Abstract
Muscle represents an important tissue target for adeno-associated viral (AAV) vector-mediated gene transfer of the factor IX (FIX) gene in hemophilia B (HB) subjects with advanced liver disease. Previous studies of direct intramuscular administration of an AAV-FIX vector in humans showed limited efficacy. Here we adapted an intravascular delivery system of AAV vectors encoding the FIX transgene to skeletal muscle of HB dogs. The procedure, performed under transient immunosuppression (IS), resulted in widespread transduction of muscle and sustained, dose-dependent therapeutic levels of canine FIX transgene up to 10-fold higher than those obtained by intramuscular delivery. Correction of bleeding time correlated clinically with a dramatic reduction of spontaneous bleeding episodes. None of the dogs (n = 14) receiving the AAV vector under transient IS developed inhibitory antibodies to canine FIX; transient inhibitor was detected after vector delivery without IS. The use of AAV serotypes with high tropism for muscle and low susceptibility to anti-AAV2 antibodies allowed for efficient vector administration in naive dogs and in the presence of low- but not high-titer anti-AAV2 antibodies. Collectively, these results demonstrate the feasibility of this approach for treatment of HB and highlight the importance of IS to prevent immune responses to the FIX transgene product.
Introduction
Adeno-associated viral (AAV) vectors have demonstrated excellent safety and efficacy profiles as gene transfer tools in numerous preclinical studies.1-10 More recently, clinical translation of these results into humans also generated promising results.11-22 Hemophilia B represents an ideal disease model for AAV-mediated gene transfer studies; results in large- and small-animal models of the disease showed sustained expression of the factor IX (FIX) therapeutic transgene and correction of the disease phenotype after AAV-mediated gene transfer to muscle4,5,23,24 or liver.6,7,10,25 Early clinical work on AAV gene transfer to muscle for hemophilia B in severely affected subjects demonstrated that this approach is feasible16,19 and led to long-term expression of the FIX transgene product.26 However, we have shown that direct intramuscular administration of an AAV2 vector encoding the FIX transgene (AAV2-FIX) does not result in therapeutic levels of circulating FIX in humans at the doses tested.19 Concurrently, studies in preclinical animal models of hemophilia B mice and dogs indicate that further dose escalation of AAV-FIX vectors injected intramuscularly is associated with higher risk of development of immune responses to the transgene product, especially if large amounts (> 1 × 1012 vector genomes [vg]) of vector are injected at a single site.24,27,28
One possible approach to overcoming the problem of reaching therapeutic levels of expression of the FIX transgene is to target a different tissue. Liver, for example, is an ideal target for the production of FIX, as it is the main site of synthesis of this protein. Results in experimental animal models and in severe hemophilia B subjects confirmed the dose advantage of liver versus muscle (direct intramuscular injection).6,20,29 In human subjects, in particular, doses of vector delivered through the hepatic artery, comparable with those that were subtherapeutic in muscle (in the range of 1012 vg/kg) resulted in levels of circulating FIX up to 12% of normal.20 However, targeting the liver for the treatment of hemophilia presents 2 major obstacles. The first is the host immune system30 ; experience in humans showed that the intravascular administration of an AAV2 vector through the hepatic artery results in only transient expression of the FIX transgene product, due to a capsid-specific CD8+ T-cell response.20,31 Although this obstacle may be overcome with the use of transient immunosuppression,10,30,32 or the use of AAV serotypes less immunogenic than AAV-2,30 another obstacle to hepatic gene transfer is represented by the disease state of the liver. Due to the widespread use of hepatitis C virus (HCV)–contaminated plasma-derived products for replacement therapy for hemophilia before 1985, more than 90% of severe hemophilia patients were infected, and many now manifest variable degrees of liver disease due to HCV infection.33 The safety of administering AAV vectors to the liver in the presence of advanced liver disease has not been established. Thus, in the presence of liver disease, muscle is still a highly attractive target tissue for AAV gene transfer for hemophilia B.
We previously showed that it is possible to transduce large areas of skeletal muscle by injecting an AAV vector through the vasculature.34 This delivery method, which relies on the permeabilization of the vascular endothelium with vasoactive drugs such as papaverine and histamine, resulted in circulating levels of canine FIX transgene product up to 15% in hemophilia B dogs at a dose of 3.7 × 1012 vg/kg. Although a similar approach would not be amenable for clinical development, as the drugs used to increase vascular permeability are not approved for human use, these results are at least a proof of principle that the approach is feasible and can lead to sustained expression of the FIX transgene at therapeutic levels.
A noninvasive pressurized infusion of vector-containing solution through the superficial saphenous vein without surgical or pharmacologic intervention has been described.35
In this study, atraumatic tourniquet placement at the groin level, combined with pressurized flow of saline from a distal catheter, caused afferent flow through the valves within the major veins of the extremity but locally retrograde flow through the valveless venules, culminating in extravasation of vector across the endothelium into the interstitium followed by widespread transduction of muscle in rats and dogs.
Here we report sustained, therapeutic canine FIX transgene expression after delivery of AAV vectors through afferent transvenular retrograde extravasation (ATVRX)35 to skeletal muscle in 16 severe hemophilia B dogs. Collectively, these data provide the basis for future translational studies in humans with hemophilia B and liver disease.
Methods
Vector production
Recombinant AAV vectors were produced by a triple transfection protocol as previously described,5 using plasmids expressing canine FIX (cFIX) under the control of the cytomegalovirus promoter/enhancer, a second plasmid supplying adenovirus helper functions, and a third plasmid containing the AAV-2 rep gene and the AAV-2 or AAV-6 cap genes. Vectors were purified by repeated CsCl density gradient centrifugation.
Animals and intravascular vector delivery procedure
A total of 16 adult hemophilia B dogs housed at the colony of the Department of Pathology and Laboratory Medicine at the University of North Carolina at Chapel Hill were used in this study. These dogs exhibit severe hemophilia B and FIX levels lower than 1%, due to a missense mutation in the F9 gene, resulting in detectable levels of mRNA transcripts but no circulating protein.36 Two normal dogs, DLAM 3 and DLAM 4, were also included in the study to test vector genome distribution within the target and contralateral limb as well as in the liver and spleen. For the ATVRX procedure, sedation was achieved with sodium pentothal (11-29 mg/kg) and anesthesia was maintained with 1% to 4% isoflurane. Pooled normal canine plasma was given to hemophilia B dogs before venous puncture at doses calculated to achieve at least 20% normal cFIX plasma levels.
Blood inflow to the target limb was transiently blocked by placing a tourniquet at the level of the groin, and further adjusting it until the femoral pulse was no longer detectable. An intravenous catheter (14-18 gauge) was placed under direct visualization into the lumen of a distal branch of the peripheral saphenous vein on the dorsum of the paw vein. At time zero, the AAV vector, diluted in 20 mL of prewarmed 37°C sterile phosphate-buffered saline per kilogram of body weight, was infused rapidly (∼ 3 minutes) under elevated hydrostatic pressure, in the range of 300 mm Hg, using a sphygnomanometer placed around the bag containing the vector solution. At 15 minutes after the pressurized vector delivery, the tourniquet was released. All the animal procedures in this study were approved by the University of North Carolina at Chapel Hill's Institutional Animal Care and Use Committee.
Clotting assays, FIX antigen, and antibodies to FIX
Whole blood clotting time (WBCT) and activated partial thromboplastin time (aPTT) were determined as previously described.27 Canine FIX (cFIX) antigen concentration was determined by enzyme-linked immunosorbent assay (ELISA) using matched-pair antibodies to cFIX (Affinity Biologicals). FIX clotting activity was determined by one-stage aPTT; plasma test samples were mixed with cFIX-deficient plasma and the aPTT values were compared with a reference standard consisting of serial dilutions of normal canine plasma mixed with canine FIX–deficient plasma. Neutralizing antibodies to canine FIX were performed by Bethesda assay as previously reported.34 Noninhibitory antibodies to FIX were measured by an ELISA specific to dog immunoglobulin G (IgG) subclasses.27
Neutralizing antibody assays to AAV-2 capsid protein
AAV2-specific neutralizing antibody titers were determined as previously described20 using serial dilutions of serum samples. Data are reported as the serum dilution at which a 50% inhibition of AAV transduction was measured.
Systemic and local toxicity
Hematologic and biochemical analysis of blood and serum samples for liver and kidney function tests and muscle enzymes were performed as previously described.5
Histology, immunohistochemistry, and muscle transduction
Muscle biopsies were analyzed as previously described.34 Briefly, serial cryosections (5-10 μm) were stained for cFIX expression using a rabbit anti-cFIX antibody (Affinity Biologicals) at a concentration of 4 μg/mL (1:500 dilution) followed by a horseradish peroxidase–conjugated swine anti–rabbit IgG (Dako) as secondary antibody used at a dilution of 1:500.
To determine vector genome copy number in muscle, and other organs for which AAV-2 vectors have high tropism, tissues were collected and snap frozen in liquid nitrogen from normal dogs 3 months after ATVRX vector delivery. Determination of transgene copy number was performed by real-time quantitative PCR using a primer and probe set on the canine FIX cDNA which did not amplify the genomic cFIX gene.
Stained sections were viewed with an Eclipse E800 microscope (Nikon) using a Plan APO 20×/0.75 objective and epifluorescent light (FITC HYQ filter). Images were captured with a CoolSnap Pro camera and analyzed with Image Pro Plus software (Media Cybernetics).
Results
Transvenular delivery of AAV-cFIX to skeletal muscle of hemophilia B dogs results in long-term correction of the disease phenotype
Hemophilia B (HB) dogs from the University of North Carolina at Chapel Hill colony, with FIX activity lower than 1% and no circulating antigen, were used in this study. In the first experiment, designed as a dose-escalation study, 8 hemophilic dogs received a single administration of an AAV-2 vector expressing cFIX through the saphenous vein via ATVRX (Table 1). Animals were divided into a low-dose cohort, receiving a vector dose of 1 × 1012 vg/kg (n = 3); a mid-dose cohort, receiving a vector dose of 3 × 1012 vg/kg (n = 3); and a high-dose cohort, receiving a vector dose of 8.5 × 1012 vg/kg (n = 2). Vector was administered under transient immunosuppression (IS) with cyclophosphamide as previously described.34 Administration of the vector-containing solution was well tolerated, and no signs of persistent local or systemic toxicity were observed. Hemostasis was controlled with infusion of pooled canine plasma before the skin incision needed for catheter placement into the saphenous vein; no excessive bleeding was observed during this procedure. Rapid fluid injection (∼ 3 minutes) resulted in transient leg swelling with increased limb circumference and tissue pressure. At 15 minutes after vector infusion, the tourniquet was released with no signs of cardiac or blood pressure abnormalities. Dogs recovered from the light sedation and showed no evidence of preferential use of the nonperfused limb during immediate (within 15-30 minutes after injection) or late ambulation.
Summary of results in naive hemophilia B dogs after ATVRX delivery of an AAV2-cFIX vector
| Group/dog ID . | Age at time of injection, mo . | Sex . | Weight, kg . | Circulating cFIX, ng/mL* . | Inhibitor (Bethesda unit) . | Bleeding episodes (expected†) . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|
| Low dose, 1 × 1012 vg/kg | |||||||
| I04 | 6 | M | 15.5 | 32 ± 7 | No | 0 (28) | 61 |
| I05 | 9 | M | 17.5 | 141 ± 18 | No | 1 (27) | 59 |
| J04 | 4.5 | M | 12.4 | 27 ± 4 | No | 1 (19) | 42 |
| Mid dose, 3 × 1012 vg/kg | |||||||
| H48 | 6.5 | M | 15 | 275 ± 75 | No | 0 (20) | 44 |
| H34 | 11 | F | 15 | 76 ± 14 | No | 1 (29) | 63 |
| I07 | 8 | F | 16 | 125 ± 39 | No | 0 (22) | 47 |
| High dose, 8.5 × 1012 vg/kg | |||||||
| H24 | 9 | M | 24 | 20 ± 3 | No | 3 (29) | 64 |
| M25 | 3.5 | F | 8.7 | 23 ± 3 | No | 1 (6) | 12 |
| Mid dose, no immunosuppression | |||||||
| J03 | 11 | M | 17.5 | 81 ± 13 | No | 1 (20) | 44 |
| J62 | 6 | M | 14 | 120‡ | Transient (1.5 BU) | 6§ (5) | 40 |
| Group/dog ID . | Age at time of injection, mo . | Sex . | Weight, kg . | Circulating cFIX, ng/mL* . | Inhibitor (Bethesda unit) . | Bleeding episodes (expected†) . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|
| Low dose, 1 × 1012 vg/kg | |||||||
| I04 | 6 | M | 15.5 | 32 ± 7 | No | 0 (28) | 61 |
| I05 | 9 | M | 17.5 | 141 ± 18 | No | 1 (27) | 59 |
| J04 | 4.5 | M | 12.4 | 27 ± 4 | No | 1 (19) | 42 |
| Mid dose, 3 × 1012 vg/kg | |||||||
| H48 | 6.5 | M | 15 | 275 ± 75 | No | 0 (20) | 44 |
| H34 | 11 | F | 15 | 76 ± 14 | No | 1 (29) | 63 |
| I07 | 8 | F | 16 | 125 ± 39 | No | 0 (22) | 47 |
| High dose, 8.5 × 1012 vg/kg | |||||||
| H24 | 9 | M | 24 | 20 ± 3 | No | 3 (29) | 64 |
| M25 | 3.5 | F | 8.7 | 23 ± 3 | No | 1 (6) | 12 |
| Mid dose, no immunosuppression | |||||||
| J03 | 11 | M | 17.5 | 81 ± 13 | No | 1 (20) | 44 |
| J62 | 6 | M | 14 | 120‡ | Transient (1.5 BU) | 6§ (5) | 40 |
Average ± SD of plateau levels > 3 months.
Based on an average of ∼ 5 bleeding episodes per year for untreated dogs.37
Measured at day > 600 after resolution of inhibitor.
Occurred from days 15 to 363, while inhibitor persisted.
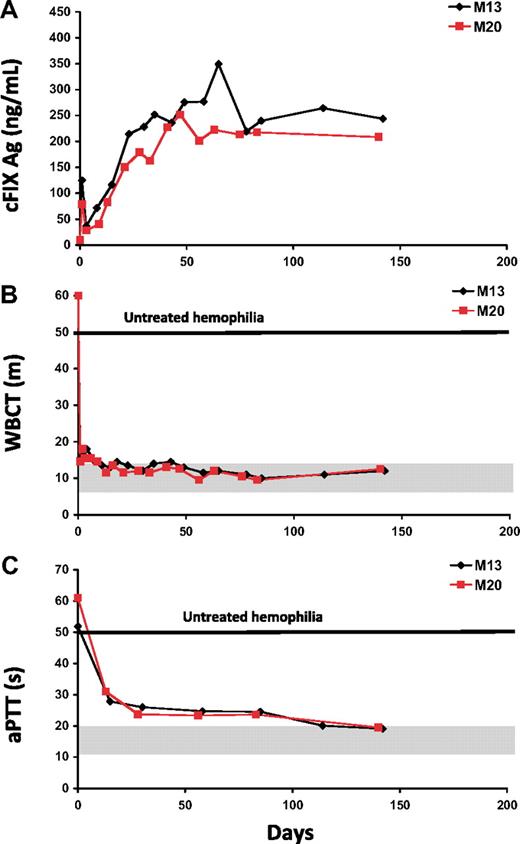
Administration of the AAV2-cFIX vector at a dose of 1 × 1012 vg/kg resulted in circulating cFIX antigen levels between lower than 1% and 3% of normal (Figure 1A). These values were in agreement with the cFIX clotting activity, as a sustained shortening of the WBCT and aPTT was observed in all animals (Figure 1B-C). Because the cFIX levels measured in the low-dose cohort were at the threshold for therapeutic efficacy for hemophilia B (1% of normal), the next dose cohort was injected with a 3-fold higher dose (3 × 1012 vg/kg). In this group, cFIX transgene antigen levels peaked at 5% to 8% of normal and at plateau ranged from 2% to 6% (Figure 1A). Detection of cFIX by ELISA was associated with a dose-dependent shortening of the WBCT and aPTT (Figure 1B-C). Long-term follow-up of the animals from the low- and mid-dose cohorts showed stable circulating FIX levels for periods of up to 4 years, with observation ongoing. This reflected a marked improvement in the clinical outcome, that is, spontaneous bleeding episodes reduced by more than 90% compared with expected37 bleeding rates (Table 1). No neutralizing antibodies to the cFIX transgene product were detected by Bethesda assay in any of the dogs from the low- and mid-dose cohorts, even after discontinuation of the immunosuppression regimen at 4 to 6 weeks after vector delivery. No antibodies to cFIX were detected, with the exception of low-titer nonneutralizing IgG2 appearing more than 6 months after vector administration (not shown). No elevation of the muscle enzyme creatinine phosphokinase (CPK) was detected in these animals at any time point (Figure 1D).
Time course of circulating canine FIX and coagulation activity in naive hemophilia B dogs after ATVRX vector delivery. (A) Canine FIX (cFIX) antigen levels measured by ELISA. (B) Activated partial thromboplastin time (aPTT); s indicates seconds. (C) Whole blood clotting time (WBCT); m indicates minutes. (D) Creatinine phosphokinase (CPK) levels. Each line represents an individual dog: red indicates dogs from the low-dose cohort (1 × 1012 vg/kg); black, dogs from the mid-dose cohort (3 × 1012 vg/kg). Shaded gray areas represent indicate range of values for hemostatically normal animals. x axis represents time in days.
Time course of circulating canine FIX and coagulation activity in naive hemophilia B dogs after ATVRX vector delivery. (A) Canine FIX (cFIX) antigen levels measured by ELISA. (B) Activated partial thromboplastin time (aPTT); s indicates seconds. (C) Whole blood clotting time (WBCT); m indicates minutes. (D) Creatinine phosphokinase (CPK) levels. Each line represents an individual dog: red indicates dogs from the low-dose cohort (1 × 1012 vg/kg); black, dogs from the mid-dose cohort (3 × 1012 vg/kg). Shaded gray areas represent indicate range of values for hemostatically normal animals. x axis represents time in days.
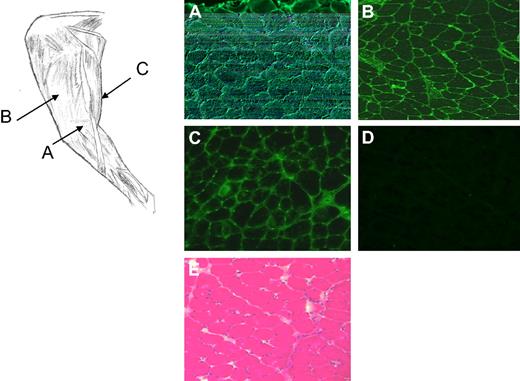
Percutaneous muscle biopsies from the vector-injected limb collected approximately 2 years after injection from dog H48 showed local expression of cFIX protein detected by immunofluorescence (Figure 2A-D). At this time point, no evidence of muscle inflammation was observed in muscle sections (Figure 2E). Complete vector distribution within skeletal muscle of the target limb versus the contralateral limb was evaluated in 2 normal dogs that received an AAV2-cFIX vector via ATVRX at a dose similar to the mid-dose cohort animals. Results are summarized in Table 2; high levels of widespread muscle transduction were detected in muscle collected from different areas of the target limb 3 months after ATVRX vector delivery. In comparison, negligible vector transduction was detectable in the contralateral (noninjected) limb and other organs with minimal dissemination to the spleen, liver, and kidney (V.H., F.M., and K.A.H., unpublished data, February 2010).
Canine FIX staining and histology of muscle biopsies from dog H48 (3 × 1012 vg/kg) 2 years after ATVRX delivery of an AAV2-cFIX vector. Left panel, sites of muscle biopsy sampling, AAV-injected target limb: (A) semimembranosus; (B) vastus lateralis; (C) semitendinosus. (A-C) cFIX immunostaining of muscle biopsies collected at the sites indicated. (D) cFIX immunostaining of muscle biopsies from the contralateral, noninjected limb. (E) Hematotoxylin and eosin staining of the muscle biopsy collected from the target leg. Images are taken at 100× magnification.
Canine FIX staining and histology of muscle biopsies from dog H48 (3 × 1012 vg/kg) 2 years after ATVRX delivery of an AAV2-cFIX vector. Left panel, sites of muscle biopsy sampling, AAV-injected target limb: (A) semimembranosus; (B) vastus lateralis; (C) semitendinosus. (A-C) cFIX immunostaining of muscle biopsies collected at the sites indicated. (D) cFIX immunostaining of muscle biopsies from the contralateral, noninjected limb. (E) Hematotoxylin and eosin staining of the muscle biopsy collected from the target leg. Images are taken at 100× magnification.
Determination of vector genome copy number after ATVRX delivery of an AAV2-cFIX vector
| Biopsy site . | DLAM 3, vg/diploid genome . | DLAM 4, vg/diploid genome . |
|---|---|---|
| Target limb | ||
| 1 | 0.163 ± 0.111 | 0.09* |
| 2 | 0.091 ± 0.012 | 0.186 ± 0.251 |
| 3 | 14.018 ± 1.479 | 9.146 ± 1.587 |
| 4 | 6.058 ± 0.591 | 0.505 ± 0.057 |
| 5 | 0.019 ± 0.005 | 5.358 ± 0.592 |
| Contralateral limb | ||
| 1 | 0.004 ± 0.001 | |
| 2 | 0.014 ± 0.015 | 0.094 ± 0.108 |
| 3 | 0.004 ± 0.003 | |
| 4 | 0.004 ± 0.001 | |
| 5 | 0.004 ± 0.004 | 0.012 ± 0.003 |
| Biopsy site . | DLAM 3, vg/diploid genome . | DLAM 4, vg/diploid genome . |
|---|---|---|
| Target limb | ||
| 1 | 0.163 ± 0.111 | 0.09* |
| 2 | 0.091 ± 0.012 | 0.186 ± 0.251 |
| 3 | 14.018 ± 1.479 | 9.146 ± 1.587 |
| 4 | 6.058 ± 0.591 | 0.505 ± 0.057 |
| 5 | 0.019 ± 0.005 | 5.358 ± 0.592 |
| Contralateral limb | ||
| 1 | 0.004 ± 0.001 | |
| 2 | 0.014 ± 0.015 | 0.094 ± 0.108 |
| 3 | 0.004 ± 0.003 | |
| 4 | 0.004 ± 0.001 | |
| 5 | 0.004 ± 0.004 | 0.012 ± 0.003 |
Results are expressed as average ± SD. All the experiments were repeated twice using 500 to 1000 ng of genomic DNA per reaction.
Single experiment with 344 ng of genomic DNA in the reaction.
High AAV2-cFIX vector doses delivered via ATVRX result in the development of nonneutralizing anti-cFIX antibodies, even in the presence of transient immunosuppression
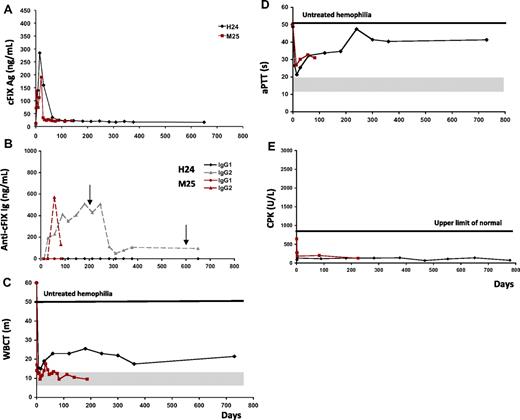
ATVRX delivery of the AAV2-cFIX vector to hemophilia B dogs in the high-dose cohort (8.5 × 1012 vg/kg; Table 1) did not result in the expected dose-response levels of cFIX. The first dog injected from this cohort, H24, showed circulating cFIX levels up to 6% at 2 weeks after vector injection; this was followed by a progressive decline to approximately 1% of normal (Figure 3A). Despite transient IS with cyclophosphamide, the animal developed a nonneutralizing IgG2 antibody specific to cFIX (Figure 3B). Serial screening for inhibitory antibodies, performed by Bethesda assay, was persistently negative (not shown). Despite the presence of nonneutralizing antibodies to cFIX, the dog showed sustained partial shortening of WBCT and aPTT values (Figure 3C-D), suggesting that the antibody enhanced the clearance of the cFIX transgene product from the bloodstream but did not inhibit its activity. During the period of observation, H24 experienced 2 bleeding episodes, which were successfully treated with pooled canine plasma (Figure 3B arrows). Recovery and half-life of the infused canine plasma, administered at a dose sufficient to achieve 25% of normal levels, were normal when measured at day 779 after ATVRX.
Transient nonneutralizing anti-cFIX antibodies develop at high doses of of AAV2-cFIX vector delivered via ATVRX. (A) Canine FIX transgene antigen levels measured by ELISA. (B) Anti-cFIX IgG1 and IgG2 subclass antibody titer measured by ELISA. (C-D) Blood coagulation activity measured by WBCT (m indicates minutes) and aPTT (s indicates seconds). (E) Creatinine phosphokinase (CPK) levels. Shaded gray areas indicate the range of values for hemostatically normal animals. Vertical arrows represent normal pooled plasma administrations. x axis represents time in days.
Transient nonneutralizing anti-cFIX antibodies develop at high doses of of AAV2-cFIX vector delivered via ATVRX. (A) Canine FIX transgene antigen levels measured by ELISA. (B) Anti-cFIX IgG1 and IgG2 subclass antibody titer measured by ELISA. (C-D) Blood coagulation activity measured by WBCT (m indicates minutes) and aPTT (s indicates seconds). (E) Creatinine phosphokinase (CPK) levels. Shaded gray areas indicate the range of values for hemostatically normal animals. Vertical arrows represent normal pooled plasma administrations. x axis represents time in days.
A second dog, M25, was injected in the same high-dose cohort, and showed similar results. Shortly after AAV2-cFIX vector delivery, 2 weeks, M25 plasma levels of cFIX peaked at 4% of normal and then reached a plateau of approximately 1% of normal (Table 1 and Figure 3A). At the same time, a nonneutralizing IgG2 antibody specific to cFIX was detected by ELISA (Figure 3B) and Western blot (data not shown). Similar to H24, M25 did not have any detectable Bethesda titer at any time point (not shown) and showed sustained partial correction of WBCT and aPTT values (Figure 3C-D). As was the case at lower doses, none of the high-dose cohort dogs had CPK elevation (Figure 3E).
Transient immunosuppression is required to prevent immune responses to the cFIX transgene product
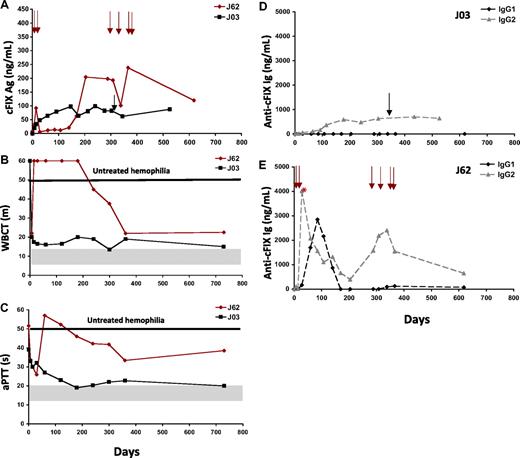
To test the role of transient IS with cyclophosphamide in preventing humoral responses to the cFIX transgene product after ATVRX gene transfer, 2 dogs were injected with the AAV2-cFIX vector via ATVRX at a dose of 3 × 1012 vg/kg with no IS (Table 1). In 1 of the 2 dogs (J03), sustained expression of cFIX antigen levels, approximately 2% at plateau, with improvement of the WBCT and aPTT, was observed (Figure 4A-C). Only one episode of spontaneous bleeding was observed during the long-term follow-up in this animal, confirming the correction of the bleeding phenotype (Table 1). Approximately 3 months after transduction, J03 developed a low-titer nonneutralizing anti-cFIX IgG2 antibody that did not influence the correction of the bleeding phenotype or the response to cFIX replacement (Figure 4D); no Bethesda titer was detected in this animal throughout the observation period.
Delivery of AAV2-cFIX via ATVRX with no IS. (A) Canine FIX antigen levels measured by ELISA. (B-C) Coagulation parameters measured by WBCT (m indicates minutes) and aPTT (s indicates seconds). (D-E) Anti-cFIX IgG1 and IgG2 subclass antibody titer measured by ELISA in J03 (D) and J62 (E). *Peak neutralizing antibody titer (1.5 BU at day 35 after ATVRX). Shaded gray areas represent range of values for hemostatically normal animals. Vertical arrows represent pooled normal plasma administrations. x axis represents time in days.
Delivery of AAV2-cFIX via ATVRX with no IS. (A) Canine FIX antigen levels measured by ELISA. (B-C) Coagulation parameters measured by WBCT (m indicates minutes) and aPTT (s indicates seconds). (D-E) Anti-cFIX IgG1 and IgG2 subclass antibody titer measured by ELISA in J03 (D) and J62 (E). *Peak neutralizing antibody titer (1.5 BU at day 35 after ATVRX). Shaded gray areas represent range of values for hemostatically normal animals. Vertical arrows represent pooled normal plasma administrations. x axis represents time in days.
A second dog (J62) from the same experimental group had a peak in cFIX transgene expression at day 14 after ATVRX injection, with subsequent return to baseline levels thereafter for a period of approximately 140 days (Figure 4A). This was followed by a slow continuous increment in cFIX antigen levels over time to plateau levels of approximately 2% of normal (Table 1 and Figure 4A). The transient loss of cFIX transgene expression was due to the formation of an inhibitory antibody that peaked at 1.5 Bethesda unit (BU) at day 35, then slowly returned to titers less than 1 BU (day 78 and thereafter). This dog developed an anti-cFIX IgG1 antibody with similar kinetics to the inhibitory antibody measured by Bethesda assay (Figure 4E).
Whether the appearance of inhibitor in this dog was associated with local muscle inflammation is not clear, however the absence of CPK elevation or evidence for long-term muscle histologic changes do not support this hypothesis. The animal also developed an IgG2 antibody response to cFIX, however this response was detected early after gene transfer (peaking at day 28) and declined slowly in titer without completely disappearing. After day 200, with the disappearance of the neutralizing antibody to cFIX, cFIX antigen levels increased and both the WBCT and the aPTT showed correction (Figure 4B-C). Infusion of pooled canine plasma to treat bleeding (the animal had 4 bleeding episodes from days 291 to 363) resulted in increasing levels of antibody titers and decreased levels of cFIX antigen (Figure 4E). Long-term follow-up of animal J62 showed cFIX antigen levels higher than 2% and partial correction of both WBCT and aPTT after clearance of the inhibitory antibody (Figure 4B-C). During this period, there were no spontaneous bleeding episodes even in the absence of pooled plasma infusion.
Although all animals developed anticapsid antibodies upon vector administration, no T-cell responses, measured with an interferon-γ enzyme-linked immunosorbent spot assay, were detectable (V.H., F.M., and K.A.H., unpublished data, February 2010).
Successful expression of cFIX via transvenular delivery of AAV-6 in naive HB dogs and HB dogs with neutralizing antibodies to AAV-2 capsid
We tested the feasibility of the readminstration of AAV-2 or AAV-6 vectors encoding cFIX to hemophilia B dogs previously injected with AAV-2 vectors intramuscularly or intravenously. Vector readministration was performed via ATVRX under transient IS with cyclophosphamide. Two dogs, exposed to AAV-2 vector by intramuscular injection more than 5 years before the ATVRX procedure, exhibited a neutralizing antibody titer (NAB) to AAV-2 of 1:300 (dog D32, 5.7 years old) and 1:30 (dog B46, 7.6 years old). Both animals at the time of readministration had circulating cFIX levels lower than 1% and received an AAV2-cFIX vector at a dose of 4 × 1012 vg/kg via ATVRX. No increase in FIX antigen or activity levels was observed after vector readministration, and bleeding episode frequency remained unchanged (Figure 5A-B and Table 3). Screening for neutralizing and nonneutralizing antibodies to cFIX was negative (not shown), and no evidence of muscle or liver damage was observed (Figure 5C and data not shown). These findings suggest that NAB to the AAV-2 capsid prevented successful gene transfer by ATVRX of the same serotype vector, AAV-2. Dog B46 died at day 175 after ATVRX of bleeding from a necrotic lesion of the nipple first diagnosed 2 years earlier, further confirming the lack of efficacy after AAV2-cFIX readministration.
Readministration of AAV vectors by ATVRX in HB dogs previously exposed to AAV-2 vectors. (A-B) Canine FIX levels measured by ELISA and coagulation activity (WBCT in minutes [m]) in dogs D32 (red) and B46 (black) upon readminstration of an AAV2-cFIX vector via ATVRX. (D-E) Canine FIX levels measured by ELISA and coagulation activity (WBCT in minutes [m]) in dogs E59 (red) and H27 (black) upon readminstration of an AAV6-cFIX vector via ATVRX. (C,F) Creatinine phosphokinase (CPK) levels. x axis represents time in days.
Readministration of AAV vectors by ATVRX in HB dogs previously exposed to AAV-2 vectors. (A-B) Canine FIX levels measured by ELISA and coagulation activity (WBCT in minutes [m]) in dogs D32 (red) and B46 (black) upon readminstration of an AAV2-cFIX vector via ATVRX. (D-E) Canine FIX levels measured by ELISA and coagulation activity (WBCT in minutes [m]) in dogs E59 (red) and H27 (black) upon readminstration of an AAV6-cFIX vector via ATVRX. (C,F) Creatinine phosphokinase (CPK) levels. x axis represents time in days.
Summary of results after delivery of an AAV6-cF.IX vector via ATVRX in hemophilia B dogs naive or with previous exposure to an AAV-2 vector
| Group/ dog ID . | Age at time of injection . | Sex . | Weight, kg . | Serotype ATVRX administration . | NAB to AAV-2 capsid* . | Circulating cFIX,† ng/mL . | Bleeding episodes (expected‡) . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|---|
| HB dogs previously exposed to AAV-2 | ||||||||
| E59 | 4.6 y | F | 21 | AAV-6 | 1:100 | 259 ± 50 | 1§ (21) | 46 |
| H27 | 2.4 y | F | 20 | AAA-6 | 1:1000 | 89 ± 47 | 0 (19) | 42 |
| D32 | 5.7 y | M | 18 | AAV-2 | 1:300 | < 10 | 8 (10) | 22 |
| B46 | 7.6 y | M | 24 | AAV-2 | 1:30 | < 10 | 4 (3) | 6 |
| Naive HB dogs | ||||||||
| M13 | 5 mo | F | 13 | AAV-6 | ND | 259 ± 37 | 0 (6) | 12 |
| M20 | 4 mo | M | 8 | AAV-6 | ND | 213 ± 25 | 0 (6) | 12 |
| Group/ dog ID . | Age at time of injection . | Sex . | Weight, kg . | Serotype ATVRX administration . | NAB to AAV-2 capsid* . | Circulating cFIX,† ng/mL . | Bleeding episodes (expected‡) . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|---|
| HB dogs previously exposed to AAV-2 | ||||||||
| E59 | 4.6 y | F | 21 | AAV-6 | 1:100 | 259 ± 50 | 1§ (21) | 46 |
| H27 | 2.4 y | F | 20 | AAA-6 | 1:1000 | 89 ± 47 | 0 (19) | 42 |
| D32 | 5.7 y | M | 18 | AAV-2 | 1:300 | < 10 | 8 (10) | 22 |
| B46 | 7.6 y | M | 24 | AAV-2 | 1:30 | < 10 | 4 (3) | 6 |
| Naive HB dogs | ||||||||
| M13 | 5 mo | F | 13 | AAV-6 | ND | 259 ± 37 | 0 (6) | 12 |
| M20 | 4 mo | M | 8 | AAV-6 | ND | 213 ± 25 | 0 (6) | 12 |
There was no inhibitor to cF.IX in any of the dogs.
NAB indicates neutralizing antibody titer; and ND, not determined.
Measured at the time of vector readministration by ATVRX; values expressed as reciprocal dilutions.
Average ± SD of plateau levels > 3 months;
Based on an average of ∼ 5 bleeding episodes/year for untreated dogs.37
Two severe bleeding episodes and 3 postpartum bleeding episodes despite prophylactic plasma transfusion before readministration.
Two additional animals previously exposed to AAV-2 vectors intravascularly received 1.5 × 1012 vg/kg of an AAV6-cFIX vector via ATVRX (Table 3). The titers of NABs to the AAV-2 capsid before the readminstration of the AAV-6 vector were 1:100 (E59, 4.6 years old) and 1:1000 (H27, 2.4 years old). Whereas the dog with a NAB titer of 1:100 (E59) showed an increase in cFIX levels from lower than 1% to approximately 5% of normal, the animal with the higher titer NAB to AAV-2 (H27, 1:1000) did not have any evidence of successful muscle transduction after AAV6 ATVRX (Figure 5D). Canine FIX expression data correlated well with WBCT in both dogs (Figure 5E), with good correction of the disease phenotype in E59 and no improvement in H27. No neutralizing antibodies to cFIX were observed in these dogs; H27 developed a low-titer nonneutralizing IgG2 antibody specific to cFIX, which resulted in increased clearance of the protein without compromising the correction of the bleeding phenotype (Table 3 and Figure 5E). No muscle (Figure 5F) or liver toxicity was documented in these dogs. Thus, AAV-6 may be an attractive strategy for muscle-directed gene transfer to overcome the presence of low to medium NAB to AAV-2; however, the strategy does not seem to be effective in the presence of high-titer NAB, probably due to cross-reactivity of neutralizing antibodies between alternate AAV serotypes.
Finally, the efficacy of AAV-6 in transducing muscle was further tested in 2 naive HB dogs receiving 3 × 1012 vg/kg of an AAV-6 vector encoding cFIX and transient IS with cyclophosphamide. In these dogs, cFIX levels peaked at 5% to 7% of normal, followed by a plateau of 4% to 5% of normal (Figure 6A and Table 3). The shortening of the WBCT and the aPTT (Figure 6B-C) demonstrates that the FIX produced by the muscle was biologically active. No spontaneous bleeding was observed during the follow-up of almost 1 year per dog, and no antibodies to cFIX were measured. Levels of cFIX transgene expression in these 2 naive dogs were comparable with those obtained in animal E59, which received a similar dose of the same AAV6-cFIX vector via ATVRX in the presence of NAB to AAV2 at a titer of 1:100 (Table 3).
Time course of canine FIX expression and coagulation activity in naive hemophilia B dogs after ATRVX delivery of an AAV6-cFIX vector. (A) Canine FIX antigen levels measured by ELISA. (B) Whole blood clotting time (WBCT); m indicates minutes. (C) Activated partial thromboplastin time (aPTT); s indicates seconds. x axis represents time in days.
Time course of canine FIX expression and coagulation activity in naive hemophilia B dogs after ATRVX delivery of an AAV6-cFIX vector. (A) Canine FIX antigen levels measured by ELISA. (B) Whole blood clotting time (WBCT); m indicates minutes. (C) Activated partial thromboplastin time (aPTT); s indicates seconds. x axis represents time in days.
Discussion
Muscle represents an ideal target tissue for AAV-mediated FIX gene transfer in hemophilia B subjects with advanced liver disease due to viral hepatitis infection. Our early studies on intramuscular delivery of an AAV-2 vector encoding FIX in hemophilia B dogs and humans showed that a major obstacle that must be overcome to achieve therapeutic transgene expression is to effect widespread muscle transduction. In this work, we show for the first time the sustained expression of the cFIX transgene product at therapeutic levels for a period of more than 4 years in a large-animal model of hemophilia B. Through a simple, noninvasive procedure, which does not require the administration of vasoactive drugs,34 we were able to reach levels of cFIX transgene expression up to 10-fold higher than those measured after the intramuscular injection of the same vector at a dose of approximately 3 × 1012 vg/kg.5 Importantly, no acute or chronic toxicity was associated with the delivery of the vector under pressure, the tourniquet placement, or long-term expression of cFIX from transduced muscle.
The delivery technique used in this study, ATVRX, was first described by Su et al in a proof-of-concept study in which both adenovirus and AAV vectors expressing β-galactosidase were used, with up to 100% efficiency of muscle transduction in rats and dogs.35 More recently, the same technique was used in nonhuman primates in an AAV gene transfer study in which a vector encoding the secreted immunomodulatory protein LEA29Y, a mutated form of CTLA4Ig, was injected at a single dose of 5 × 1012 vg/kg. In this study, expression levels of transgene were stable for at least 34 months and 4- to 8-fold higher after intravascular (via ATVRX) versus intramuscular delivery of vector.38 A subsequent study from the same group used the LEA29Y transgene delivered intramuscularly or intravascularly to prevent responses to the transactivator protein rtTA-M2,39 showing better results when the vector encoding LEA29Y was administered via ATVRX. Several other reports show successful muscle transduction with viral and nonviral vectors using intravascular routes with or without pressure delivery.40-42 For example, Qiao et al showed expression of the myostatin propeptide gene in normal dogs after hydrodynamic injection of an AAV-8 vector43 with no apparent toxicity. However, a preliminary report from the same group44 showed muscle inflammation and atrophy 11 weeks after the administration of an AAV-9 vector encoding minidystrophin in neonatal Duchenne muscular dystrophy dogs. This suggests that careful evaluation of the immunogenic profile of the therapeutic transgene expressed in muscle will be required in the context of the specific disease target. This is particularly relevant for those diseases characterized by a lack of tolerance to the transgene product, such as hemophilia, or by inflammation of the target organ, such as muscular dystrophy. For these diseases, immunomodulation may be necessary to prevent harmful responses to the transgene product (reviewed in Arruda et al32 ).
The study described here represents the first ATVRX dose-escalation study in which expression levels of the therapeutic transgene were followed long term in a large-animal model of disease. We showed that ATVRX administration of an AAV2 vector expressing cFIX under the control of a constitutive promoter led to a dose-dependent detection of circulating cFIX, suggesting that the muscle tissue biosynthetic machinery can produce active FIX; this is in accordance with what we have previously described.45 At high vector doses (8 × 1012 vg/kg), dose dependence of transgene expression is lost, likely due to the development of a low-titer nonneutralizing antibody to cFIX. What makes this finding intriguing is that at high vector doses the immune response to the transgene product develops despite the use of transient immunosuppression with cyclophosphamide. However, one important observation is that, in the dogs treated at the highest vector dose with immunosuppression, the measured antibody is not neutralizing, thus the humoral response measured is somewhat benign, as it diminishes levels of cFIX without neutralizing its activity, resulting in partial correction of the disease phenotype, and preservation of the ability to respond to infused protein, albeit likely requiring higher doses.
In earlier work, we showed that intravascular delivery of AAV vectors to skeletal muscle of hemophilia B dogs with coadministration of vasoactive drugs requires transient immunosuppression with cyclosphosphamide to prevent the formation of neutralizing antibodies to the cFIX transgene.34 In the absence of immunosuppression, vector administration through this procedure resulted in high-titer inhibitory antibodies to AAV (up to ∼30 BU). Here, 2 hemophilia B dogs received an AAV2-cFIX vector via ATVRX with no immunomodulation; 1 dog expressed the transgene at levels comparable with the immunosuppressed dogs that received the same dose, whereas the other dog developed a transient inhibitory antibody to the cFIX transgene peaking at 1.5 BU. This dog developed a predominantly Th1 response to the cFIX antigen, with an increase in IgG1 antibodies coinciding with the detection of inhibitor; after that, a nonneutralizing antibody response (Th2 driven, with production of anti-cFIX IgG2) became predominant with detectable cFIX in the circulation and correction of the disease phenotype. Although this would argue for a lower immunogenicity of ATVRX compared with the intramuscular38,46 and intravascular34 routes, in terms of safe translation of this approach to the clinics IS seems to be a requirement in the context of muscle-directed gene transfer. This is particularly true for loss-of-function diseases, in which absence of a gene product prevents thymic deletion of autoreactive T-cell clones. The use of immunosuppression in the context of AAV-mediated muscle gene transfer has already been safely tested in other disease models,47,48 including human subjects undergoing AAV1 intramuscular gene transfer for lipoprotein lipase deficiency49,50 and AAV6 gene transfer in dog model of Duchenne muscular dystrophy.47 Although the purpose of IS has been mostly to explore to prevent capsid-specific T-cell responses there are examples transient immunosuppression coupled with AAV delivery to skeletal muscle the approach is feasible and safe. When designing muscle-targeted AAV gene transfer strategies, the risk of immune responses to the vector transgene product should be evaluated on a case-by-case basis, taking into account the role of danger signals, such as pre-existing inflammation in the muscle, and the level of tolerance to the transgene at the time of gene transfer. Finally, the risk of incurring harmful T-cell responses to the gene transfer vector itself should also be taken into consideration when an intravascular route of administration is chosen, as systemic exposure upon tourniquet release may result in enhanced immunogenicity in the target tissue or other organs. For example, there are modest levels of vector biodistribution to the liver after ATVRX in dogs, presumably due to systemic exposure after the tourniquet is released (V.H., F.M., and K.A.H., unpublished data, February 2010).
Results with the ATVRX delivery of an AAV6-cFIX vector presented here have multiple implications. First, in the presence of anti–AAV-2 NAB titers of 1:100, which can be considered high, efficient muscle transduction can be achieved. This is an important result, as antibodies to AAV serotype 2 are highly prevalent in the general population and switching serotypes is a feasible approach to vector readministration. Second, using AAV serotypes that more efficiently transduce muscle, combined with ATVRX, closes the gap in terms of levels of transgene expression in muscle- versus liver-directed AAV gene transfer. Third, an earlier objection to muscle-directed gene transfer, that it would likely require transient immunosuppression in the setting of a genetic disease in which the host may not be tolerant to the transgene product, may also be lessened if it becomes clear that AAV-mediated gene transfer to liver will also require short-term immunosuppression.10,31 These considerations bolster the case for continued development of ATVRX as a delivery method for AAV-mediated gene transfer in the setting of hemophilia.
In summary, our results showed that the intravascular delivery of an AAV vector to muscle results in sustained expression of the FIX transgene product at therapeutic levels. This procedure has proven to be safe and effective in correcting the disease phenotype in a large-animal model of hemophilia B, thus laying the foundation for future clinical development of this novel therapeutic approach in humans with advanced liver disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Mr Junwei Sun for his support with paper preparation and Mr Alex Tai and Mr Yi Zhao for their assistance with AAV vectors preparation.
This work was supported by a grant from the National Institutes of Health (HL64190 to K.A.H, and Project 1 of HL64190 to V.R.A.), the Howard Hughes Medical Institute, and the Center for Cellular and Molecular Therapeutics at the Children's Hospital of Philadelphia.
National Institutes of Health
Authorship
Contribution: V.R.A. directed design and execution of the experiments and drafted the paper; H.H.S provided insights on the surgical protocol design and execution; V.H., G.B., S.B., P.F., and Y.C. participated in the animals' laboratory evaluations and follow-up; S.Z. and J.F.W. provided the AAV vectors used in the study; L.B.C., H.J., and G.F.P. provided assistance in experimental design; T.C.N., H.G.F., and D.A.B. performed the vector administrations and surgical procedures, provided care to the animals, and assisted with experimental design and interpretation; F.M. provided insights on protocol design and drafted the paper; and K.A.H. directed experimental design, conducted data analysis and interpretation, and drafted the paper.
Conflict-of-interest disclosure: J.F.W., F.M., and K.A.H. are consultants for companies that are developing AAV-based therapeutics not in the field of hemophilia and hold patents related to AAV gene therapy. The remaining authors declare no competing financial interests.
Correspondence: Katherine High, The Children's Hospital of Philadelphia, 3501 Civic Center Blvd, 5060 Colket Center for Translational Research, Philadelphia, PA 19104; e-mail: high@email.chop.edu.


![Figure 5. Readministration of AAV vectors by ATVRX in HB dogs previously exposed to AAV-2 vectors. (A-B) Canine FIX levels measured by ELISA and coagulation activity (WBCT in minutes [m]) in dogs D32 (red) and B46 (black) upon readminstration of an AAV2-cFIX vector via ATVRX. (D-E) Canine FIX levels measured by ELISA and coagulation activity (WBCT in minutes [m]) in dogs E59 (red) and H27 (black) upon readminstration of an AAV6-cFIX vector via ATVRX. (C,F) Creatinine phosphokinase (CPK) levels. x axis represents time in days.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/23/10.1182_blood-2009-12-261156/4/m_zh89991053360005.jpeg?Expires=1769085041&Signature=2QO1HN6aUMUMumMk1TNWo28f4NB9EPShUlmxeZxhBl-LMWZkHLOjMcwGBYpgHFlmRsaNYXqrOk3ijpM6GozNw-BvezcLRCJufZ3JsIFNCW1g~Jem-FATUcEB~zhbl-UC9Y-W1vggJ5CE6mLoKy-BKFUALPY1zR2yjDlyppLxyglmbRuEHYFYzeDepHyoFCZOBMA-nuqn0dUU3SZWvgIgHiwSJf58SEsL8joUHshZ0rYtLFcowPxHBuKgMoMQBU5W34jYvShBtnOUPjkrdDxiEwnVsAVK~D22wHKB0aF8VrwDGg44vMXY8kWxHpH0MlbOUvjLpYvtwp1sfLLgeGGNBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)