Abstract
Through its binding with protein S (PS), a key element of the coagulation/fibrinolysis cascade, the C4b-binding protein (C4BP) has been hypothesized to be involved in the susceptibility to venous thrombosis (VT). To identify genetic factors that may influence the plasma levels of the 3 C4BP existing isoforms, α7β1, α6β1, and α7β0, we conducted a genome-wide association study by analyzing 283 437 single nucleotide polymorphisms (SNPs) in the Genetic Analysis of Idiopathic Thrombophilia (GAIT) study composed of 352 persons. Three SNPs at the C4BPB/C4BPA locus were found genome-wide significantly associated with α7β0 levels. One of these SNPs was further found to explain approximately 11% of the variability of mRNA C4BPA expression in the Gutenberg Heart Study composed of 1490 persons, with no effect on C4BPB mRNA expression. The allele associated with increased α7β0 plasma levels and increased C4BPA expression was further found associated with increased risk of VT (odds ratio [OR] = 1.24 [1.03-1.53]) in 2 independent case-control studies (MARseille THrombosis Association study [MARTHA] and FActeurs de RIsque et de récidives de la maladie thromboembolique VEineuse [FARIVE]) gathering 1706 cases and 1379 controls. This SNP was not associated with free PS or total PS. In conclusion, we observed strong evidence that the C4BPB/C4BPA locus is a new susceptibility locus for VT through a PS-independent mechanism that remains to be elucidated.
Introduction
Protein S (PS), a vitamin K–dependent plasma protein, is a key regulator of the coagulation/fibrinolysis cascade. PS has no enzymatic activity but acts mainly as a cofactor for activated protein C (APC).1 It participates in the inactivation of factors Va (FVa) and FVIIIa, leading to inhibition of thrombin generation.2-4 Even in absence of APC, PS could still inhibit thrombin generation by decreasing the conversion of prothrombin to thrombin and that of FX to FXa.5 PS is also a cofactor for tissue factor pathway inhibitor (TFPI) for inhibiting FXa.6
In plasma, PS circulates either in a free form (fPS; ∼ 40%) or as a complex with the C4b-binding protein (C4BP; ∼ 60%).7-10 It was initially thought that the C4BP-bound PS was not active and that only the circulating fPS was.7-9 However, more recent works shed new light on a more complex mechanism, as C4BP-PS complex was shown to directly participate in the FVa and FVIIIa inactivation,11 and C4BP could inhibit APC-catalyzed FVa inactivation even in the absence of PS.12 Such complex and elusive mechanisms could explain why, despite the key role of PS in the regulation of thrombin generation, its involvement in the susceptibility of venous thrombosis (VT) remains obscure. Although PS deficiency is considered a risk factor for VT,13-15 total PS and fPS show very little correlation with VT.16,17
C4BP is a large plasma protein existing in 3 different isoforms, α7β1, α7β0, and α6β1, according to the number of identical α-chains (6 or 7) and the presence/absence of a single β-chain.18 The main isoform is α7β1, whereas the α7β0 isoform lacking the β-chain represents approximately 17% of the C4BP molecules.7,18 Whereas the α-chain binds for many ligands,19 the β-chain is specific for the binding of PS. As a consequence, the amount of β-chain–containing isoforms can be viewed as a surrogate marker for fPS levels. Plasma levels of all 3 isoforms have documented high heritability estimates (∼ 40% each),20 but little is know about their genetic determinants other that they seem to segregate with the C4BPA and C4BPB genes in 1q32.21
The objective of this work was to search for single nucleotide polymorphisms (SNPs) that could influence plasma levels of C4BP isoforms and to investigate whether such SNPs could influence the risk of VT. Our working hypothesis was that SNP(s) associated with both plasma levels of some specific C4BP isoform(s) and VT risk would provide new insights into the mechanisms relating PS (either fPS, total PS, or both) to VT susceptibility. For this purpose, a genome-wide association study (GWAS) of plasma levels of C4BP isoforms was first performed in Spanish pedigrees from the Genetic Analysis of Idiopathic Thrombophilia (GAIT) study22 in parallel with a SNP-expression association analysis of mRNA levels of the C4BPA and C4BPB genes coding for the α- and β-chains, respectively, in the Gutenberg Heart Study.23 These analyses were followed up in 3 independent samples with an in silico GWAS and 2 case-control candidate gene studies for VT.24
Methods
Subjects
All studies were approved by the institutional ethics committees of all participating institutions and their extensive descriptions have been provided elsewhere: in Souto et al22 for the GAIT study; in Trégouët et al24 for the GWAS, MARseille THrombosis Association study (MARTHA), and FActeurs de RIsque et de récidives de la maladie thromboembolique VEineuse (FARIVE) studies; and in Zeller et al23 for the Gutenberg Heart Study. A brief description of these studies follows.
GAIT study.
The GAIT study is composed of 21 extended Spanish pedigrees including 352 persons. Twelve families were ascertained though a proband with idiopathic thrombophilia, whereas 9 families were randomly selected. Thrombophilia was defined either as multiple thrombotic events, a single spontaneous episode with at least 1 affected first-degree relative, or an early age of onset (< 45 years). Idiopathic status of a person was defined as absence of known risk factors such as antithrombin, protein C or protein S deficiencies, activated PC resistance, factor V Leiden, and lupus anticoagulant. In the entire sample, 33 persons had venous thrombosis. Participant age ranged from 1 to 87 years, with a median of 38 years.
Gutenberg Heart Study.
This report was based on the analysis of 1490 unrelated healthy controls of German origin who participated in the community-based Gutenberg Heart Study (GHS) conducted in the Rhein-Mainz region in western mid-Germany. All subjects were aged between 35 and 74 years. Participants ascertained from the registers of the local registry offices were invited for a 5-hour baseline examination where clinical examinations and collection of blood samples were performed. DNA and mRNA were collected for all persons.
In silico GWAS data.
For this part, we used data that were already available (in silico) from our previous GWAS on VT.24 Briefly, 419 VT cases were compared with 1228 healthy controls, all expected to be of European origin. Cases were patients with documented early age of onset (< 50 years) objectively diagnosed VT—including deep vein thrombosis (DVT) and/or pulmonary embolism (PE)—recruited from 4 different French medical centers (Grenoble, Marseille, Montpellier, and Paris) between 1999 and 2006. Exclusion criteria were acquired risk factors and known genetic risk factors, that is, antithrombin, protein C, or protein S deficiencies, and homozygosity for factor V Leiden or FII 20210A. Controls were French persons selected from the Suvimax study.25
MARTHA study.
The MARTHA study is a case-control study including 1150 cases and 801 controls. Cases were unrelated whites consecutively recruited from the Thrombophilia center of La Timone hospital between January 1994 and October 2005. Cases were patients without known genetic risk factor (In silico GWAS data) and lupus anticoagulant. Thrombotic events including DVT and PE were documented by venography, Doppler ultrasound, spiral computed tomographic scanning angiography, and/or ventilation/perfusion lung scan. Controls were healthy French persons without a personal history of cardiovascular disease (including VT) selected from the Marseille area and from the national health examination centers of French Social Security in collaboration with the Hemostasis and Thrombosis Study Group.
FARIVE study.
The FARIVE study is a multicenter case-control study involving consecutive subjects treated as inpatients or outpatients for a first episode of proximal DVT and/or PE. Six hundred seven VT cases were compared with 607 healthy persons. Diagnosis for DVT was made through venography and compression-ultrasonography, and for PE through spiral computed tomography, high-probability ventilation/perfusion lung scan, pulmonary angiography, and compatible physical findings in a patient with proven DVT. Patients younger than 18 years, having already had VT, a diagnosis of active cancer, or a history of malignancy fewer than 5 years previously, or having a short life expectancy because of other causes were excluded. The control group consisted of age- and sex-matched persons and had to be free of personal and family history of venous and arterial thrombotic disease, and free of cancer and liver or kidney failure.
C4BP measurements
A detailed description of C4BP measurements has previously been reported.20 Briefly, plasma levels of C4BPα and C4BPβ were obtained using mouse anti–human antibodies. The percentage of C4BP β-chain lacking isoform, %α7β0, was estimated from the ratio (C4BPα − C4BPβ)/C4BPα. It can be viewed as the molar excess of C4BPα-chain. In addition to C4BP measurements, fPS are total PS levels were also measured through the use of enzyme-linked immunosorbent assays from Diagnostica Stago.22
Genotyping
Pedigrees members of the GAIT study were typed with the Illumina Infinium 317 Beadchip as previously described.26 Single nucleotide polymorphisms (SNPs) with a call rate less than 0.95, a minor allele frequency less than 0.025 or deviating from Hardy-Weinberg equilibrium (HWE) at P less than 5 × 10−7 were excluded, resulting into 283 437 SNPs left for analysis.
GHS participants were typed with the Affymetrix Genome-Wide Human SNP Array 6.0 as previously described.23 SNPs with a call rate less than 0.98, a minor allele frequency less than 0.01, or deviating from Hardy-Weinberg equilibrium at P less than 10−4 were excluded from the analysis.
Persons for the initial GWAS analysis on VT were typed with the Illumina Sentrix HumanHap300 Beadchip with genotyping quality control criteria previously described.24 In MARTHA and FARIVE, rs3813948 genotyping was performed using TaqMan technology (Applied Biosystems) with genotype success rate of 98.7% and 99.9%, respectively.
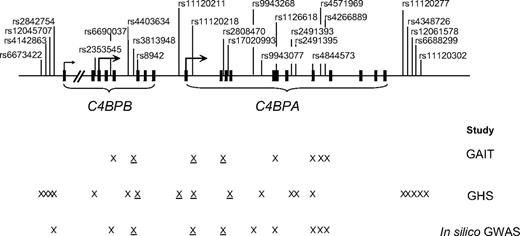
The location of all C4BPB/C4BPA SNPs studied in this report is shown in Figure 1.
Location of the studied C4BPB/C4BPA polymorphisms. Exons are denoted as squares. X represents polymorphisms that were genotyped in a given study. Underlined X denotes polymorphisms showing association with any of the studied phenotypes.
Location of the studied C4BPB/C4BPA polymorphisms. Exons are denoted as squares. X represents polymorphisms that were genotyped in a given study. Underlined X denotes polymorphisms showing association with any of the studied phenotypes.
mRNA expression analysis
Detailed description of monocyte isolation and RNA extraction and preparation can be found in Zeller et al.23 Monocytes RNA profiles were generated using Illumina Human HT-12 expression V3 BeadChips. The preprocessing of the expression data and the application of several filters resulted in the identification of 12 808 genes expressed in monocytes.23 Among the genes were C4BPB, C4BPA, and PROS1 (the gene encoding for protein S).
Statistical analysis
In all studies, deviation from Hardy-Weinberg equilibrium (HWE) was investigated using a standard χ2 with 1 degree of freedom. In the GAIT study, HWE was tested using parental data only.
In the GAIT study, association between SNPs and C4BP-related phenotype levels was tested using a measured genotype association analysis assuming additive allele effects. This analysis was carried out using the variance-components methodology implemented in the SOLAR Version 4.0 software (Southwest Foundation for Biomedical Research, http://solar.sfbrgenetics.org/download.html).27 Analyses were adjusted for age, sex, and smoking, and in females, for oral contraception as well. Any SNP was considered significantly associated with a C4BP phenotype when its corresponding P value was less than 1.8 × 10−7; this threshold corresponds to a family-wise error rate of .05 after applying the Bonferroni correction for the number of tested SNPs.
In GHS, association between SNPs and mRNA expression levels was tested by 1-way analysis of variance with 2 degrees of freedom. Single locus association analyses of SNPs with mRNA expression were followed by haplotype analyses to handle linkage disequilibrium (LD) between SNPs. For this purpose, THESIAS software (Inserm UMR_S 937, http://genecanvas.ecgene.net/downloads.php?cat_id=1) was used.28 Haploview software (Broad Institute of MIT and Harvard, http://www.broadinstitute.org/haploview/haploview)29 was used to quantify and display the extent of linkage disequilibrium (LD) between studied SNPs.
In the GWAS and the 2 independent case-control studies for VT, the Cochran-Armitage trend test30 was used to test for the association of studied SNPs with VT. When appropriate, the homogeneity of the genetic effects across MARTHA and FARIVE, and according to subgroups defined by categoric variables such sex, FV Leiden, FII 20210 mutations, and ABO blood group, was tested using the Mantel-Haenszel method.31
Results
Family-based genome-wide association analysis of C4BP phenotypes
Results of the genome-wide association analyses are summarized in Figure 2. No SNP was found associated with plasma C4BPα or C4BPβ at a genome-wide significance level (Figure 2A and B, respectively). However, analysis of %α7β0 revealed 3 SNPs achieving the a priori genome-wide significance threshold of 1.8 × 10−7 (Figure 2C). These SNPs were all located on chromosome 1q32.2 in the C4BPB/C4BPA gene cluster. One of them, rs3813948, is located within the C4BPB gene, whereas the others, rs11120218 and rs2808470, are located in the C4BPA (Table 1), all intronic SNPs. For each of these 3 SNPs, the minor allele was significantly associated with increased levels of %α7β0. In addition, the minor allele of each of the 2 C4BPA SNPs showed association with increased C4BPα levels (P ∼ 10−3) but did not reach genome-wide statistical significance (Table 1). In contrast, these SNPs were not associated with fPS or with total PS levels (Table 1).
Manhattan plots obtained from the genome-wide association analyses of the plasma levels of C4BPα (A), C4BPβ (B), and %α7β0 (C) performed in the GAIT study. The solid horizontal line indicates the genome-wide statistical significance level of 1.8 × 10−7.
Manhattan plots obtained from the genome-wide association analyses of the plasma levels of C4BPα (A), C4BPβ (B), and %α7β0 (C) performed in the GAIT study. The solid horizontal line indicates the genome-wide statistical significance level of 1.8 × 10−7.
Association* of the C4BPB/C4BPA SNPs identified through the GAIT GWAS with plasma levels of C4BPα, C4BPβ, α7β0, free PS, and total PS
| Gene/rsID (position, bp)/genotype . | Phenotype . | ||||
|---|---|---|---|---|---|
| C4BPα . | C4BPβ . | %α7β0 . | fPS . | Total PS . | |
| C4BPB | |||||
| rs3813948 (205 336 481) | |||||
| TT (n = 313) | 323.1 (79.37) | 256.4 (51.94) | 19.3 (10.24) | 97.0 (21.27) | 102.2 (19.73) |
| TC (n = 37) | 335.5 (80.67) | 231.1 (42.06) | 30.0 (7.35) | 92.7 (19.25) | 94.5 (19.12) |
| CC (n = 2) | 354.8 (63.92) | 238.0 (58.69) | 33.3 (4.53) | 97.0 (16.97) | 93.5 (16.26) |
| R2, % | 0.87 | 1.56 | 10.98 | 0.01 | 0.71 |
| P | .0687 | .0326 | 4.37 × 10−10 | .9321 | .1842 |
| C4BPA | |||||
| rs11120218 (205 345 074) | |||||
| GG (n = 235) | 315.1 (77.42) | 252.9 (52.01) | 18.5 (10.41) | 97.5 (21.35) | 101.4 (19.35) |
| GA (n = 112) | 341.9 (77.95) | 255.6 (50.34) | 24.2 (9.34) | 94.7 (22.44) | 101.4 (20.78) |
| AA (n = 5) | 383.8 (124.03) | 244.7 (62.06) | 35.3 (5.76) | 93.0 (19.45) | 97.4 (19.50) |
| R2, % | 3.81 | 0.01 | 10.62 | 0.41 | 0.00 |
| P | 5.64 × 10−4 | .8979 | 1.55 × 10−9 | .1999 | .5523 |
| rs2808470 (205 353 810) | |||||
| CC (n = 221) | 315.8 (77.21) | 254.0 (52.17) | 18.3 (10.64) | 97.0 (20.91) | 102.1 (18.95) |
| CT (n = 122) | 338.6 (79.96) | 253.8 (50.71) | 23.9 (8.99) | 95.9 (21.55) | 101.9 (21.25) |
| TT (n = 9) | 345.2 (108.26) | 234.6 (52.41) | 30.2 (10.96) | 97.3 (20.34) | 101.4 (21.15) |
| R2, % | 2.77 | 0.01 | 9.80 | 0.12 | 0.02 |
| P | 1.35 × 10−3 | .9615 | 3.36 × 10−9 | .4676 | .6502 |
| Gene/rsID (position, bp)/genotype . | Phenotype . | ||||
|---|---|---|---|---|---|
| C4BPα . | C4BPβ . | %α7β0 . | fPS . | Total PS . | |
| C4BPB | |||||
| rs3813948 (205 336 481) | |||||
| TT (n = 313) | 323.1 (79.37) | 256.4 (51.94) | 19.3 (10.24) | 97.0 (21.27) | 102.2 (19.73) |
| TC (n = 37) | 335.5 (80.67) | 231.1 (42.06) | 30.0 (7.35) | 92.7 (19.25) | 94.5 (19.12) |
| CC (n = 2) | 354.8 (63.92) | 238.0 (58.69) | 33.3 (4.53) | 97.0 (16.97) | 93.5 (16.26) |
| R2, % | 0.87 | 1.56 | 10.98 | 0.01 | 0.71 |
| P | .0687 | .0326 | 4.37 × 10−10 | .9321 | .1842 |
| C4BPA | |||||
| rs11120218 (205 345 074) | |||||
| GG (n = 235) | 315.1 (77.42) | 252.9 (52.01) | 18.5 (10.41) | 97.5 (21.35) | 101.4 (19.35) |
| GA (n = 112) | 341.9 (77.95) | 255.6 (50.34) | 24.2 (9.34) | 94.7 (22.44) | 101.4 (20.78) |
| AA (n = 5) | 383.8 (124.03) | 244.7 (62.06) | 35.3 (5.76) | 93.0 (19.45) | 97.4 (19.50) |
| R2, % | 3.81 | 0.01 | 10.62 | 0.41 | 0.00 |
| P | 5.64 × 10−4 | .8979 | 1.55 × 10−9 | .1999 | .5523 |
| rs2808470 (205 353 810) | |||||
| CC (n = 221) | 315.8 (77.21) | 254.0 (52.17) | 18.3 (10.64) | 97.0 (20.91) | 102.1 (18.95) |
| CT (n = 122) | 338.6 (79.96) | 253.8 (50.71) | 23.9 (8.99) | 95.9 (21.55) | 101.9 (21.25) |
| TT (n = 9) | 345.2 (108.26) | 234.6 (52.41) | 30.2 (10.96) | 97.3 (20.34) | 101.4 (21.15) |
| R2, % | 2.77 | 0.01 | 9.80 | 0.12 | 0.02 |
| P | 1.35 × 10−3 | .9615 | 3.36 × 10−9 | .4676 | .6502 |
R2 represents the percentage of variance explained by the genotype.
GAIT indicates Genetic Analysis of Idiopathic Thrombophilia; fPS, free Protein S; GWAS, genome-wide association study; and PS, Protein S.
Mean (SD).
Association of C4BPB/C4BPA locus with mRNA expression in monocytes
Nineteen SNPs spanning the C4BPB/C4BPA locus were genotyped in GHS (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and all of them were in HWE. Four SNPs were strongly associated (P < 10−11) with C4BPA expression, but little association was observed with C4BPB expression (supplemental Table 1). The SNP most strongly associated with C4BPA monocyte expression was the C4BPA rs11120211 (R2 = 10.7%, P = 6.7 × 10−36), the rs11120211-A allele being associated with higher levels in a fairly additive model (Figure 3). Among the 3 other SNPs that were associated with C4BPA expression, rs11120218 (P = 3.53 × 10−17) was also associated with plasma levels of %α7β0 and C4BPα in the GAIT study (Table 1). LD and in-depth haplotype analyses revealed that the effect of these 3 other SNPs was due to their LD with rs11120211, and that no other effect on C4BPA mRNA expression levels was observed at the C4BPB/C4BPA locus beyond that of the rs11120211 (supplemental Tables 1-2). It is worth pointing out that C4BPB and C4BPA mRNA expressions were not correlated (r = 0.00, P = .98) and that none of the 19 genotyped SNPs was associated with C4BPB mRNA expression, either through single locus (supplemental Table 1) or haplotype (supplemental Table 2) analyses. Similarly, no association was observed between C4BPB/C4BPA SNPs and PROS1 mRNA expression levels (supplemental Tables 1-2), and PROS1 mRNA expression was not correlated with C4BPA mRNA levels either (r = −0.03, P = .29).
Association between the C4BPA rs11120211 and C4BPA mRNA expression in the GHS. Box plots of the distribution of arcsinh-transformed expression data. The arcsinh transformation was used to stabilize variance across expression levels.32
Association between the C4BPA rs11120211 and C4BPA mRNA expression in the GHS. Box plots of the distribution of arcsinh-transformed expression data. The arcsinh transformation was used to stabilize variance across expression levels.32
Genotype distribution of the C4BPB rs3813948 according to VT status in the MARTHA and FARIVE case-control studies
| rs3813948 . | MARTHA . | FARIVE . | MARTHA and FARIVE . | |||
|---|---|---|---|---|---|---|
| Controls, n = 789 (%) . | Cases, n = 1110 (%) . | Controls, n = 590 (%) . | Cases, n = 596 (%) . | Controls, n = 1379 (%) . | Cases, n = 1706 (%) . | |
| TT | 672 (85) | 922 (83) | 501 (85) | 491 (82) | 1173 (85) | 1413 (83) |
| TC | 110 (14) | 175 (16) | 85 (14) | 101 (17) | 195 (14) | 276 (16) |
| CC | 7 (1) | 13 (1) | 4 (1) | 4 (1) | 11 (1) | 17 (1) |
| MAF | 0.079 | 0.091 | 0.079 | 0.091 | 0.079 | 0.091 |
| OR* | 1.171 (0.911-1.506); P = .217 | 1.204 (0.884-1.639); P = .238 | 1.181 (0.972-1.434); P = .093 | |||
| OR† | 1.239 (0.936-1.639); P = .134 | 1.239 (0.900-1.705); P = .189 | 1.239 (1.003-1.530); P = .046 | |||
| rs3813948 . | MARTHA . | FARIVE . | MARTHA and FARIVE . | |||
|---|---|---|---|---|---|---|
| Controls, n = 789 (%) . | Cases, n = 1110 (%) . | Controls, n = 590 (%) . | Cases, n = 596 (%) . | Controls, n = 1379 (%) . | Cases, n = 1706 (%) . | |
| TT | 672 (85) | 922 (83) | 501 (85) | 491 (82) | 1173 (85) | 1413 (83) |
| TC | 110 (14) | 175 (16) | 85 (14) | 101 (17) | 195 (14) | 276 (16) |
| CC | 7 (1) | 13 (1) | 4 (1) | 4 (1) | 11 (1) | 17 (1) |
| MAF | 0.079 | 0.091 | 0.079 | 0.091 | 0.079 | 0.091 |
| OR* | 1.171 (0.911-1.506); P = .217 | 1.204 (0.884-1.639); P = .238 | 1.181 (0.972-1.434); P = .093 | |||
| OR† | 1.239 (0.936-1.639); P = .134 | 1.239 (0.900-1.705); P = .189 | 1.239 (1.003-1.530); P = .046 | |||
MARTHA indicates MARseille THrombosis Association study; FARIVE, FActeurs de RIsque et de récidives de la maladie thromboembolique VEineuse; MAF, minor allele frequency; and OR, odds ratio.
Crude odds ratio for VT associated with carrying the C allele (dominant model).
OR adjusted for age, sex, FV and FII mutations, and ABO blood group.
Interestingly, looking at the HapMap II database33 revealed that the rs11120211 located in the C4BPA proximal promoter was in complete association with the rs3813948 identified in the GAIT study but was not available in the DNA array used for genotyping GHS participants. In this database, the rs11120211-A allele associated with increased C4BPA mRNA levels in GHS corresponds to the rs3813948-C allele associated with increased plasma levels of C4BPα and %α7β0 in the GAIT study.
In silico GWAS analysis of VT at the C4BPB/C4BPA locus
Because of the hypothesized link between C4BP isoforms and VT risk, we investigated whether SNPs at the C4BPB/C4BPA locus were associated with VT using an in silico association analysis of previously published GWAS data for VT.24 Ten SNPs available in the DNA chip array used in this GWAS were located on the chromosome 1 region spanning the 205 320 000- to 205 400 000-bp region harboring the C4BP/C4BPA gene cluster (supplemental Table 3). Among these 10 SNPs, the 3 SNPs that were associated with %α7β0 in the GAIT study, that is, rs3813948, rs11120218, and rs2808470, showed association with VT at P less than .05 (supplemental Table 3).
Interestingly, the SNP that was most strongly associated with VT was the rs3813948, its minor C allele being more frequent in VT cases than in controls (0.093 vs 0.067). This allele was the most strongly associated with increased %α7β0 levels in the GAIT study (see “Family-based genome-wide association analysis of C4BP phenotypes”) and in complete association with the rs11120211-A allele associated with increased C4BPA expression in GHS. Because LD analysis revealed that the effect of the rs11120218 and rs2808470 on VT risk was due to their LD with the rs3813948 (D′ = +0.99, r2 = 0.423, and D′ = +0.99, r2 = 0.31, respectively), the influence of the rs3813948 on VT risk was further investigated for replication in 2 independent case-control studies.
Case-control association analysis of rs3813948 with VT risk
We then genotyped the rs3813948 in 2 case-control studies for VT, MARTHA and FARIVE. Genotype distribution of the rs3813948 followed HWE law in both studies (Table 2). In both studies, the rs3813948-C allele tended to be slightly more frequent in cases than in controls (0.091 vs 0.079), without reaching the significance level of .05 in any of the 2 studies. However, marginal significance association (P = .046) was obtained when the results of the 2 studies were combined. In the pooled samples, the rs3813948-C allele was associated with a slight increased risk of VT (OR = 1.239 [1.003-1.530]; P = .046). This effect of rs3813948 on VT risk did not show heterogeneity with respect to sex, ABO blood group, and FV and FII Leiden mutations (data not shown). It is noteworthy that the combined sample of 1706 cases and 1379 controls provided limited power (ie, 56%) to detect such genetic effect at a .05 significance level.
Discussion
Using a multistep strategy relying on different study designs, we were able to show that the C4BPB/C4BPA gene cluster is a new susceptibility loci for VT, likely through an effect on C4BPA mRNA expression.
The starting point of this work was to search for SNPs associated with both plasma levels of specific(s) C4BP isoform(s) and VT risk, with the aim of getting a better understanding of the mechanisms relating PS to VT susceptibility. We were successful at finding 1 SNP influencing plasma levels of the percentage of α7β0 relative to the amount of C4BPα-chain, which was also associated with VT risk, but independently of PS as initially hypothesized. Indeed, in the GAIT study, this SNP did not show any evidence for association with fPS or total PS, despite its location within the previously identified linkage peak for free PS.34 These results suggest a pleiotropic effect of the locus on different phenotypes related to C4BP.16 To sum up, the effect of this SNP was observed on C4BPA mRNA expression, plasma levels of %α7β0, to a lesser extent on those of total C4BPα, and on VT risk but not on fPS or total PS.
These observations suggest that molecular excess of C4BPα-chains unbound to C4BPβ-chain could be a new disease-associated mechanism relating C4BPB/C4BPA locus to VT susceptibility independently of PS regulation. This hypothesis is supported by several studies showing that increased plasma levels of C4BPα-chains, but not of C4BPβ-chains, are observed during inflammation,35,36 a biologic process whose role in thrombosis is increasingly advocated.37-40 Interestingly, the C4BPα-chain has binding sites for many ligands such as CD40, CRP, and heparin,41,42 which are key molecules involved in inflammatory and coagulation pathways.38,39 The hypothesis raised by our work suggesting the implication of C4BP independently of PS regulation is further supported by the observation that C4BP participates in FVa inactivation even in the absence of PS.12
Several SNPs spanning the C4BPB/C4BPA locus were studied in this report and, because some of them were in complete association with each other, it is not possible from the current report to draw any definitive conclusions about the functional variant responsible for the observed associations. This could be the rs3813948 lying in the C4BPB gene intron 4, the rs11120211 in the C4BPA proximal promoter, or any other SNP in complete association with them. According to HapMap database, additional candidates could be the rs17020634 and rs12063780 located in C4BPA proximal promoter or the C4BPA intronic rs12057769 and rs17020983. Because SNPs of interest explained up to 11% of C4BPA mRNA expression with no effect of C4BPB mRNA, it is more likely that the C4BPA promoter harbors the culprit. This hypothesis should further be investigated using in vitro functional studies. It could be argued that investigating C4BP mRNA expressions in monocytes, as we did here, is not relevant in the context of VT. However, the increasingly recognized role of C4BP in inflammation,36,41,43,44 where monocytes are major players, justifies our rationale of using this type of cell for determining SNPs associated with C4BPB/C4BPA mRNA expressions.
Despite the rather strong association of the identified SNP with both C4BPA mRNA expression and plasma levels of α7β0, this SNP explaining approximately 11% of their variability, it was associated with only a modest increased risk of VT (OR = 1.24). This effect was not identified from our previous GWAS on VT24 due to mainly lack of power related to the use of stringent statistical threshold and moderate sample sizes. This highlights some limitations of the current GWAS approach and the need for gathering well-powered and carefully designed epidemiologic studies, and using alternative and complementary research strategies, as we did here, to identify the factors underlying genetic susceptibility to VT.45
The main limitation of this work is that neither α7β0 plasma levels nor C4BPA mRNA expression was available in our VT case-control samples. Without such information, it is not possible to definitively claim that the observed association between C4BPB/C4BPA locus and VT risk is mediated through an effect on CBPA mRNA, and then through an excess α7β0 isoform. However, the concomitant association of the same allele with both increased levels of C4BPA and α7β0 and VT risk strongly favors this hypothesis.
In conclusion, this work provides strong evidence that the C4BPB/C4BPA locus is a new susceptibility locus for VT, whose functional variant(s) remains to be identified. In addition, the biologic mechanisms linking this locus to VT appears independent of the protein S regulation, opening a new research area focusing on C4BP regulatory pathway.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The GAIT study was partially supported by grants no. 2 R01 HL070751-05 from the National Institutes of Health, PI-08/0420, PI-08/0756, SAF2008/01859, and Spanish Society on Thrombosis and Hemostasia (SETH). The FARIVE study was supported by grants from the Fondation pour la Recherche Médicale, the Program Hospitalier de recherche Clinique, the Fondation de France, and the Leducq Foundation. The MARTHA study was supported by a grant from the Program Hospitalier de la Recherche Clinique. The Gutenberg Heart Study is funded through the government of Rheinland-Pfalz (Stiftung Rheinland Pfalz für Innovation, contract no. AZ 961-386261/733), the research programs Wissen schafft Zukunft and Schwerpunkt Vaskuläre Prävention of the Johannes Gutenberg-University of Mainz and its contract with Boehringer Ingelheim and PHILIPS Medical Systems including an unrestricted grant for the Gutenberg Heart Study. Specifically, the research reported in this article was supported by the National Genome Network NGFNplus (contract no. project A3 01GS0833) by the Federal Ministry of Education and Research, Germany.
J.-M.S. was supported by Programa d'Estabilització d'Investigadors de la Direcció d'Estrategia i Coordinació del Departament de Salut (Generalitat de Catalunya); M.G., by a grant funded by the Agence Nationale pour la Recherche (Project ANR-07-MRAR-021). F.G. holds a Canada Research Chair.
National Institutes of Health
Authorship
Contribution: A.B. and D.-A.T. carried out the main statistical analyses and wrote the paper; M.G. and M.R. participated in data storing and statistical analyses; N.S. and T.Z. performed genetic laboratory analyses; T.Z., M.R., and F.C. performed and coordinated mRNA expression analyses; J.C.S., S.R.C., J.F., L.A., and J.-M.S. coordinated GAIT data collection; M.L., M.-C.A., J.E., and P.-E.M. coordinated case-control studies for VT; P.W., T.Z., T.M., L.T., F.C., and S.B. coordinated GHS; D.-A.T., F.G., J.-M.S., and P.-E.M. designed this research study; and F.G., J.-M.S., J.F., L.T., and P.-E.M. drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David-Alexandre Trégouët, Inserm UMR_S 937, Faculty of Medicine, Pierre & Marie Curie University, 91 Blvd de l'Hôpital, 75013 Paris Cedex, France; e-mail: david.tregouet@upmc.fr.