Abstract
Endothelial cell–specific chemotaxis receptor (ECSCR) is a cell surface protein expressed by blood endothelial cells with roles in endothelial cell migration and signal transduction. We investigated the function of ecscr in the development of the zebrafish vasculature. Zebrafish ecscr is expressed in angioblasts and in axial vessels during angioblast migration and vasculogenesis. Morpholino-directed ecscr knockdown resulted in defective angioblast migration in the posterior lateral plate mesoderm, a process known to depend on vascular endothelial-derived growth factor (VEGF). In cultured cells, transfected ECSCR localized to actin-rich membrane protrusions, colocalizing with kinase insert domain protein receptor (KDR)/VEGF receptor 2 in these regions. ECSCR-silenced cells show reduced VEGF-induced phosphorylation of KDR but not of FMS-like tyrosine kinase 1 (FLT1)/VEGF receptor 1. Finally, chemical inhibition of VEGF receptor activity in zebrafish resulted in angioblast deficiencies that partially overlap with those seen in ecscr morphants. We propose that ecscr promotes migration of zebrafish angioblasts by enhancing endothelial kdr sensitivity to VEGF.
Introduction
Formation of the embryonic vasculature involves multiple cellular processes including specification of angioblasts, their differentiation into endothelial cells, and the proliferation, migration, and assembly of angioblasts and endothelial cells into vessels. In zebrafish, angioblasts arise within the lateral plate mesoderm (LPM) positioned in orderly longitudinal rows.1,2 Between stage 10 somites (som) and 20 hours after fertilization (hpf), posterior angioblasts undergo a series of rearrangements, culminating in migration to the midline, aggregation into a nonlumenized vascular cord, and reorganization to yield the trunk axial vessels, the dorsal aorta (DAs), and posterior cardinal vein (PCV)3 (C.Z.C., R.R., personal communication, October 15, 2009).
Multiple cell-autonomous and extrinsic signals control specification, differentiation, and migration of angioblasts.4,5 Of these, vascular endothelial growth factor (VEGF) and its receptors are critical governors of angioblast numbers, migration, and assembly3,6-9 (and C.Z.C., G.V.S., R.R. Angioblasts proliferate at the lateral plate mesoderm during early steps of vasculogenesis in zebrafish. Manuscript submitted). Migration of angioblasts to the vascular cord occurs under the influence of tissues and secreted factors and is controlled by the Shh-VEGF-Notch signaling axis8 (C.Z.C., R.R., personal communication, October 15, 2009). However, many gene products associated with early migration of angioblasts remain poorly characterized.
Endothelial cell–specific chemotaxis receptor (ECSCR), also known as endothelial cell–specific molecule 2 and Apoptosis Regulator through modulating IAp expression (ARIA),10,11 was initially identified in a bioinformatic search for novel gene products enriched in endothelium.11 The ECSCR EST encoded a conceptual single-pass transmembrane protein of 205 amino acids, without significant homology to any other protein. Armstrong et al12 reported the cloning and analysis of human ECSCR. ECSCR expression was restricted to endothelial cells, both in vivo and in culture. ECSCR was also shown to be a marker of endothelial progenitor cells.10,12,13 Predicted plasma membrane expression was confirmed by cell surface expression of an ECSCR-GFP fusion protein in endothelial cells. The conserved cytoplasmic tail links ECSCR to several intracellular processes, including actin cytoskeleton remodeling,12 EGF receptor signal transduction,14 and proteasome activity.10 However, the function of ECSCR in embryonic development is not known.
Here, we examine the expression of the zebrafish homolog of ECSCR and analyze the impact of ecscr loss of function on the migration of angioblasts in the posterior LPM. ecscr message was detected in angioblasts and the forming vasculature. Loss of ecscr function in zebrafish embryos resulted in poor positioning of posterior angioblasts at developmental stages and embryonic sites correlating with angioblast migration to the midline. In cultured human endothelial cells, transfected ECSCR localized to actin-rich membrane protrusions, where it colocalized with VEGF receptor kinase insert domain protein receptor (KDR). Knockdown of ECSCR function in cultured endothelial cells resulted in deficient migration in response to serum and to purified VEGF, as well as reduced apoptosis and proliferation. ECSCR-deficient endothelial cells showed reduced KDR phosphorylation levels, without changes in FMS-like tyrosine kinase 1 (FLT1)/VEGF receptor 1 tyrosine phosphorylation. Finally, chemical inhibition of VEGF receptors in zebrafish also resulted in reduced midline angioblasts, a deficiency partially resembling with the result of ecscr silencing. Taken together, these observations suggest a model in which zebrafish ecscr acts at the cell surface to potentiate KDR-dependent migration of angioblasts to the midline.
Methods
Antisera and reagents
Anti-V5 Tag antibody and phalloidin were from Invitrogen. The antibodies to FLT1, phospho-KDR (Y996), and phospho-KDR (Y951) were from Santa Cruz Biotechnology. Anti-ARPC2 (Arp2/3 component) and anti–phospho-KDR (Y1059) were from Millipore/Upstate. Phospho-KDR (Y1175) and anti-phospho–phospholipase Cβ3 (PLCβ3; S537 and S1105) were from Cell Signaling Technology. Antibodies to KDR were from Santa Cruz Biotechnology and Cell Signaling Technology. Class V KDR inhibitor and KDR kinase inhibitor III SU5416 were from Calbiochem. VEGF was from Peprotech.
Zebrafish husbandry and microinjection and analysis
All zebrafish studies were performed according to Medical College of Wisconsin animal protocol guidelines under protocol 312-06-2. Wild-type TuAb zebrafish lines were maintained by standard protocols.15 Fertilized eggs from crosses of appropriate fish were injected in embryo buffer and allowed to develop at 28°C. Ecscr morpholinos were purchased from Gene Tools Inc. Morpholino injections are performed as follows: Morpholino (MOs) were reconstituted in nuclease-free water to a stock concentration of 16.5 ng/nL. Appropriate dilutions were made in 5× injection dye, and 3 nL of MOs (6-14 ng) was injected at the 1- to 4-cell stage. MO1 sequence is CATCAGTAGAAAACCTACCAAAGGC, and MO2 sequence is CATGGCTAAGTCCAAATGACGTTCA. Zebrafish ecscr and human ECSCR clones were purchased from Open Biosystems. Plasmids were linearized and transcribed by the Messenger Machine kit (Ambion). For rescue of MO2, 75 pg of human capped mRNA was coinjected with 10 ng of MO2 into 1-cell stage embryos. In situ hybridizations were performed using the dig-labeled kit (Roche) and standard methods.15 Zebrafish ecscr probe was generated from sequence shared by both observed transcripts (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Some embryos were further processed for sectioning by embedding in JB-4 methacrylate and sectioning at 0.4 μm. The etsrp probe has been published previously.16
Cell culture
Endothelial cells were maintained on tissue culture plastic unless indicated otherwise. Endothelial cells were cultured in endothelial basal medium-2 plus endothelial supplements (Lonza) for 5 to 7 passages. Pulmonary artery endothelial cells were maintained in F11 + penicillin/streptomycin/glutamine plus 10% fetal bovine serum. Transfection of both cell types was performed by Lipofection (Invitrogen). ECSCR siRNA was generated using the Dicer method17 using the Block-iT kit from Invitrogen. siRNA transfection of human umbilical vein endothelial cells (HUVECs) was performed at 80% confluence using 500 ng of siRNA and Lipofectamine 2000 Reagent (Invitrogen).18 The proliferation assay was performed as published previously.19 Briefly, HUVECs were serum starved before stimulation with VEGF 10 ng/mL. After 20 additional hours, [3H]thymidine was added to each well; 4 hours later, washed cells were measured for trichloroacetic acid–precipitable radioactivity. Experiments were repeated at least 3 times each time in triplicate. The apoptosis assay was performed using the annexin V detection of surface phosphatidylserine20 as follows: HUVECs were contact inhibited for 5 days. Upon release, cells were transiently transfected with 200 or 500 ng of lacZ and ECSCR siRNA. At 12 to 16 hours after transfection, cells were stained with annexin V–fluorescein isothiocyanate and propidium iodide. Apoptotic cells were measured as the number of annexin V–positive and propidium iodide–negative cells as determined by flow cytometric analysis. Motility of cells was assayed using 24-well Boyden chamber with filters having 8-μm pore size. Human pulmonary artery endothelial cells (HPAECs) or HUVECs were transiently transfected with 500 ng of ECSCR or lacZ siRNA as above. Cells were resuspended in serum-free media and starved for 1 to 2 hours before the assay. Boyden chambers were placed over a lower chamber containing 350 μL of 10% fetal calf serum and endothelial cell growth supplements (Lonza), or purified VEGF (25 ng/mL), in endothelial basal medium. Upper chambers were seeded at a density of 2 × 104 cells per well in 500 μL of serum-free media. Cells were allowed to migrate for 5 hours at 37°C in 5% CO2. Migrated cells were counted in triplicate wells from 5 different fields using 20× or 10× resolution. These experiments were repeated at least twice.
KDR and FLT1 biochemistry
HUVECs (plated on 25 ng/mL collagen) were transiently transfected with 500 ng of ECSCR or LacZ siRNA in a 10-cm cell culture dish using Lipofection. After 24 hours of transfection, the cells were serum starved overnight, hours before stimulation with 25 ng/mL VEGF165 for 2 or 5 minutes. Stimulation was stopped by adding ice cold 1× radioimmunoprecipitation assay buffer supplemented with 1× protease inhibitor cocktail and 1× phosphatase inhibitor (HALT; Thermo Fisher). Cell lysis was performed on ice for 30 to 60 minutes, followed by centrifugation of the cells, at 11 000g at 4°C for 5 minutes. The supernatant, containing proteins, was collected and stored at −80°C until use. Protein was estimated by DC Protein Assay (Bio-Rad). KDR and phosphoantigens were assayed by Western blot of total lysates; phospho-FLT1 was assayed by anti-FLT1 immune precipitation followed by phosphotyrosine immunoblot (4G10; Millipore/Upstate).
Quantitative PCR
Zebrafish ecscr, kdrl, and β-actin PrimeTime polymerase chain reaction (PCR) assay probe sets were custom designed using software on the Integrated DNA Technologies web page (http://www.idtdna.com/SciTools/SciTools.aspx?cat=DesignAnalyze). Ecscr probe sequence was /56-FAM/AAC AGC GTC CCT TGA GGT TGA CA/3IABlk_FQ/ and primers are GCGGTTTTTAATACTCTCGTG and GACTCACTTTACTAGCCTTTGG. Kdrl probe sequence was /56-FAM/CAG CTT TCA AGT GGC TAA AGG CAT GG/3IABlk_FQ/ and primers are CCAGATCACGGTGGATACAT and AAGAGGAGGAAGAGCAAGAG. Transcript levels were assayed using a Bio-Rad IQ5 platform. β-actin reference transcript was used to normalize transcript levels using the delta-deltaCt method. β-actin probe sequence was /56-FAM/TCA GCA ATG CCA GGG TAC ATG GT/3IABlk_FQ/ and primers were GGGAGCAATGATCTTGATCTTC and GTATGCCAACACAGTGCT.
Results
Embryonic expression of zebrafish ecscr is restricted to the vasculature
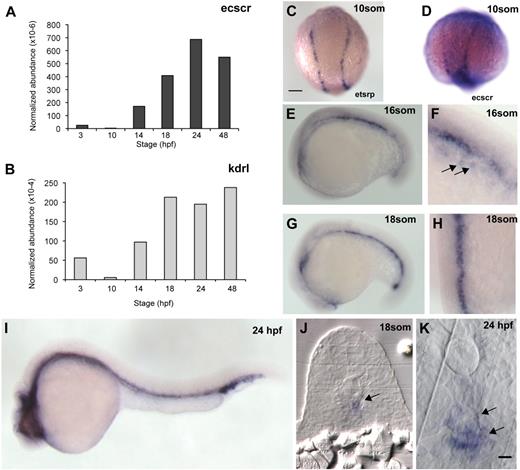
To investigate the expression levels of ecscr in zebrafish, we performed quantitative reverse-transcription (RT)–PCR measurement for ecscr and the endothelial marker kdr-like (kdrl)21 RNAs obtained from embryos of various developmental stages (Figure 1A-B). Developmental changes in normalized ecscr abundance roughly paralleled those of kdrl. From 10 to 24 hpf, the developmental interval in which the axial vessels are formed, ecscr message increases rapidly. ecscr message remained high in 7-day-old larvae, the latest stage tested (not shown). Using a semiquantitative RT-PCR and agarose gel analysis, we detected variable amounts of a second, smaller, PCR product in addition to the amplicon expected from the reported sequence12 of zebrafish ecscr (supplemental Figure 1). We designed our quantitative PCR (qPCR) assay and in situ probe (see next paragraph) to detect both endogenous ecscr transcripts.
Expression of zebrafish ECSCR. (A) qPCR measurement of ecscr transcript levels, normalized to beta-actin transcripts, during various stages of zebrafish development. Primer design is indicated in supplemental Figure 1. (B) Normalized qPCR of kdrl transcripts. (C) Dorsal view, with posterior up, of a 10-som embryo processed for ISH for the ets transcription factor etsrp16 showing the distribution of angioblasts in the posterior LPM. Scale bars for panels C through E, G, and I represent 200 μm. (D-I) Whole-mount ISH for ecscr message in zebrafish of the indicated stages. (D) 10-som, showing expression on premigratory angioblasts similar to etsrp+ cells. (E) Lateral view at 16 som. (F) Oblique view near the tail at 16 som, showing faint hybridization to migrating angioblasts ( ). (G) Lateral view at 18 som. (H) Dorsal view at 18 som. Hybridization is detected at the midline vascular cord. (I) Lateral view at 24 hpf. (J-K) Methacrylate sections of whole-mount in situs showing ecscr transcripts localized to midline vascular structures. (J) 18 som. (K) Higher magnification view of a 24-hpf section.
). (G) Lateral view at 18 som. (H) Dorsal view at 18 som. Hybridization is detected at the midline vascular cord. (I) Lateral view at 24 hpf. (J-K) Methacrylate sections of whole-mount in situs showing ecscr transcripts localized to midline vascular structures. (J) 18 som. (K) Higher magnification view of a 24-hpf section.  point to DA and PCV. Scale bar represents 10 μm.
point to DA and PCV. Scale bar represents 10 μm.
Expression of zebrafish ECSCR. (A) qPCR measurement of ecscr transcript levels, normalized to beta-actin transcripts, during various stages of zebrafish development. Primer design is indicated in supplemental Figure 1. (B) Normalized qPCR of kdrl transcripts. (C) Dorsal view, with posterior up, of a 10-som embryo processed for ISH for the ets transcription factor etsrp16 showing the distribution of angioblasts in the posterior LPM. Scale bars for panels C through E, G, and I represent 200 μm. (D-I) Whole-mount ISH for ecscr message in zebrafish of the indicated stages. (D) 10-som, showing expression on premigratory angioblasts similar to etsrp+ cells. (E) Lateral view at 16 som. (F) Oblique view near the tail at 16 som, showing faint hybridization to migrating angioblasts ( ). (G) Lateral view at 18 som. (H) Dorsal view at 18 som. Hybridization is detected at the midline vascular cord. (I) Lateral view at 24 hpf. (J-K) Methacrylate sections of whole-mount in situs showing ecscr transcripts localized to midline vascular structures. (J) 18 som. (K) Higher magnification view of a 24-hpf section.
). (G) Lateral view at 18 som. (H) Dorsal view at 18 som. Hybridization is detected at the midline vascular cord. (I) Lateral view at 24 hpf. (J-K) Methacrylate sections of whole-mount in situs showing ecscr transcripts localized to midline vascular structures. (J) 18 som. (K) Higher magnification view of a 24-hpf section.  point to DA and PCV. Scale bar represents 10 μm.
point to DA and PCV. Scale bar represents 10 μm.
To determine the spatial distribution of ecscr message in the developing embryo, we performed whole-mount in situ hybridization (ISH) on zebrafish embryos of various stages (Figure 1D-K). In 10-somite (som) embryos, we detect faint ecscr message in the LPM (Figure 1D), at positions where endothelial precursors expressing the ets-related protein-1 (etsrp)16 are observed (Figure 1C). We readily detected ecscr message at midline vascular structures from 16 to 18 som (Figure 1E-H). Faint ecscr signal is also detected on angioblasts approaching the midline (Figure 1F arrow). At 24 hpf (Figure 1I), ecscr message is detected on both trunk axial vessels, the DA and PCV. We did not detect ecscr in the intersomitic vessels at 24 hpf. This failure to detect ecscr in intersomitic vessels using ISH may be due to a low level of ecscr transcript, as has been observed previously with other vascular gene transcripts such as unc5b22 and syx.23 Cross sections of 18-som and 24-hpf fish (Figure 1J-K) showed ecscr expression restricted to midline endothelial structures, including the DA and PCV at 24 hpf (arrows in Figure 1K). Taken together, RT-PCR and whole-mount ISH confirmed the zebrafish ecscr expression by angioblasts and endothelial cells, as reported for the human and mouse orthologs.
Loss of ecscr function results in aberrantly positioned angioblasts in posterior LPM
We used an antisense morpholino (MO) approach24 to study the loss of ecscr function in developing zebrafish. We designed 2 morpholinos: one targeting the 5′ splice donor sequence of predicted exon 5 (MO1; supplemental Figure 1) and a second targeting the translational start codon (MO2). Both morpholinos target sequences shared by the 2 endogenous ecscr transcripts. Using semiquantitative RT-PCR, we found that injection of 10 ng of MO1 caused reduction of the wild-type ecscr transcript and forced production of alternative transcripts (supplemental Figure 1).
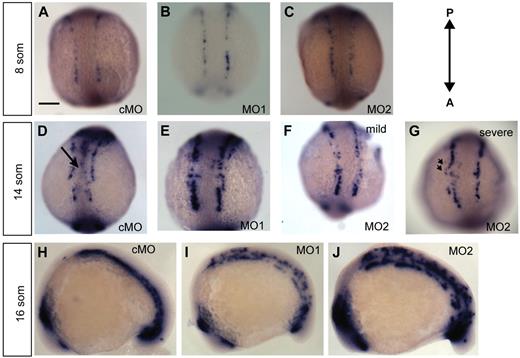
To assess the developing vasculature in morphant fish from 10 to 18 som, we performed whole-mount ISH using the early angioblast marker etsrp16 (C.Z.C., R.R., personal communication, October 15, 2009) (Figure 2A-F). Whole-mount ISH for etsrp revealed normal patterning and positioning of angioblasts in ecscr morphants at 8 som (Figure 2A-C). Numbers of angioblasts in ecscr morphants were not significantly different (28 ± 5 for cMO- versus 28 ± 4 for MO2-injected embryos; n = 5). At 14 som, when posterior angioblasts begin migrating toward the midline3 (C.Z.C., R.R., personal communication, October 15, 2009), cMO-injected fish showed angioblasts arriving at the developing midline vascular cord (Figure 2D black arrow). In contrast, escr MO1- and MO2-injected fish showed reduced or absent midline etsrp+ angioblasts (Figure 2D-F). In addition to the reduction of midline etsrp+ cells, MO2-injected embryos also showed etsrp+ cells positioned more laterally than expected, suggesting incorrect direction of movement (Figure 2G arrowheads). Malpositioned angioblasts were seen principally in the rostral portion of the posterior angioblast field. Anterior and tail bud angioblasts showed similar configuration between cMO- and MO2-injected embryos (supplemental Figure 2). Thus, the deficiency correlated with the location of the onset of centripetal angioblast migration.3,25
Knockdown of ECSCR2 results in aberrant positioning of migrating angioblasts. (A-G) Dorsal views of the posterior of control MO (cMO)–injected or ecscr morpholino (MO1, MO2) zebrafish embryos of the indicate stages subjected to whole-mount ISH for etsrp. (A-C) 8 som. Angioblasts are detected in apparently normal numbers and position in morpholino-injected embryos. Scale bar represents 200 μm. (D-G) 14 som. cMO-injected embryos show etsrp+ angioblasts arriving at the midline ( ) and convergence of the premigratory rows toward the midline, whereas anti-ecscr morphants show a greater gap between angioblast rows and few midline angioblasts. MO2 morphants showed a greater range of morpholino phenotypes, with more severely affected morphants (G) showing an irregular row of angioblasts with occasional angioblasts positioned away from the midline (
) and convergence of the premigratory rows toward the midline, whereas anti-ecscr morphants show a greater gap between angioblast rows and few midline angioblasts. MO2 morphants showed a greater range of morpholino phenotypes, with more severely affected morphants (G) showing an irregular row of angioblasts with occasional angioblasts positioned away from the midline ( in panel G). (H-J) Lateral view of 16-som embryos. cMO-injected embryos and the bulk of MO1- and MO2-injected embryos show a consolidated midline vascular cord3 (H), whereas approximately 20% of MO1 and MO2 morphants (I-J) show increased numbers of premigratory angioblasts lateral and dorsal to the consolidating vascular cord. Results at each embryonic stage are representative of at least 20 embryos from 2 independent injections.
in panel G). (H-J) Lateral view of 16-som embryos. cMO-injected embryos and the bulk of MO1- and MO2-injected embryos show a consolidated midline vascular cord3 (H), whereas approximately 20% of MO1 and MO2 morphants (I-J) show increased numbers of premigratory angioblasts lateral and dorsal to the consolidating vascular cord. Results at each embryonic stage are representative of at least 20 embryos from 2 independent injections.
Knockdown of ECSCR2 results in aberrant positioning of migrating angioblasts. (A-G) Dorsal views of the posterior of control MO (cMO)–injected or ecscr morpholino (MO1, MO2) zebrafish embryos of the indicate stages subjected to whole-mount ISH for etsrp. (A-C) 8 som. Angioblasts are detected in apparently normal numbers and position in morpholino-injected embryos. Scale bar represents 200 μm. (D-G) 14 som. cMO-injected embryos show etsrp+ angioblasts arriving at the midline ( ) and convergence of the premigratory rows toward the midline, whereas anti-ecscr morphants show a greater gap between angioblast rows and few midline angioblasts. MO2 morphants showed a greater range of morpholino phenotypes, with more severely affected morphants (G) showing an irregular row of angioblasts with occasional angioblasts positioned away from the midline (
) and convergence of the premigratory rows toward the midline, whereas anti-ecscr morphants show a greater gap between angioblast rows and few midline angioblasts. MO2 morphants showed a greater range of morpholino phenotypes, with more severely affected morphants (G) showing an irregular row of angioblasts with occasional angioblasts positioned away from the midline ( in panel G). (H-J) Lateral view of 16-som embryos. cMO-injected embryos and the bulk of MO1- and MO2-injected embryos show a consolidated midline vascular cord3 (H), whereas approximately 20% of MO1 and MO2 morphants (I-J) show increased numbers of premigratory angioblasts lateral and dorsal to the consolidating vascular cord. Results at each embryonic stage are representative of at least 20 embryos from 2 independent injections.
in panel G). (H-J) Lateral view of 16-som embryos. cMO-injected embryos and the bulk of MO1- and MO2-injected embryos show a consolidated midline vascular cord3 (H), whereas approximately 20% of MO1 and MO2 morphants (I-J) show increased numbers of premigratory angioblasts lateral and dorsal to the consolidating vascular cord. Results at each embryonic stage are representative of at least 20 embryos from 2 independent injections.
To quantify the effects of loss of ecscr function, angioblast defects in morpholino-injected embryos were scored as mild (no accumulation of midline angioblasts; Figure 2F) or severe (mislocalized angioblasts lateral to the orderly angioblast rows; arrow in Figure 2G). Mild and severely affected embryos were observed with increasing frequency upon injection with increasing doses of MO2 (Table 1). To confirm the specificity of the MO2 morpholino effect, we coinjected 100 pg of capped human ECSCR mRNA, which is not targeted by the zebrafish morpholinos, together with 10 ng of ecscr MO2. Coinjection resulted in amelioration of the morphant phenotype, including increased frequency of embryos scored unaffected and mildly affected (supplemental Figure 3 and Table 1). Furthermore, ISH for myeloblast determination protein (myoD) showed correct numbers of somites in cMO- and MO3-injected embryos (supplemental Figure 2), indicating a selective effect of morpholinos targeting ecscr on angioblast development.
Angioblast phenotypes in ECSCR loss of function
| Injection . | Phenotype, % of observed . | |||
|---|---|---|---|---|
| Normal . | Mild . | Severe . | Toxic . | |
| cMO 14 ng (36) | 83 | 6 | 0 | 11 |
| MO1 10 ng (20)* | 31 | 54 | 0 | 15 |
| MO2 10 ng (18)* | 22 | 61 | 17 | 0 |
| MO2 14 ng (17) | 29 | 24 | 24 | 24 |
| MO2 10 ng + hECSCR mRNA (37)† | 62 | 30 | 0 | 8 |
| Injection . | Phenotype, % of observed . | |||
|---|---|---|---|---|
| Normal . | Mild . | Severe . | Toxic . | |
| cMO 14 ng (36) | 83 | 6 | 0 | 11 |
| MO1 10 ng (20)* | 31 | 54 | 0 | 15 |
| MO2 10 ng (18)* | 22 | 61 | 17 | 0 |
| MO2 14 ng (17) | 29 | 24 | 24 | 24 |
| MO2 10 ng + hECSCR mRNA (37)† | 62 | 30 | 0 | 8 |
Zebrafish were injected with morpholinos as indicated, and scored at 14 som for etsrp+ angioblast positioning. Numbers in parentheses indicate the total number scored, and the incidence of each possible phenotype is given as a percentage of all scored embryos. Criteria: Embryos were scored as normal if there was evidence of a nascent midline vascular cord; mildly affected if midline angioblasts were sparse or absent; severely affected if individual angioblasts appeared lateral to the main row (ie, wrong direction); and toxic if the entire embryo appeared to be adversely affected.
ECSCR indicates endothelial-cell specific chemotaxis receptor.
Significant differences of observed distributions are indicated as follows: *P < .001 versus cMO injected;
P < .001 versus MO2 10 ng injected, using χ2 test excluding embryos scored as toxic.
By 16 som, most MO1- and MO2-injected fish showed etsrp+ angioblasts at the well-formed vascular cord, indicating recovery of the formation of midline structures. However, 31% of MO2 morphants and 42% of MO1 morphants still showed unmigrated or mislocalized angioblasts (Figure 2I-J), at a stage in which control embryos showed completed migration (Figure 2H)3 (C.Z.C., R.R., personal communication, October 15, 2009). Taken together, these results indicated that loss of ecscr function in zebrafish results in aberrantly positioned angioblasts and delayed arrival at the midline, without developmental delay in other structures.
ECSCR colocalizes with KDR at lamellar protrusions
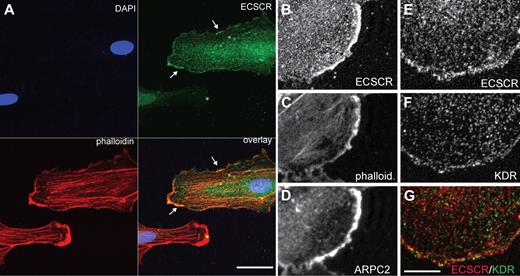
Migrating endothelial cells navigate their surroundings using surface receptors localized to specialized actin-rich membrane protrusions, including filopodia and lamellipodia.26 Protrusive lamellae depend on the actin reorganizing activity of the Arp2/3 complex.27,28 ECSCR has been shown to localize to the cell surface,10,12 raising the possibility that it could enhance migration through colocalization with receptors at specialized membrane domains. To investigate this possibility, we asked whether ECSCR localizes at lamellipodia (Figure 3). We overexpressed C-terminal–tagged full-length ECSCR in cultured HUVECs (supplemental Figure 3) and analyzed its localization using immunocytochemistry and confocal microscopy. ECSCR localized to actin-rich membrane protrusions (Figure 3A green and Figure 3B) as stained by phalloidin (Figure 3A red and Figure 3C). These actin-rich regions were further strongly immune reactive for p34ARPC2, a component of the Arp2/3 complex (Figure 3D), suggesting that they are sites of actin polymerization and active lamellipodial protrusion.
Transfected ECSCR and endogenous KDR colocalize at the edge of cultured endothelial cells. Human ECSCR cDNA was generated with a C-terminal V5 tag and overexpressed in HUVECs. (A) Triple label confocal micrograph comparing transfected ECSCR (green) to phalloidin (red) and DAPI (4,6 diamidino-2-phenylindole; blue). Transfected ECSCR is enriched in actin-rich membrane protrusions. Scale represents 40 μm. (B-D) Higher magnification view of a different cell labeled with anti-V5 (top), phalloidin (center), and ARPC2, a component of the Arp2/3 complex (bottom). ECSCR localizes to areas of active membrane protrusion. (E-G) Comparison of ECSCR with KDR. Confocal antitag (panel D and red signal in panel F) and antiendogenous KDR (panel E and green signal in panel F) cytochemistry. The punctate vesicular pattern of KDR overlaps with ECSCR at convex membrane protrusions where KDR approaches the membrane (seen as yellow), but not away from the cell edge. Scale bar represents 10 μm. Results were replicated in 3 independent experiments.
Transfected ECSCR and endogenous KDR colocalize at the edge of cultured endothelial cells. Human ECSCR cDNA was generated with a C-terminal V5 tag and overexpressed in HUVECs. (A) Triple label confocal micrograph comparing transfected ECSCR (green) to phalloidin (red) and DAPI (4,6 diamidino-2-phenylindole; blue). Transfected ECSCR is enriched in actin-rich membrane protrusions. Scale represents 40 μm. (B-D) Higher magnification view of a different cell labeled with anti-V5 (top), phalloidin (center), and ARPC2, a component of the Arp2/3 complex (bottom). ECSCR localizes to areas of active membrane protrusion. (E-G) Comparison of ECSCR with KDR. Confocal antitag (panel D and red signal in panel F) and antiendogenous KDR (panel E and green signal in panel F) cytochemistry. The punctate vesicular pattern of KDR overlaps with ECSCR at convex membrane protrusions where KDR approaches the membrane (seen as yellow), but not away from the cell edge. Scale bar represents 10 μm. Results were replicated in 3 independent experiments.
We also compared tagged ECSCR protein localization to that of endogenous KDR. KDR immune reactivity showed a punctate pattern throughout the cell (Figure 3F and Figure 3G green),29,30 with immune reactivity approaching the cell margin near membrane protrusions (LeClaire et al27 and Figure 3G green). ECSCR also showed a punctate pattern away from the cell edge. These ECSCR+ puncta did not overlap with KDR+ puncta (compare red and green in Figure 3G) except at the convex, protrusive membrane region (Figure 3G yellow).
Loss of ECSCR in cultured endothelial cells causes decreased motility, reduced apoptosis, and reduced proliferation
To inactivate ECSCR in cultured cells, we used BLOCK-iT Dicer RNAi reagents to generate small inhibitory RNAs spanning the ECSCR cDNA or control bacterial lacZ gene.17 We transfected siRNA duplexes into human umbilical vein (HUVECs) or pulmonary artery (HPAECs) endothelial cells. qPCR analysis showed that transfection of ECSCR siRNA resulted in reduction of ECSCR message by 70% relative to lacZ control (supplemental Figure 3). We first corroborated previous reports10,12,14 showing a role for ECSCR in cell migration in response to serum. Boyden chamber analysis showed that knockdown of ECSCR resulted in impaired serum-stimulated trans-migratory activity of both HUVECs and HPAECs (Figure 4A). We then performed additional analyses on HUVECs. ECSCR-silenced HUVECs showed a 35% reduction in migration in response to purified VEGF (Figure 4B). ECSCR has been reported to trigger proteasomal activation and ultimately enhance apoptosis.10 Consistent with this report, we found a highly significant reduction in annexin binding, an early cellular phenotype of apoptosis, in HUVECs lacking ECSCR compared with controls (Figure 4C). Finally, ECSCR knockdown cells showed a strong reduction in VEGF (10 ng/mL)–stimulated [3H]thymidine incorporation, indicating a decreased proliferative response in knockdown cells. We conclude that ECSCR functions to potentiate motility as well as increasing proliferation and proapoptotic events in cultured endothelial cells.
Knockdown of ECSCR in HUVECs results in reduced migration, apoptosis, and proliferation. (A) HUVECs and HPAECs were transfected with ECSCR siRNA or control lacZ siRNA and assayed for transmigration activity in Boyden chambers. ECSCR knockdown cells showed reduced transmigration activity relative to controls in response to 10% fetal bovine serum (HUVECs: 38% of control; HPAECs: 57% of control; P < .05 for both, Student paired t test). (B) ECSCR siRNA or control lacZ siRNA-transfected HUVEC transmigration activity in response to purified VEGF (25 ng/mL). (ECSCR knockdown, 117% of unstimulated vs 157% of unstimulated for control knockdown; P < .05.) (C) Knockdown cells were contact inhibited then analyzed for annexin translocation by flow cytometry. ECSCR knockdown resulted in strongly reduced apoptosis compared with lacZ controls (11% annexin positive vs 36% in controls; P < .01). (D) HUVECs were transfected with siRNA constructs, contact inhibited, serum starved, and then released from inhibition in the presence or absence of VEGF. Proliferation was assayed by incorporation of [3H]thymidine. ECSCR knockdown resulted in greatly decreased VEGF-stimulated thymidine incorporation (104% of unstimulated vs 281% in control cells; P < .001). Results are representative of 3 independent experiments.
Knockdown of ECSCR in HUVECs results in reduced migration, apoptosis, and proliferation. (A) HUVECs and HPAECs were transfected with ECSCR siRNA or control lacZ siRNA and assayed for transmigration activity in Boyden chambers. ECSCR knockdown cells showed reduced transmigration activity relative to controls in response to 10% fetal bovine serum (HUVECs: 38% of control; HPAECs: 57% of control; P < .05 for both, Student paired t test). (B) ECSCR siRNA or control lacZ siRNA-transfected HUVEC transmigration activity in response to purified VEGF (25 ng/mL). (ECSCR knockdown, 117% of unstimulated vs 157% of unstimulated for control knockdown; P < .05.) (C) Knockdown cells were contact inhibited then analyzed for annexin translocation by flow cytometry. ECSCR knockdown resulted in strongly reduced apoptosis compared with lacZ controls (11% annexin positive vs 36% in controls; P < .01). (D) HUVECs were transfected with siRNA constructs, contact inhibited, serum starved, and then released from inhibition in the presence or absence of VEGF. Proliferation was assayed by incorporation of [3H]thymidine. ECSCR knockdown resulted in greatly decreased VEGF-stimulated thymidine incorporation (104% of unstimulated vs 281% in control cells; P < .001). Results are representative of 3 independent experiments.
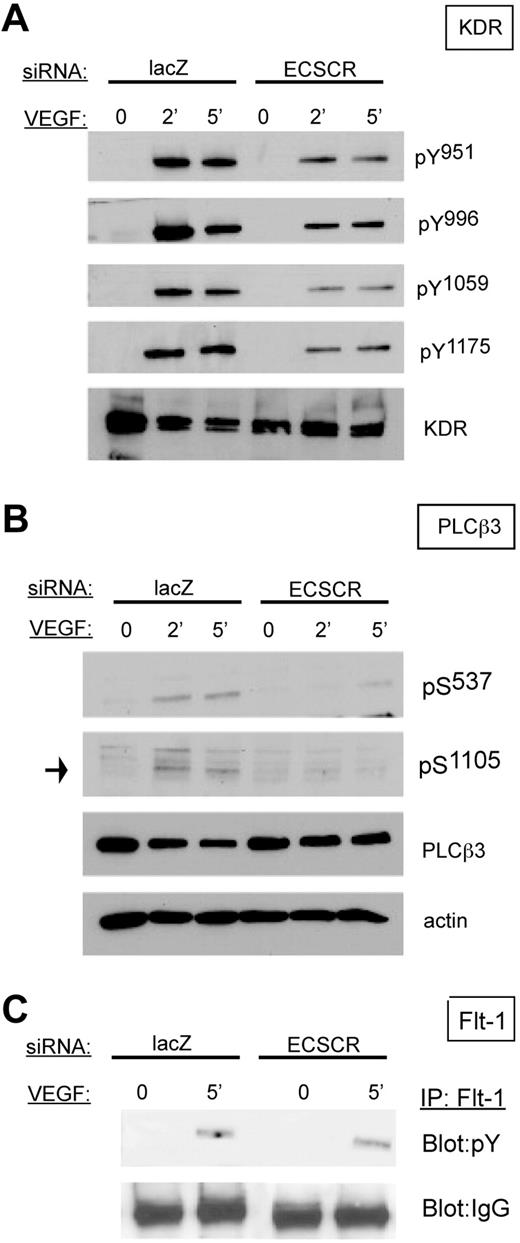
ECSCR-deficient cells show reduced KDR phosphorylation, but not FLT1 phosphorylation, upon VEGF stimulation
Because VEGF receptor activation is a potent promigratory stimulus for endothelial cells, and KDR partially colocalizes with ECSCR in transfected cells, we examined the biochemical response of ECSCR-silenced HUVECs to VEGF (Figure 5). In starved control cells, stimulation with VEGF for 2 or 5 minutes resulted in robust increases of KDR tyrosine phosphorylation at tyrosines 951, 996, 1059, and 1175 (Figure 5A). In contrast, ECSCR-silenced cells showed markedly reduced tyrosine phosphorylation at these residues. Furthermore, activation of PLCβ3, a promigratory effector,31 was sharply reduced (Figure 5B). In contrast, FLT1 tyrosine phosphorylation was not affected (Figure 5C). Thus loss of ECSCR affects VEGF-stimulated activation of KDR, but not of FLT1.
Knockdown of ECSCR in HUVECs results in reduced VEGF-induced phosphorylation of VEGF receptor 2/KDR and downstream PLCβ3, but not tyrosine phosphorylation of VEGF receptor 1/FLT1. (A) HUVECs transfected with the indicated siRNAs were serum starved overnight and stimulated with 25 ng/mL VEGF for the indicated periods. Total lysates were blotted using the indicated phosphoepitope-specific antisera. ECSCR knockdown cells show strongly reduced tyrosine phosphorylation of KDR on multiple epitopes. (B) Total lysates as described in panel A were blotted using anti-PLCβ3 phosphoepitopes. Activating phosphorylation of serines 537 and 1105 was reduced in ECSCR knockdown cells. (C) FLT1 was immune precipitated from unstimulated and VEGF-stimulated cells (5 minutes), and immune precipitates were blotted for phosphotyrosine. Tyrosine-phosphorylated FLT1 was present at similar levels in VEGF-stimulated control lacZ and ECSCR knockdown cells. Results were observed in at least 2 independent experiments.
Knockdown of ECSCR in HUVECs results in reduced VEGF-induced phosphorylation of VEGF receptor 2/KDR and downstream PLCβ3, but not tyrosine phosphorylation of VEGF receptor 1/FLT1. (A) HUVECs transfected with the indicated siRNAs were serum starved overnight and stimulated with 25 ng/mL VEGF for the indicated periods. Total lysates were blotted using the indicated phosphoepitope-specific antisera. ECSCR knockdown cells show strongly reduced tyrosine phosphorylation of KDR on multiple epitopes. (B) Total lysates as described in panel A were blotted using anti-PLCβ3 phosphoepitopes. Activating phosphorylation of serines 537 and 1105 was reduced in ECSCR knockdown cells. (C) FLT1 was immune precipitated from unstimulated and VEGF-stimulated cells (5 minutes), and immune precipitates were blotted for phosphotyrosine. Tyrosine-phosphorylated FLT1 was present at similar levels in VEGF-stimulated control lacZ and ECSCR knockdown cells. Results were observed in at least 2 independent experiments.
Treatment of zebrafish embryos with VEGF receptor inhibitors results in reduction in angioblast numbers and reduced midline angioblasts at 14 hpf
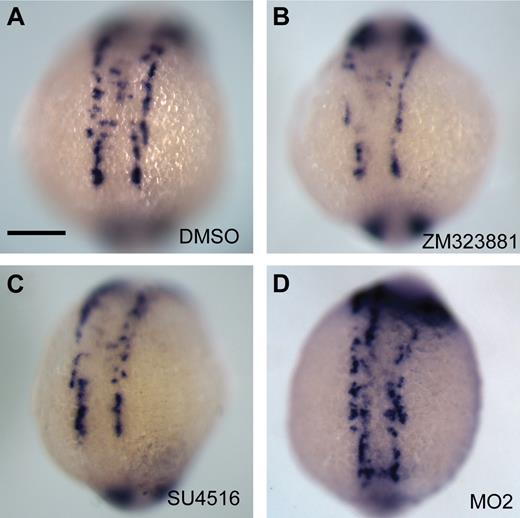
Morpholino knockdown of ecscr in zebrafish resulted in deficiencies during vasculogenesis, including delayed arrival at the midline and delayed consolidation of the midline cord, which are suggestive of loss of angioblast sensitivity to VEGF.3,6 To examine this resemblance in greater detail, we treated zebrafish embryos with the VEGF receptor inhibitors SU5416 (10 μm), which inhibits VEGF and PDGF receptors, and ZM323881 (1 μm), a selective inhibitor of VEGF-R2. We analyzed angioblast positioning at 14 som using whole-mount ISH for etsrp (Figure 6). Similarly to ecscr morphant embryos, in more than 80% of embryos treated with either compound, etsrp+ angioblasts were absent from the midline. However, this observation is complicated by a reduction of angioblast numbers in inhibitor-treated embryos, which was not seen in ecscr morphants. Counts of etsrp+ posterior angioblasts in embryos treated with either inhibitor compound showed significant reductions in numbers of angioblasts compared with embryos treated with dimethyl sulfoxide (DMSO; 46.8 ± 2.4 etsrp+ profiles in DMSO-treated embryos versus 31.0 ± 4.1 in embryos treated with ZM323881 and 38.4 ± 3.0 in embryos treated with SU5416; P < .01 for ZM323881 and P < .05 for SU5416; n = 5 for all groups). In contrast, ecscr morphant fish showed 43.2 (± 4.3) angioblasts, which is not significantly different from DMSO-treated fish. These results are consistent with the possibility that angioblasts in ecscr morphants have lost sensitivity to VEGF for migration, but not for maintenance of angioblast number (see also “Discussion”).
Inhibition of VEGF receptors in zebrafish results in reduced etsrp+ angioblast numbers including reduced numbers at the midline. Embryos were treated from 10 to 16 hpf with vehicle control DMSO (A) or VEGF receptor inhibitors ZM323881 (1 μm; B) or SU4516 (10 μm; C), and angioblast positions were analyzed by whole-mount ISH for etsrp. Inhibitor-treated embryos show reduced angioblast numbers, and strongly reduced etsrp+ cells at the midline. (D) ecscr morphants show disrupted anterior vascular cord. Each result is representative of more than 20 embryos from 2 independent experiments. Scale bar in panel A represents 200 μm.
Inhibition of VEGF receptors in zebrafish results in reduced etsrp+ angioblast numbers including reduced numbers at the midline. Embryos were treated from 10 to 16 hpf with vehicle control DMSO (A) or VEGF receptor inhibitors ZM323881 (1 μm; B) or SU4516 (10 μm; C), and angioblast positions were analyzed by whole-mount ISH for etsrp. Inhibitor-treated embryos show reduced angioblast numbers, and strongly reduced etsrp+ cells at the midline. (D) ecscr morphants show disrupted anterior vascular cord. Each result is representative of more than 20 embryos from 2 independent experiments. Scale bar in panel A represents 200 μm.
Discussion
In this study, we have shown that ECSCR, a cell surface protein selectively expressed on endothelial cells, affects migration and other responses to VEGF. Deficient migration resultant from ECSCR loss of function is seen in both cultured mature endothelial cells and in angioblasts in vivo. ECSCR-deficient cells show reduced VEGF-stimulated tyrosine phosphorylation of KDR/VEGF receptor 2, but not FLT-1/VEGF receptor 1. We further suggest the plasma membrane of protrusive lamellae as a site of local interaction between ECSCR and KDR.
The expression pattern for zebrafish ecscr function and phenotype of morphant embryos indicates that ecscr functions during vasculogenic migration of angioblasts to the midline. Ecscr is expressed on angioblasts and developing blood vessels. Knockdown of ecscr results in delayed angioblast arrival at the trunk midline at 14 som, with more severely affected embryos showing malpositioned angioblasts lateral to the orderly main rows. Importantly, the anterior angioblast pool appears unaffected by loss of ecscr function, underscoring the emerging concept of heterogeneity in angioblast pools along the anteroposterior axis,32-34 including possible distinct contributions from the VEGF/VEGF receptor axis34,35 (C.Z.C., R.R., personal communication, October 15, 2009). Our knockdown results suggest that ecscr function is required before consolidation of the midline vascular cord, and is consistent with a recent study of ECSCR biology in tube formation by cultured endothelial cells.12 Normalized expression of ecscr continues to increase, in tandem with kdrl expression, throughout the embryonic segmentation stages (Figure 1A-B) and is seen in adult endothelium,10,12 suggesting a continued role of this gene product.
The migration of angioblasts to form the vascular cord remains a poorly understood process. Of extrinsic factors attracting angioblasts, VEGF is of critical importance. Inhibition of VEGF signaling can modulate angioblast differentiation and survival36 as well as specific cellular behaviors of migrating angioblasts,37 and ectopic VEGF is sufficient to attract angioblasts in vivo.9 The phenotype of ecscr morphants (Figure 2 and supplemental Figure 2) and results with knockdown cultured cells suggest reduced responsiveness to VEGF in cells lacking ECSCR. In support of this concept, embryos treated with inhibitors of VEGF receptors also show reduced numbers of midline angioblasts. However, inhibitor-treated embryos also showed strongly reduced angioblast numbers, which was not seen in ecscr morphants (Figure 6). Inhibited signaling by VEGF receptors could thus account for the altered migration of angioblasts, if not the angioblast numbers, seen in ecscr morphant embryos.
Silencing of ECSCR in cultured HUVECs results in decreased migration, but proliferation, apoptosis, and KDR phosphorylation in response to VEGF are affected. The differential results seen in our fish and our cultured cell experiments may indicate a context dependence of ecscr signaling distinguishing angioblasts from differentiated endothelial cells. In support of this idea, mouse ecscr can trigger degradation of the antiapoptotic proteins cellular inhibitor of apoptosis 1 (cIAP-1) and cIAP-2 in mature endothelial cells, thereby promoting apoptosis.10 However, the zebrafish homolog of the cIAPs, birc2, is not detected until 60 hpf,38 well after the ecscr functions investigated here. To clarify the signaling properties of ecscr during angioblast migration, it will be important to identify molecular criteria that distinguish angioblasts from differentiated endothelial cells.34,39
Transfected ECSCR is detected on the entire perimeter of transfected endothelial cells, with some enrichment in actin-rich protrusive lamellae (Figure 4). These same structures are the principal site of colocalization of ECSCR with KDR. Interestingly, VEGF stimulation promotes lamellar protrusions in angioblasts37 and in sprouting endothelial cells.40,41 Thus, ECSCR via its cytoplasmic interactors,10,12,14 or via extracellular interactions, could locally influence KDR activation.
In conclusion, the data presented here identify a new cell surface protein important for vasculogenic migration. Embryonic vasculogenesis is important both in its own right and for its potential relevance for adult processes such as malformations.42-44 It will be of considerable interest to relate ecscr to the hierarchy of known signals responsible for the generation of the initial vasculature in fish and mammals.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Suresh Kumar of the Children's Research Institute Imaging core for assistance with confocal microscopy.
This work was supported by the Children's Research Institute at the Medical College of Wisconsin (seed funds), National Institutes of Health grant HL090712 (R.R.), National Institutes of Health grants HL072178 and HL70567, and by a generous gift from Bruce and Martha Atwater (D.M.). G.V.S. is a recipient of the State of Wisconsin Breast Cancer Research Tax Write-off Program Award. C.Z.C is partly supported by funds from Advancing Healthier Wisconsin grant awarded to R.R.
National Institutes of Health
Authorship
Contribution: A.V., R.B., R.Q.M., D.M., R.R., and G.A.W. designed research; A.V., R.B., I.R., K.L., K.P., G.V.S., M.H, C.Z.C, B.Z., and E.W performed research; A.V., R.B., and G.A.W. analyzed the data; and G.A.W. and R.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George Wilkinson, Department of Pediatrics, CRI Developmental Vascular Biology Program, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: gwilkins@mcw.edu.

![Figure 4. Knockdown of ECSCR in HUVECs results in reduced migration, apoptosis, and proliferation. (A) HUVECs and HPAECs were transfected with ECSCR siRNA or control lacZ siRNA and assayed for transmigration activity in Boyden chambers. ECSCR knockdown cells showed reduced transmigration activity relative to controls in response to 10% fetal bovine serum (HUVECs: 38% of control; HPAECs: 57% of control; P < .05 for both, Student paired t test). (B) ECSCR siRNA or control lacZ siRNA-transfected HUVEC transmigration activity in response to purified VEGF (25 ng/mL). (ECSCR knockdown, 117% of unstimulated vs 157% of unstimulated for control knockdown; P < .05.) (C) Knockdown cells were contact inhibited then analyzed for annexin translocation by flow cytometry. ECSCR knockdown resulted in strongly reduced apoptosis compared with lacZ controls (11% annexin positive vs 36% in controls; P < .01). (D) HUVECs were transfected with siRNA constructs, contact inhibited, serum starved, and then released from inhibition in the presence or absence of VEGF. Proliferation was assayed by incorporation of [3H]thymidine. ECSCR knockdown resulted in greatly decreased VEGF-stimulated thymidine incorporation (104% of unstimulated vs 281% in control cells; P < .001). Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/22/10.1182_blood-2009-10-248856/4/m_zh89991050180004.jpeg?Expires=1769182575&Signature=4GpszKFVFZTGIwE2NG7u9dQvlnl1QpR8g-fyTkNJyupqzeDRk4OqBpsq806-wsK18~13oV05zkYzJhhSuOyHhg23OjrBGQ73mGHvIBD80dPbcU6ai7nwK5SlXsN3AeKR~H561v7g2izO3vLrWATmDAn8B4QHanaSvhae2aPS20z1~FM7yLRIwccFBhQIkWNCrCB1j7fHgRH7Uo~-gPBQU-vs1zISxTeJdFeZDpSjTZeIB0mwyFvUA0B4yUaxRKl-Hen0yF1bjfwqhek1~97~j0IpkPTVdzbHkhdnSG2KAIS19fGjtAJ9qoS3PYKcbiJKT8E0Q0Ie7MUhkQbb84wWmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)