluate background binding. Binding is shown as mean percentage of WT MFI ± SD (mean of at least 3 different transfections). *P < .01. (B) Surface expression of human-αvβ3 measured in cells expressing WT αvβ3, αvβ3-261Ala, or αvβ3-261Lys using 23C6 or LM609 antibodies that bind to human αvβ3 at a higher affinity than to the chimera αv(hamster)β3(human). Fibrinogen binding was measured after anti-LIBS6 activation (L+Fb). Binding is expressed as mean percentage of WT MFI ± SD (N ≥ 3).
Flow cytometry of BHK cells harboring mutants at position Arg261 of β3. (A) Surface expression of αIIb (CA3 antibody) and αIIbβ3 (P2 antibody) was measured in cells expressing WT αIIbβ3, αIIbβ3-261Ala, or αIIbβ3-261Lys. Fibrinogen binding was determined after artificial activation by activating antibodies PT25-2 (PT25+Fb) or anti-LIBS6 (L+Fb). Cells harboring only the vectors pCEP and pcDNA3 were used to evaluate background binding. Binding is shown as mean percentage of WT MFI ± SD (mean of at least 3 different transfections). *P < .01. (B) Surface expression of human-αvβ3 measured in cells expressing WT αvβ3, αvβ3-261Ala, or αvβ3-261Lys using 23C6 or LM609 antibodies that bind to human αvβ3 at a higher affinity than to the chimera αv(hamster)β3(human). Fibrinogen binding was measured after anti-LIBS6 activation (L+Fb). Binding is expressed as mean percentage of WT MFI ± SD (N ≥ 3).
Effect of β3-Arg261Lys substitution on αIIbβ3 and αvβ3 outside-in signaling–related functions
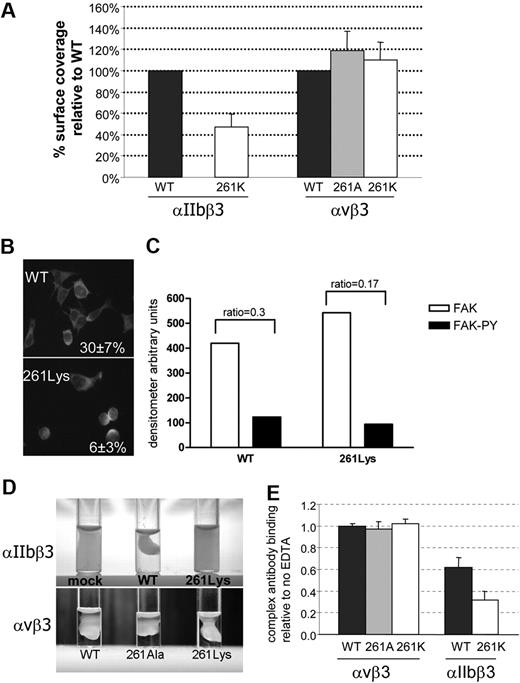
Because replacement of β3-Arg261 by lysine did not affect αIIbβ3 complex formation or ligand binding, it seemed dubious that this residue is so highly conserved in β3 of different species (Figure 2C). To further explore why Arg261 is so essential for αIIbβ3 function, we examined outside-in signaling–related functions in mutants containing Lys261. Compared with WT cells, cells harboring αIIbβ3-Lys261 exhibited impaired adhesion to immobilized fibrinogen (Figure 6A). Surface coverage after 30-minute incubation revealed a 55% reduction in cell adherence compared with WT cells. Staining cells with antiactin FITC-conjugated antibody showed that most mutant cells that adhered were less spread and usually rounded compared with WT cells (Figure 6B). Almost 30% of WT cells were fully spread, whereas in αIIbβ3-Lys261 cells only 6% were fully spread. Adhesion to immobilized VWF was also examined and was approximately 50% compared with WT cells (supplemental Figure).
The effect of β3 substitutions on αIIbβ3 and αvβ3 outside-in signaling–related functions and complex stability. (A) Cell adhesion to fibrinogen-coated wells was evaluated after 30-minute incubation. The cells were fixed and stained by May-Grünwald eosin–methylene blue solution. Surface coverage was measured using Impact R analyzer. Results present as percentage surface coverage relative to WT cells ± SD (N ≥ 3). (B) Spreading was evaluated in cells harboring WT αIIbβ3 or αIIbβ3-261Lys and by staining with anti-actin FITC-conjugated antibody after 30-minute incubation. The actin-stained cells of 10 fields were counted and percentage of cells fully spread was calculated out of total adhered cells (presented in the right corner ± SD). (C) Band intensity of immunoblotting of anti-Fak and anti-Fak phosphorylated (FAK-PY) on WT αIIbβ3 and αIIbβ3-261Lys cell lysates. Measurements of band intensity were done using the EzQuant densitometry program. Index phosphorylation was calculated as the ratio between FAK-PY and FAK band intensity (presented on the top of the bars). (D) Clot retraction of cells resuspended in DMEM in the presence of fibrinogen and thrombin after incubation for 18 hours at 37°C. (E) Surface expression of integrin complexes was measured by flow cytometry using P2-FITC antibody against αIIbβ3 and LM609-FITC against αvβ3. Cells were incubated with or without 5mM EDTA for 30 minutes at room temperature. The results are presented as the proportion of MFI in the presence and absence of EDTA (n ≥ 3).
The effect of β3 substitutions on αIIbβ3 and αvβ3 outside-in signaling–related functions and complex stability. (A) Cell adhesion to fibrinogen-coated wells was evaluated after 30-minute incubation. The cells were fixed and stained by May-Grünwald eosin–methylene blue solution. Surface coverage was measured using Impact R analyzer. Results present as percentage surface coverage relative to WT cells ± SD (N ≥ 3). (B) Spreading was evaluated in cells harboring WT αIIbβ3 or αIIbβ3-261Lys and by staining with anti-actin FITC-conjugated antibody after 30-minute incubation. The actin-stained cells of 10 fields were counted and percentage of cells fully spread was calculated out of total adhered cells (presented in the right corner ± SD). (C) Band intensity of immunoblotting of anti-Fak and anti-Fak phosphorylated (FAK-PY) on WT αIIbβ3 and αIIbβ3-261Lys cell lysates. Measurements of band intensity were done using the EzQuant densitometry program. Index phosphorylation was calculated as the ratio between FAK-PY and FAK band intensity (presented on the top of the bars). (D) Clot retraction of cells resuspended in DMEM in the presence of fibrinogen and thrombin after incubation for 18 hours at 37°C. (E) Surface expression of integrin complexes was measured by flow cytometry using P2-FITC antibody against αIIbβ3 and LM609-FITC against αvβ3. Cells were incubated with or without 5mM EDTA for 30 minutes at room temperature. The results are presented as the proportion of MFI in the presence and absence of EDTA (n ≥ 3).
Phosphorylation of FAK is known to be related to outside-in signaling. Immunoblots of WT and αIIbβ3-Lys261 cells that adhered to fibrinogen-coated wells were stained by anti-FAK and anti–FAK-PY. The index of phosphorylation was calculated as the ratio between anti–FAK-PY and anti-FAK band intensities (Figure 6C). In the adhered cells harboring αIIbβ3-Lys261, the phosphorylation index was 37% plus or minus 5% lower than in WT cells. Hence, the substitution of Arg261 by lysine not only reduced cell adhesion but also impaired outside-in signaling events such as FAK phosphorylation in the adhered cells.
Clot retraction was absent in cells expressing αIIbβ3-Lys261 (Figure 6D). Thus, although substitution of Arg261 by lysine did not impair surface expression and ligand binding, it remarkably impaired outside-in signaling–related functions.
In cells expressing αvβ3, substitutions of Arg261 by alanine or lysine exhibited normal adhesion to immobilized fibrinogen and normal clot retraction (Figure 6A,D). Hence, it appears that outside-in signaling–related functions are intact in these αvβ3 mutants.
Effect of β3 Arg 261Lys substitution on αIIbβ3 and αvβ3 complex stability
Similarly to αIIbβ3, αvβ3 is a calcium-dependent complex that can disintegrate upon incubation in EDTA in a temperature- and time-dependent manner but to a much lesser extent than αIIbβ3.36 Incubation of WT cells, or cells harboring αvβ3-261Ala or 261Lys in 5mM EDTA at room temperature for 30 minutes did not cause dissociation of αvβ3 as measured by an anti-αvβ3 antibody (LM609; Figure 6E). Under the same conditions, WT αIIbβ3 complex exhibited a 40% decrease in binding of the P2 antibody, and in cells bearing Arg261Lys, αIIbβ3 was reduced by 70% (Figure 6E). These results suggest that Arg261 is essential for stabilization of αIIbβ3 but not for αvβ3.
Structural analysis of the interaction between βA domain of β3 and β-propeller of αIIb or αv subunits
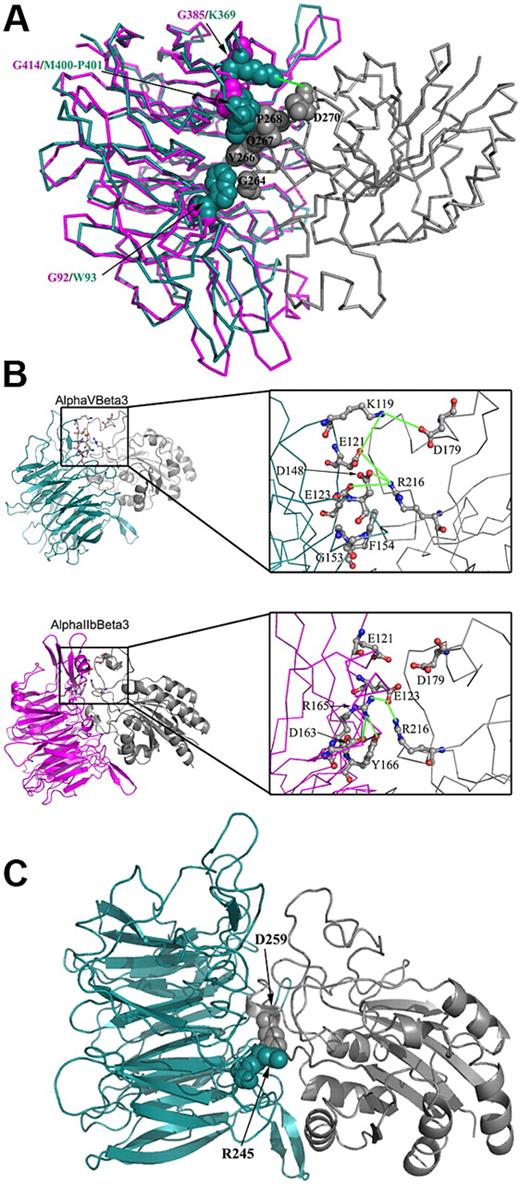
Although αIIb and αv are highly homologous in their β-propeller domain, there are some differences in the α/β interface. Comparison between αIIb and αv residues in their interface with β3 was performed to appraise the stability of the αIIbβ3 and αvβ3 complexes. Several significant differences between the head domains of αIIbβ3 and αvβ3 were discerned as follows: (1) In αv, there are 3 hydrophobic residues (Trp93, Met400, Pro401) that are in close contact with β3 residues (Gly264, Val266, Gln267, Pro268). In αIIb, the corresponding residues are Gly92 and Gly414 that lack similar contacts with β3 (Figure 7A). (2) An acidic residue, Asp270 in β3, can form a salt bridge with Lys369 from αv but not with the corresponding residue Gly385 in αIIb (Figure 7A). (3) In the ligand binding region, αvβ3 has a polar network composed of β3-Arg216 (basic residue) that interacts with αv-Glu121, Glu123, and Asp148 (acidic residues). In αIIb, this network is slightly different; β3-Arg216 interacts only with one residue, αIIb-Glu123; the latter residue is also involved in intrasubunit interactions with 3 residues, αIIb-Tyr166, -Asp163, and -Arg165 (the corresponding residues in αv are nonpolar residues Phe154, Gly151, and Gly153, respectively; Figure 7B). (4) A salt bridge between Asp179 from β3 and Lys119 from αv is disturbed in αIIbβ3 because the acidic amino acid Asp179 from β3 is facing another acidic amino acid Glu121 (Figure 7B). (5) A salt bridge between β3-Asp259 and αv-Arg245 is disrupted in αIIbβ3 because in αIIb, instead of Arg245 there is Thr259, which cannot interact with β3-Asp259 (Figure 7C). Together, these differences indicate that the αvβ3 complex is more stable than the αIIbβ3 complex, which apparently makes it more tolerant to changes in the Arg261 position.
Comparison of residues located at the interface of β3 and αIIb or β3 and αv that contribute to the stability of these complexes. The analysis was preformed using 1TXV PDB code for αIIbβ3 and 1JV2 for αvβ3. Only the β-propeller domain from the α subunits and the β-A domain from the β3 are displayed for clarity. αIIb, αv, and β3 are pink, cyan, and gray, respectively. In panels A and C, several residues located at the α-β interface are displayed as space-filled atoms, and in panel B, several residues are displayed in a balls-and-sticks representation and colored by atom type. Electrostatic connections are displayed as green lines. Amino acid abbreviations are shown in 1-letter codes. (A) Whereas residues Trp93, Met400-Pro401, and Lys369 located on αv form interactions with β3, their corresponding residues in αIIb are occupied by glycine residues (Gly92, Gly414, and Gly385, respectively) that lack similar contacts with β3. (B) The right panel displays a close view of the interface in the same orientation as presented in the left panel. The figure displays a network of electrostatic interactions connecting αv and β3. This network includes interactions between Lys119 (αv) and Asp179 (β3), and among Glu121, Glu123, and Asp148 in αv and Arg216 in β3. In contrast, the corresponding region at the αIIbβ3 interface exhibits a network of polar interactions that is mostly intrinsic to αIIb; Arg165 interacts with Glu123, Asp163, and Tyr166, all in αIIb. Only one intersubunit electrostatic interaction is conserved in αIIbβ3, that is, Glu123 (αIIb) and Arg216 (β3). (C) A salt bridge between αv-Arg245 and β3-Asp259. This electrostatic interaction is absent in the αIIbβ3 complex because the corresponding position in αIIb is occupied by the uncharged residue Thr259.
Comparison of residues located at the interface of β3 and αIIb or β3 and αv that contribute to the stability of these complexes. The analysis was preformed using 1TXV PDB code for αIIbβ3 and 1JV2 for αvβ3. Only the β-propeller domain from the α subunits and the β-A domain from the β3 are displayed for clarity. αIIb, αv, and β3 are pink, cyan, and gray, respectively. In panels A and C, several residues located at the α-β interface are displayed as space-filled atoms, and in panel B, several residues are displayed in a balls-and-sticks representation and colored by atom type. Electrostatic connections are displayed as green lines. Amino acid abbreviations are shown in 1-letter codes. (A) Whereas residues Trp93, Met400-Pro401, and Lys369 located on αv form interactions with β3, their corresponding residues in αIIb are occupied by glycine residues (Gly92, Gly414, and Gly385, respectively) that lack similar contacts with β3. (B) The right panel displays a close view of the interface in the same orientation as presented in the left panel. The figure displays a network of electrostatic interactions connecting αv and β3. This network includes interactions between Lys119 (αv) and Asp179 (β3), and among Glu121, Glu123, and Asp148 in αv and Arg216 in β3. In contrast, the corresponding region at the αIIbβ3 interface exhibits a network of polar interactions that is mostly intrinsic to αIIb; Arg165 interacts with Glu123, Asp163, and Tyr166, all in αIIb. Only one intersubunit electrostatic interaction is conserved in αIIbβ3, that is, Glu123 (αIIb) and Arg216 (β3). (C) A salt bridge between αv-Arg245 and β3-Asp259. This electrostatic interaction is absent in the αIIbβ3 complex because the corresponding position in αIIb is occupied by the uncharged residue Thr259.
Discussion
In this study, we analyzed interactions between αIIb or αv and β3 in the head domain of αIIbβ3 and αvβ3. Tables 1 and 2 summarize the effects of αIIb and β3 substitutions on the various functions of αIIbβ3 and αvβ3. For the study of αIIbβ3, we focused on 2 aromatic residues, Trp110 and Phe171, located in the center of the β-propeller of αIIb and Arg261 of β3; the latter residue was shown to protrude into the center of the β-propeller.10 αIIbβ3 complex formation and surface expression were totally abolished only when alanine substituted the 2 αIIb aromatic residues (Figure 1B) or β3-Arg261 (Figure 5A). Preservation of normal surface expression of αIIbβ3 and normal fibrinogen binding was demonstrated upon substitution of Trp110 or Arg261 by the corresponding residues of other integrins (Figures 3 and 5A). These substitutions, however, had a remarkable deleterious effect on αIIbβ3 outside-in signaling–related functions; they caused impaired adhesion, spreading, and clot retraction (Figures 4 and 6). These findings indicate that β3-Arg261 and its interaction with αIIb-Trp110 are essential for normal outside-in signaling–related functions of αIIbβ3. Disruptions of outside-in functions albeit normal ligand binding in transgenic mice have been described so far only in β3 cytoplasmic tail mutations and were found to be protective against arterial thrombosis.37 Our results show that disruption of outside-in signaling associated with normal ligand binding can also be achieved by changes in the head domain of αIIbβ3, which conceivably can be a target for developing new antithrombotic agents.
Effect of substitutions of αIIb-Trp110 and -Phe171 on inside-out and outside-in signaling–related functions of αIIbβ3
| αIIb substitution . | Surface expression . | Soluble fibrinogen binding . | Percent mature of total αIIb formation . | Outside-in signaling–related functions* . | Stability in EDTA . | |
|---|---|---|---|---|---|---|
| Adhesion to immobilized fibrinogen . | Clot retraction . | |||||
| Trp110Ala | Reduced | Negligible | Reduced | ND | ND | ND |
| Phe171Ala | Reduced | Reduced† | Reduced | ND | ND | ND |
| Trp110Ala and Phe171Ala | Negligible | Negligible | Negligible | ND | ND | ND |
| Trp110Leu or Trp110Arg | Normal | Normal | Normal | Impaired | Impaired | Reduced |
| Phe171Ile or Phe171Val | Reduced | negligible | Reduced | ND | ND | ND |
| αIIb substitution . | Surface expression . | Soluble fibrinogen binding . | Percent mature of total αIIb formation . | Outside-in signaling–related functions* . | Stability in EDTA . | |
|---|---|---|---|---|---|---|
| Adhesion to immobilized fibrinogen . | Clot retraction . | |||||
| Trp110Ala | Reduced | Negligible | Reduced | ND | ND | ND |
| Phe171Ala | Reduced | Reduced† | Reduced | ND | ND | ND |
| Trp110Ala and Phe171Ala | Negligible | Negligible | Negligible | ND | ND | ND |
| Trp110Leu or Trp110Arg | Normal | Normal | Normal | Impaired | Impaired | Reduced |
| Phe171Ile or Phe171Val | Reduced | negligible | Reduced | ND | ND | ND |
ND indicates not done.
Outside-in signaling–related functions were examined only when there was adequate surface expression.
Reduced to the same extent of αIIbβ3 surface expression.
Effect of β3-Arg261 substitutions on surface expression, ligand binding, and outside-in signaling–related functions in αIIbβ3 and αvβ3
| β3 substitution . | Surface expression . | Soluble fibrinogen binding . | Outside-in signaling–related functions* . | Stability in EDTA . | |
|---|---|---|---|---|---|
| Adhesion to immobilized fibrinogen and spreading . | Clot retraction . | ||||
| αIIbβ3 | |||||
| Arg261Ala | Negligible | Negligible | ND | ND | ND |
| Arg261Lys | Normal | Normal | Impaired | Impaired | Reduced |
| αvβ3 | |||||
| Arg261Ala | Normal | Normal | Normal | Normal | Normal |
| Arg261Lys | Normal | Normal | Normal | Normal | Normal |
| β3 substitution . | Surface expression . | Soluble fibrinogen binding . | Outside-in signaling–related functions* . | Stability in EDTA . | |
|---|---|---|---|---|---|
| Adhesion to immobilized fibrinogen and spreading . | Clot retraction . | ||||
| αIIbβ3 | |||||
| Arg261Ala | Negligible | Negligible | ND | ND | ND |
| Arg261Lys | Normal | Normal | Impaired | Impaired | Reduced |
| αvβ3 | |||||
| Arg261Ala | Normal | Normal | Normal | Normal | Normal |
| Arg261Lys | Normal | Normal | Normal | Normal | Normal |
ND indicates not done.
Outside-in signaling–related functions were examined only when there was adequate surface expression.
Substituting only one aromatic residue, Trp110 or Phe171, by alanine abrogated the conformation of αIIbβ3 head domain as displayed by reduced binding of an αIIbβ3 complex–specific P2 antibody (∼15% of WT) (Figure 1). Binding of an αIIb-specific antibody displayed higher values (∼ 40% of WT), indicating that there were more αIIbβ3 complexes than detected by the P2 antibody. Similar results for Trp110Ala were obtained using a different antibody specific for αIIbβ3 complex.38 Fibrinogen binding was profoundly impaired in the Trp110Ala mutant (Figure 1A) as also shown by Kamata et al.31 This impaired binding probably stemmed from the location of Trp110 in a unique loop of αIIb that is included in the ligand binding specific region.11 In contrast, the Phe171Ala mutant, which was surface expressed in a reduced amount, still bound fibrinogen (Figure 1B). Data obtained from substitutions of Phe171 or Trp110 by corresponding hydrophobic residues present in other α subunits underscored the difference between the 2 residues. Whereas substitution of Trp110 by arginine or leucine yielded almost normal surface expression of αIIbβ3 and normal ligand binding, substitutions of Phe171 by isoleucine or valine impaired complex maturation and abolished fibrinogen binding (Figure 3). These findings indicate that the highly conserved Phe171 is essential for the interaction of αIIb with β3 probably because of its strategic position in the lower ring cage motif of the β-propeller. The nonconserved Trp110 of blade 2 is most likely more tolerant to substitutions because it is not part of the cage motif although it is located at the center of the β-propeller.
Because Arg261 is unique for β3 (Figure 2B) and conserved among β3 subunits from various species (Figure 2C), we assumed that it plays a specific role in β3 structure and function. We examined the role of the positively charged Arg261 by replacing it first with a short neutral residue, alanine, and secondly by a similarly charged residue, lysine, which is present in most other β subunits at this position. We demonstrated that Arg261Ala substantially impaired the maturation of αIIbβ3, whereas substituting Arg261 by lysine yielded normal surface expression and normal soluble fibrinogen binding, indicating that a positively charged residue is essential at this position (Figure 5A).
The fact that clot retraction was substantially impaired for mutants that displayed normal fibrinogen binding (αIIb-Trp110Arg and -Trp110Leu or β3-Arg261Lys) (Figures 4B and 6D) suggests that fibrinogen and fibrin do not share the same binding sites. An opposite example of a mutation that did impair fibrinogen binding but did not harm clot retraction was demonstrated in a naturally GT type II mutation Leu262Pro in β3.39
Because β3 can form a complex with αv to yield αvβ3, we examined whether our mutants in the β3 subunits affected αvβ3 surface expression and ligand binding. As in αIIbβ3, substituting Arg261 by lysine resulted in normal surface expression and ligand binding (Figure 5A). In contrast, there was a striking difference from αIIbβ3 upon substituting Arg261 by alanine yielding normal surface expression and ligand binding. Such a discrepancy in αIIbβ3 and αvβ3 formation caused by a mutation in β3 was also observed in patients with GT; platelets carrying β3-His280Pro contained only 6% of normal αIIbβ3 but approximately 50% of normal αvβ3.40 Similarly, Ser162Leu and Arg216Gln mutations identified in 2 GT patients displayed complete abolishment of αIIbβ3 expression yet normal αvβ3 expression, as shown in an expression assay in 293 cells. Taken together, these data suggest αIIbβ3 formation is more sensitive to changes than αvβ3.
Another significant difference between αIIbβ3 and αvβ3 was demonstrated in outside-in functions. Substitution of β3-Arg261 by lysine or alanine yielded normal adhesion of αvβ3 to immobilized fibrinogen and normal clot retraction (Figure 6) unlike αIIbβ3 that harbored the same mutations. αIIbβ3 and αvβ3 complex stability in the presence of EDTA was also different. When Arg261 was substituted by lysine in the αIIbβ3, complex stability was significantly reduced compared with WT (Figure 6E). In contrast, there was no reduction in the stability of αvβ3-261Lys or even in αvβ3-261Ala in the presence of EDTA as in WT αvβ3.
The difference in the complex stability between αvβ3 and αIIbβ3 could be explained by structural analysis that identified interactions within the αvβ3 interface that are absent in the αIIbβ3 interface (Figure 7). There are 3 salt bridges and 3 hydrophobic interactions that exist in αvβ3 but are absent in αIIbβ3. Moreover, a polar network that comprises 3 acidic residues of αv and a basic residue (Arg216) of β3 is weak in αIIbβ3 because in αIIb there is only one acidic residue, Glu123, which also interacts with a basic residue Arg165 of αIIb (Figure 7C). The importance of the latter interaction is underscored by a β3-Arg216Gln mutation causing GT40 that gives rise to substantially reduced surface expression of αIIbβ3 but normal expression of αvβ3. Thus, the additional interactions in αvβ3 may contribute to its higher stability compared with αIIbβ3. In αIIbβ3, the absence of these supporting contacts makes the interaction of β3-Arg261 and the aromatic residues in the β-propeller of αIIb critical for αIIbβ3 complex formation and integrity.
In summary, we have shown that disrupting the interaction between αIIb-Trp110 and β3-Arg261 abrogates outside-in functions but preserves normal ligand binding. This effect is not observed in αvβ3. Our results underscore a new site in the head domain, which can potentially be targeted in the development of new antithrombotic agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Peter Newman for providing αIIb and β3 cDNAs and Dr Mark Ginsberg of the University of California, San Diego for providing anti-LIBS6 antibody.
Authorship
Contribution: H.H. designed and performed the study and participated in interpretation of the findings and writing of the paper; M.L. analyzed the crystal structures of αIIbβ3 and αvβ3, performed multiple sequence alignments, and participated in interpretation of the findings and writing of the paper; U.S. designed the study and participated in interpretation of data and writing of the paper; and N.R. designed the study and participated in interpretation of the findings and writing the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Uri Seligsohn, Amalia Biron Research Institute of Thrombosis and Hemostasis, Chaim Sheba Medical Center, Tel Hashomer, Israel 52621; e-mail: seligson@sheba.health.gov.il.