Abstract
Transforming growth factor-β (TGF-β) has an essential role in the generation of inducible regulatory T (iTreg) and T helper 17 (Th17) cells. However, little is known about the TGF-β–triggered pathways that drive the early differentiation of these cell populations. Here, we report that CD4+ T cells lacking the molecular adaptor tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) exhibit a specific increase in Th17 differentiation in vivo and in vitro. We show that TRAF6 deficiency renders T cells more sensitive to TGF-β–induced Smad2/3 activation and proliferation arrest. Consistent with this, in TRAF6-deficient T cells, TGF-β more effectively down-regulates interleukin-2 (IL-2), a known inhibitor of Th17 differentiation. Remarkably, TRAF6-deficient cells generate normal numbers of Foxp3-expressing cells in iTreg differentiation conditions where exogenous IL-2 is supplied. These findings show an unexpected role for the adaptor molecule TRAF6 in Smad-mediated TGF-β signaling and Th17 differentiation. Importantly, the data also suggest that a main function of TGF-β in early Th17 differentiation may be the inhibition of autocrine and paracrine IL-2–mediated suppression of Th17 cell generation.
Introduction
A major research effort has been focused on the identification of cytokines, signaling mediators, and transcription factors that regulate T helper 17 (Th17) differentiation.1 It is well recognized that the synergistic effects of transforming growth factor-β (TGF-β) and interleukin-6 (IL-6) are required for the generation of Th17 cells in mice and humans.2,3 However, it is not well understood how these cytokines induce the Th17 differentiation program.
TGF-β is essential to maintain high IL-6 receptor surface levels in T-cell receptor (TCR)–activated cells and also induces the expression of the Th17 lineage-specific factor retinoic acid–related orphan receptor γ, isoform t (RORγt).4,5 In addition, IL-23 receptor expression, which is required to stabilize the Th17 cell phenotype, can be induced or repressed by TGF-β in a concentration-dependent manner.5 It is largely unknown which TGF-β pathways (Smad-dependent vs Smad-independent) mediate these events or what their early molecular targets are during Th17 differentiation.
Several studies have shown that IL-6 stimulation inhibits TGF-β–driven Foxp3 expression and inducible regulatory T (iTreg) development from naive CD4+ T cells, possibly acting as a switch between Th17 and Treg cell differentiation in vivo.6,7 The reciprocal developmental relationship between both cell subsets is also evidenced by the effect of IL-2 in promoting Foxp3 expression and Treg development while down-regulating RORγt levels and Th17 differentiation.7,8 It has been speculated that TGF-β favors the generation of Th17 cells by inhibiting IL-2 production, although other mechanisms (eg, down-regulation of Th1 and Th2 responses) are also possible.8-10
Tumor necrosis factor (TNF) receptor–associated factor 6 (TRAF6) is a unique member of the TRAF family of adaptor proteins that mediates signaling downstream of a variety of receptors.11 In a mouse model of T cell–specific TRAF6 deficiency (TRAF6-ΔT) we previously demonstrated a CD4+ T cell–intrinsic role for TRAF6 in the maintenance of peripheral tolerance and anergy induction.12-14 We report now that after inflammatory stimuli, both in vitro and in vivo, IL-17–producing cells are selectively increased in TRAF6-ΔT mice. The enhanced generation of Th17 cells is associated with increased TGF-β–induced Smad2/3 activation and TGF-β–dependent IL-2 down-regulation. Interestingly, IL-2–dependent iTreg generation is intact in TRAF6-deficient T cells. Collectively, these findings show an unexpected role for the adaptor molecule TRAF6 in Smad-mediated TGF-β signaling and Th17 differentiation. In addition, the data imply that during early Th17 differentiation TGF-β plays a significant role in the down-regulation of IL-2, a known inhibitor of Th17 cell generation.
Methods
Mice
The TRAF6-ΔT mice (TRAF6flox/flox CD4-Cre) have been previously described. All experiments were performed with the use of 4- to 12-week-old mice backcrossed to the C57BL/6 background for at least 10 generations. Littermate TRAF6flox/flox or heterozygous mice (TRAF6flox/+ CD4-Cre) were used as controls. Congenic CD45.1 C57BL/6 mice used in this study were purchased from Taconic. Animal procedures were approved by the subcommittees on research animal care at the University of Pennsylvania.
Antibodies and cytokines
Purified monoclonal antibodies for CD3 (2C11), CD28 (37N.51), IL-2 (S4B6), IL-4 (11B11), and interferon-γ (IFN-γ; XMG1.2) were produced in-house. The following fluorophore- and biotin-conjugated monoclonal antibodies for surface and intracellular cell staining were obtained from BD Biosciences: CD4 (RM4-5), CD25 (PC61), CD44 (IM7), CD62L (MEL-14), IFN-γ (XMG1.2), IL-17 (TC11-18H10). Fluorophore-conjugated anti–IL-13 (eBio13A) and anti-Foxp3 (FJK-16s) were purchased from eBioscience. Recombinant mIL-1, hIL-2, mIL-4, mIL-12, mIL-6, and hTGF-β1 were obtained from PeproTech. Anti-biotin magnetic beads were purchased from Miltenyi Biotec. CFSE (5,6-carboxyfluorescein diacetate, succinimidyl ester) was purchased from Invitrogen. Western blot antibodies specific for phospho–signal transducer and activator of transcription 1 (STAT1; 58D6), phospho-STAT3 (3E2), phospho-p38, phospho-SMAD2 (138D4), phospho-SMAD3 (C25A9), STAT1, STAT3, p38, Smad2, and Smad3 were from Cell Signaling; the antibody specific for TRAF6 was from Epitomics.
Colitis induction
Four-week-old weight- and sex-matched TRAF6-ΔT and control littermates were used in the studies. Dextran sodium sulfate (DSS; molecular weight, 36 000-50 000 kDa; MP Biochemicals) was supplied ad libitum for 7 days at a 2% (wt/vol) concentration. Mouse survival was assessed daily for up to 20 days. Alternatively, mice were killed on day 7 to evaluate disease severity. Blood was collected for serum isolation, colons were removed for measurement and lamina propria lymphocyte isolation; distal colons were fixed in 10% buffered formalin and embedded in paraffin. To assess colon pathology, 2 nonconsecutive slides/sample were processed for routine hematoxylin and eosin (H&E) staining before visualization for cell infiltrates and tissue damage. Images were acquired on a Nikon Diaphot 300 inverted microscope (10×/0.25 NA objective lens) using a Spot RT color camera (Diagnostic Instruments). Images were processed with Adobe Photoshop 7.0.1 (Adobe Systems).
Isolation of lamina propria lymphocytes
Colons were opened longitudinally, washed with phosphate-buffered saline to remove fecal content, and shaken in 5mM EDTA (ethylenediaminetetraacetic acid) in Hanks balanced salt solution for 20 minutes at 37°C to remove epithelial cells. Tissues were minced into small pieces (< 2 mm2) and incubated with RPMI 1640 containing 4% fetal bovine serum, 1 mg/mL collagenase type II (Invitrogen), 1 mg/mL dispase (Invitrogen), and 40 μg/mL DNase I (Roche Diagnostics) for 1 hour at 37°C while shaking. Digested tissues were washed with 5mM EDTA in Hanks balanced salt solution, resuspended in a 40% Percoll (GE Healthcare) solution, and layered over an 80% Percoll solution. Lamina propria lymphocytes were collected at the interphase of the Percoll gradient after centrifugation at 750g for 20 minutes. Cells were washed with RPMI 1640 and used immediately for experiments.
Cell purification and in vitro differentiation
Enriched CD4+ T-cell cultures were isolated from spleen and lymph nodes after red blood cell lysis and depletion of CD8+ and NK1.1+ cells by magnetic bead (NEB) separation. Cells were plated in 96-well round-bottomed plates (Costar) at 1 × 106 cells/mL and stimulated with anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL). When indicated, cells were cultured in Th1 (IL-12 [10 ng/mL] plus anti–IL-4 [10 μg/mL]), Th2 (IL-4 [5 ng/mL] plus anti–IFN-γ [10 μg/mL]), Th17 (TGF-β [1 ng/mL] and IL-6 [50 ng/mL] plus anti–IFN-γ [10 μg/mL] and anti–IL-4 [10 μg/mL]) or iTreg (TGF-β [1 ng/mL] plus anti–IFN-γ [10 μg/mL] and anti–IL-4 [10 μg/mL]) conditions. Cells were supplemented with fresh medium and reagents on day 3 and were collected on day 4.
When indicated, naive CD4+ T cells (CD62LhiCD44loCD25−) were sort-purified on a FACSAria (BD Biosciences). Naive cells were stimulated in flat-bottom plates with plate-coated anti-CD3 (1 μg/mL) and soluble anti-CD28 (2 μg/mL) for 3 to 5 days.
Analysis of cytokine production
CD4+ T cells were restimulated with phorbol myristate acetate (50 ng/mL) and ionomycin (500 ng/mL) plus Brefeldin A (BD Biosciences) at 37°C for 4 ours. The intracellular cytokine content was determined with the Cytofix/Cytoperm Kit Plus (BD Biosciences) according to the manufacturer's instructions and flow cytometric analysis in a FACSCalibur (BD Biosciences).
Enzyme-linked immunoabsorbent assay
Enzyme-linked immunoabsorbent assay (ELISA) kits were purchased from BD Biosciences (IL-2, IL-17, IL-5, IFN-γ) and Bethyl Laboratories (mouse albumin).
Western blotting
Cells were isolated by positive selection with magnetic beads (Miltenyi Biotec) and stimulated in flat-bottom plates with plate-coated anti-CD3 (1 μg/mL) and soluble anti-CD28 (2 μg/mL) for 3 days. Cells were washed and expanded in 96-well round-bottom plates in complete RPMI media containing IL-2 (50 U/mL) for 18 to 72 hours. Cells were purified by a Ficoll-Paque Plus gradient (GE Healthcare) before assessing their purity by flow cytometry. After stimulation, cells were harvested with ice-cold phosphate-buffered saline and lysed in buffer containing 20mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4), 150mM NaCl, 1.0% Triton X-100, 10% glycerol, and protease and phosphatase inhibitors. Lysates were incubated on ice for 20 minutes, clarified by centrifugation at 20 000g for 10 minutes, and boiled with sodium dodecylsulfate loading buffer. Samples were run on 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore). Blots were probed with primary antibodies in 5% milk dissolved in Tris (tris(hydroxymethyl)aminomethane)–buffered saline with 0.1% Tween-20, followed by secondary horseradish peroxidase–conjugated anti–rabbit or anti–mouse immunoglobulin G (Promega). Western blots were incubated with enhanced chemiluminescence (ECL) substrate (Pierce Chemical) and exposed to film. Films were digitally scanned to allow quantitation of optical densities of individual bands with the use of Scion Image software (National Institutes of Health).
Quantitative real-time PCR
Total cellular RNA was extracted using TRIzol (Invitrogen), and cDNA was synthesized with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). TaqMan probes for Foxp3, IL-17, t-bet, GATA-3, 18S ribosomal RNA were purchased from Applied Biosystems. Quantitative real-time polymerase chain reaction (PCR) was performed on an ABI Prism 7000 Sequence Detection system. All samples were run in triplicate, and relative mRNA expression levels were determined after normalizing all values to 18S RNA.
T-cell proliferation assays
CFSE-labeled cells were cultured in 96-well flat-bottom plates and stimulated with plate-bound anti-CD3 (1 μg/mL) and soluble anti-CD28 (2 μg/mL) with different doses of TGF-β1. Cell proliferation was assessed by flow cytometry after 72 hours of culture. Alternatively, proliferation was determined in unlabeled cells cultured in identical conditions by measuring thymidine incorporation during the final 12 hours of a 72-hour culture.
Statistical analysis
An unpaired Student t test was used to determine statistical significance, and P values of less than .05 were considered significant. When indicated, statistical significance was determined with a paired Student t test.
Results
Increased IL-17 levels in TRAF6-ΔT mice with DSS-induced acute colitis
TRAF6-ΔT mice spontaneously develop a progressive inflammatory phenotype characterized by intestinal mononuclear cell infiltrates that can be detected as early as 6 weeks of age.14 To better dissect these events and investigate the role of T cell–expressed TRAF6 during experimentally induced in vivo inflammation, we used TRAF6-ΔT mice in a DSS-induced colitis model. To ensure that the interpretation of our experiments would not be complicated by the development of spontaneous inflammation, young mice (4 weeks old) were used in all colitis experiments.
Acute colitis was induced in TRAF6-ΔT and control littermate mice by oral DSS administration. Four-week-old control mice were highly susceptible to this treatment, because 73% (11 of 15) died between days 5 and 12 after starting DSS. In contrast, only 36% (4 of 11) of TRAF6-ΔT mice died during this period (Figure 1A). All mice surviving after day 12 recovered to their normal physical appearance and stool consistency, and they were killed 30 days after treatment. Disease severity was assessed at day 7 by analysis of colon length, fecal albumin content, and distal colon histology. Consistent with decreased disease severity, TRAF6-ΔT mice exhibited a significant attenuation of colon shortening (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and lower levels of fecal albumin (supplemental Figure 1B). Histologic analysis at day 7 of DSS treatment showed cell infiltrates and perturbed surface epithelium in the distal colon of all control mice analyzed, whereas none of the TRAF6-ΔT mice exhibited visible cell infiltrates or perturbed epithelium (Figure 1B).
TRAF6-ΔT mice show resistance to DSS-induced acute colitis with an accumulation of IL-17–producing lamina propria lymphocytes. Acute colitis was induced in weight-matched TRAF6-ΔT and control 4-week-old mice by administering DSS in drinking water (2% wt/vol) for 7 days. (A) Mice survival was followed for 20 days. (B) Distal colon histology was assessed at day 7 of DSS treatment. A total of 5 DSS-treated mice/group and 2 water-treated mice/group were analyzed. (C) Lamina propria lymphocytes isolated from the large intestine of day 7 DSS-treated mice were stimulated in vitro to analyze intracellular cytokine content by flow cytometry. Representative flow cytometric plots show cells gated as Thy1.2+CD4+. (D) Serum was isolated from day 7 DSS- and water-treated TRAF6-ΔT and control mice. IL-17 levels in the sera were determined by ELISA. (C-D) Charts represent data from 2 independent experiments. *P < .05.
TRAF6-ΔT mice show resistance to DSS-induced acute colitis with an accumulation of IL-17–producing lamina propria lymphocytes. Acute colitis was induced in weight-matched TRAF6-ΔT and control 4-week-old mice by administering DSS in drinking water (2% wt/vol) for 7 days. (A) Mice survival was followed for 20 days. (B) Distal colon histology was assessed at day 7 of DSS treatment. A total of 5 DSS-treated mice/group and 2 water-treated mice/group were analyzed. (C) Lamina propria lymphocytes isolated from the large intestine of day 7 DSS-treated mice were stimulated in vitro to analyze intracellular cytokine content by flow cytometry. Representative flow cytometric plots show cells gated as Thy1.2+CD4+. (D) Serum was isolated from day 7 DSS- and water-treated TRAF6-ΔT and control mice. IL-17 levels in the sera were determined by ELISA. (C-D) Charts represent data from 2 independent experiments. *P < .05.
These observations prompted us to analyze how T cell–specific TRAF6 deficiency affected T-cell differentiation in DSS-treated mice. Several recent studies together show a complex role for IL-17 in the attenuation of DSS-induced acute colitis.15-17 IL-17 is produced by a CD4+ T-cell subset (Th17), which is constitutively localized to the intestinal lamina propria. Interestingly, intracellular cytokine staining of lamina propria lymphocytes isolated from the large intestine of DSS-treated mice showed a specific increase in IL-17–producing CD4+ T cells in TRAF6-ΔT mice (Figure 1C-D).
TRAF6-deficient T cells exhibit enhanced Th17 differentiation in vitro
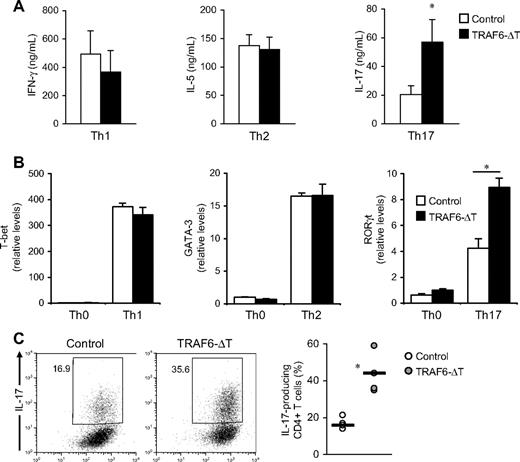
To investigate the mechanistic basis of the increased numbers of IL-17–producing cells found in TRAF6-ΔT mice, we cultured control and TRAF6-deficient CD4+ T cells under Th1, Th2, and Th17 conditions. As previously shown,12 in vitro Th1 and Th2 differentiation (determined as percentages of IFN-γ and IL-13 cytokine-expressing cells, respectively) are not affected by the absence of TRAF6. In contrast, TRAF6-deficient cells exhibited increased numbers of IL-17–expressing cells (supplemental Figure 2) and contained higher IL-17 levels in culture supernatants (Figure 2A) when differentiated under Th17 conditions.
TRAF6-deficient CD4+ T cells exhibit increased Th17 differentiation in vitro. (A) CD4+ T cells isolated from TRAF6-ΔT and control mouse spleens were activated with anti-CD3 and anti-CD28 with accessory cells in Th1, Th2, and Th17 conditions. Intracellular cytokine content was determined by flow cytometry after 4-day culture. Cells were cultured in triplicate, and the experiment was repeated with similar results. (B) Relative RNA levels of transcription factors t-bet, GATA-3, and RORγt were determined by real-time PCR analysis. The experiment was repeated with similar results. (C) Naive (CD62L+CD44loCD25−) CD4+ T cells isolated from lymph nodes and spleen were stimulated with plate-coated anti-CD3 and soluble anti-CD28 under optimal Th17 conditions. Intracellular cytokine content was determined by flow cytometry after 5-day stimulation. Right panel shows results from 4 independent experiments. *P < .05.
TRAF6-deficient CD4+ T cells exhibit increased Th17 differentiation in vitro. (A) CD4+ T cells isolated from TRAF6-ΔT and control mouse spleens were activated with anti-CD3 and anti-CD28 with accessory cells in Th1, Th2, and Th17 conditions. Intracellular cytokine content was determined by flow cytometry after 4-day culture. Cells were cultured in triplicate, and the experiment was repeated with similar results. (B) Relative RNA levels of transcription factors t-bet, GATA-3, and RORγt were determined by real-time PCR analysis. The experiment was repeated with similar results. (C) Naive (CD62L+CD44loCD25−) CD4+ T cells isolated from lymph nodes and spleen were stimulated with plate-coated anti-CD3 and soluble anti-CD28 under optimal Th17 conditions. Intracellular cytokine content was determined by flow cytometry after 5-day stimulation. Right panel shows results from 4 independent experiments. *P < .05.
Real-time PCR analysis showed higher RORγt transcript levels in Th17-differentiated TRAF6-deficient cells, confirming that the increased IL-17 levels observed in ELISA and flow cytometric experiments were a result of enhanced Th17 differentiation (Figure 2B). As expected, we detected no significant changes in the expression levels of other lineage-specific transcription factors when cells were cultured in Th1 and Th2 conditions.
TRAF6-ΔT mice have increased numbers of activated CD4+ T cells.14 To rule out the possibility that cells in this population are “primed” to differentiate into the Th17 subset and to exclude any potential influence from TRAF6-ΔT accessory cells, we stimulated sorted naive (CD4+CD44loCD62L+CD25−) cells with anti-CD3/anti-CD28 antibodies in Th17 conditions. After restimulation, we again found that TRAF6-deficient cells exhibited increased numbers of IL-17 producers (Figure 2C), indicating that this was a T cell–specific effect.
To determine whether the enhanced generation of Th17 cells was caused by altered kinetics of cell differentiation, naive CD4+ T cells isolated from control and TRAF6-ΔT mice were labeled with CFSE and activated under Th17 conditions. After 3 days, relative numbers of IL-17–producing cells were assessed by intracellular cytokine staining, whereas cell division number was determined by CFSE dilution. More TRAF6-deficient cells produced IL-17 at each individual cell division number, indicating that enhanced differentiation is not caused by faster proliferation and subsequent accumulation of Th17 cells (supplemental Figure 3).
Extrinsic factors affect the differentiation of TRAF6-deficient CD4+ T cells
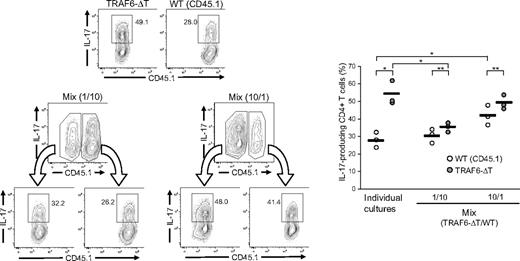
To determine whether the TRAF6-dependent inhibitory mechanism is mediated by extrinsic factors, congenic CD45.1+ wild-type and CD45.2+ TRAF6-deficient CD4+ T cells were cultured separately or cocultured under Th17 conditions. Coculture experiments were carried out at 1:10 and 10:1 ratios of TRAF6-deficient to wild-type cells. At the end of the assay, cells were stained for intracellular IL-17 and discriminated by CD45.1/45.2 expression. Compared with homogeneous cultures, TRAF6-deficient cells differentiated in a wild-type–enriched culture generated fewer Th17 cells, although still more than wild-type cells. Likewise, wild-type cells in cultures enriched with TRAF6-deficient cells generated more Th17 cells than when differentiated independently (Figure 3). However, TRAF6-deficient cells consistently generated more Th17 cells than wild-type cells in the same culture. Altogether, the results indicate that the differentiation of TRAF6-deficient cells is affected predominantly by cell extrinsic factors, suggesting an alteration in the regulation of cytokines that affect Th17 differentiation.
Extrinsic factors affect the differentiation of TRAF6-deficient Th17 cells. CD4+ T cells isolated from TRAF6-ΔT and congenic (CD45.1) wild-type mice were stimulated alone or mixed at 1:10 and 10:1 ratios (Mix) under Th17-skewing conditions. After 4 days of culture, cells were restimulated and stained for flow cytometric analysis. Numbers in flow cytometry plots indicate percentages of IL-17–expressing CD4+ T cells. Right panel represents results from triplicate wells in 1 of 2 experiments. Lines represent average values of the triplicate wells. *P < .05 (unpaired analysis), **P < .05 (paired analysis).
Extrinsic factors affect the differentiation of TRAF6-deficient Th17 cells. CD4+ T cells isolated from TRAF6-ΔT and congenic (CD45.1) wild-type mice were stimulated alone or mixed at 1:10 and 10:1 ratios (Mix) under Th17-skewing conditions. After 4 days of culture, cells were restimulated and stained for flow cytometric analysis. Numbers in flow cytometry plots indicate percentages of IL-17–expressing CD4+ T cells. Right panel represents results from triplicate wells in 1 of 2 experiments. Lines represent average values of the triplicate wells. *P < .05 (unpaired analysis), **P < .05 (paired analysis).
TRAF6-deficient T cells are more sensitive to TGF-β–induced signaling
Among the cytokines known to be important for Th17 induction are IL-6, IL-2, and TGF-β. Activation of STAT3 is an essential component of the Th17-inducing mechanism triggered by IL-6. Western blot experiments showed no differences in STAT3 activation between TRAF6-deficient and control cells stimulated with IL-6 alone or together with TGF-β (supplemental Figure 4), suggesting that TRAF6 does not have a main role in the modulation of early signaling events downstream of the IL-6 receptor.
IL-2 inhibits RORγt and Th17 differentiation through a STAT5-mediated pathway.8 To determine whether IL-2 inhibits the generation of TRAF6-deficient Th17 cells, we first blocked IL-2 activity in Th17 cultures with the use of a combination of neutralizing anti–IL-2 and blocking anti-CD25 antibodies. After restimulation, both control and TRAF6-deficient cell cultures generated roughly equal numbers of IL-17–producing cells (Figure 4). Notably, control cells exhibited a higher fold increase in the numbers of Th17 cells. The addition of recombinant human IL-2 resulted in strong suppression of IL-17–producers from both genotypes. In conclusion, IL-2 can inhibit Th17 differentiation of TRAF6-deficient cells.
IL-2 inhibits Th17 differentiation of TRAF6-deficient CD4+ T cells. CD4+ T cells were isolated from TRAF6-ΔT and control mice and stimulated under optimal Th17-polarizing conditions for 4 days (Th17). Neutralizing antibody against murine IL-2 (S4B6, 10 μg/mL) plus blocking antibody against CD25 (3C7, 10 μg/mL) or recombinant human IL-2 (100 U/mL) were added to the cultures as indicated. Numbers in flow cytometry plots indicate percentages of IL-17–expressing CD4+ T cells. Dot plot panels of conditions with added recombinant IL-2 (+ IL-2) are from a separate experiment. Bottom panels show the percentages of IL-17–positive cells from separate experiments using triplicate cultures per experimental condition. Values on top of bars indicate fold increase relative to cells differentiated in regular Th17 conditions. Experiments were repeated twice with similar results.*P < .01, **P < .05, ***P > .1.
IL-2 inhibits Th17 differentiation of TRAF6-deficient CD4+ T cells. CD4+ T cells were isolated from TRAF6-ΔT and control mice and stimulated under optimal Th17-polarizing conditions for 4 days (Th17). Neutralizing antibody against murine IL-2 (S4B6, 10 μg/mL) plus blocking antibody against CD25 (3C7, 10 μg/mL) or recombinant human IL-2 (100 U/mL) were added to the cultures as indicated. Numbers in flow cytometry plots indicate percentages of IL-17–expressing CD4+ T cells. Dot plot panels of conditions with added recombinant IL-2 (+ IL-2) are from a separate experiment. Bottom panels show the percentages of IL-17–positive cells from separate experiments using triplicate cultures per experimental condition. Values on top of bars indicate fold increase relative to cells differentiated in regular Th17 conditions. Experiments were repeated twice with similar results.*P < .01, **P < .05, ***P > .1.
TGF-β signaling is required for the differentiation of Th17 cells, although its downstream mediators are not well characterized in this context. To analyze whether TRAF6 deletion has an effect on TGF-β activity in T cells, naive TRAF6-deficient and control CD4+ T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 antibodies with increasing concentrations of TGF-β. Under these conditions, TRAF6-deficient cells exhibited a higher sensitivity to TGF-β–induced proliferation arrest than control cells, as determined by 3H-thymidine uptake after 3-day culture (Figure 5A). Similar results were obtained by determining the number of cell divisions in CFSE dilution experiments. Similar numbers of 7-amino-actinomycin D–negative cells in both cultures confirmed that differences in CFSE dilution were due to differences in proliferation rather than cell survival (supplemental Figure 5).
TRAF6-deficient CD4+ T cells are more sensitive to SMAD-mediated TGF-β signaling. CD4+ naive T cells were stimulated in 96-well flat-bottom plates with plate-coated anti-CD3 and soluble anti-CD28 with the indicated concentrations of TGF-β1. (A) Cell proliferation was measured by tritium incorporation during the last 8 hours of a 72-hour culture. Error bars represent SD from triplicate wells in 1 of 3 experiments. (B) IL-2 RNA levels were determined by real-time PCR analysis after 48 hours of stimulation. The experiment was repeated with similar results. (C) IL-2 protein levels in the supernatants were determined by ELISA after 72 hours of culture. Error bars represent SD from triplicate wells in 1 of 2 experiments. (D) CD4+ T cells were stimulated for 24 hours with plate-coated anti-CD3 and soluble anti-CD28. After 16 hours of resting, cells were stimulated with TGF-β1 (1 ng/mL) for the indicated times. Phosphorylated and total Smad2/3 proteins were detected in Western blot assays of cell lysates (left). Graphs represent relative amounts of phospho-Smad2 and phospho-Smad3 after normalization to total Smad2 and Smad3, respectively. Receptor levels were determined in cells before TGF-β1 stimulation (right). Figure shows a representative result from 3 independent experiments. (E) CFSE-stained naive CD4+ T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 in the presence of TGF-β1 (1 ng/mL) with or without IL-2. CFSE dilution due to cell proliferation was determined by flow cytometric analysis after 3 days of stimulation. Numbers and gates in the flow cytometry plots indicate percentage of cells with lower CFSE staining levels than undivided cells. The experiment was repeated with similar results. *P < .01, **P < .05.
TRAF6-deficient CD4+ T cells are more sensitive to SMAD-mediated TGF-β signaling. CD4+ naive T cells were stimulated in 96-well flat-bottom plates with plate-coated anti-CD3 and soluble anti-CD28 with the indicated concentrations of TGF-β1. (A) Cell proliferation was measured by tritium incorporation during the last 8 hours of a 72-hour culture. Error bars represent SD from triplicate wells in 1 of 3 experiments. (B) IL-2 RNA levels were determined by real-time PCR analysis after 48 hours of stimulation. The experiment was repeated with similar results. (C) IL-2 protein levels in the supernatants were determined by ELISA after 72 hours of culture. Error bars represent SD from triplicate wells in 1 of 2 experiments. (D) CD4+ T cells were stimulated for 24 hours with plate-coated anti-CD3 and soluble anti-CD28. After 16 hours of resting, cells were stimulated with TGF-β1 (1 ng/mL) for the indicated times. Phosphorylated and total Smad2/3 proteins were detected in Western blot assays of cell lysates (left). Graphs represent relative amounts of phospho-Smad2 and phospho-Smad3 after normalization to total Smad2 and Smad3, respectively. Receptor levels were determined in cells before TGF-β1 stimulation (right). Figure shows a representative result from 3 independent experiments. (E) CFSE-stained naive CD4+ T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 in the presence of TGF-β1 (1 ng/mL) with or without IL-2. CFSE dilution due to cell proliferation was determined by flow cytometric analysis after 3 days of stimulation. Numbers and gates in the flow cytometry plots indicate percentage of cells with lower CFSE staining levels than undivided cells. The experiment was repeated with similar results. *P < .01, **P < .05.
A main mechanism of TGF-β–induced proliferation arrest is the Smad-mediated block of IL-2 transcription in CD4+ T cells.18-20 Consistent with this mechanism, TRAF6-deficient cells expressed lower IL-2 mRNA (Figure 5B) and intracellular protein levels (supplemental Figure 6) than controls after stimulation with a range of concentrations of TGF-β. Analysis of culture supernatants by ELISA also showed a stronger inhibition of IL-2 production in TRAF6-ΔT cells (Figure 5C), although this was detected at only one of the concentrations of TGF-β tested. We believe that IL-2 consumption and stability in the supernatant could account for the differences between ELISA and intracellular staining or reverse transcription–PCR results. Consistent with enhanced Smad-mediated inhibition of IL-2 transcription, stimulation of TRAF6-deficient cells with TGF-β resulted in stronger and more sustained Smad2 and Smad3 phosphorylation (Figure 5D). At the same time, both control and TRAF6-deficient CD4+ T cells expressed similar TGF-β receptor levels (Figure 5D). Finally, the addition of IL-2 to the culture restored proliferation of TRAF6-deficient cells to levels similar to control cells (Figure 5E), suggesting that their higher sensitivity to TGF-β–induced arrest was the result of altered IL-2 regulation. Taken together, our data indicate that TRAF6 acts as a negative regulator of Smad-mediated TGF-β signaling in T cells and its deletion results in increased sensitivity to the biologic effects of TGF-β.
IL-2 is down-regulated during early Th17 differentiation and can drive normal iTreg differentiation from TRAF6-deficient cells when added exogenously
We reasoned that enhanced Th17 differentiation in TRAF6-deficient T cells could be the result of increased sensitivity to TGF-β signaling and associated inhibition of IL-2 expression. To test this possibility, we measured IL-2 transcript levels at an early time point (48 hours) during Th17 differentiation from naive CD4+ T cells. We found that IL-2 mRNA was down-regulated very early during Th17 differentiation and that this effect was magnified in TRAF6-deficient cells even at suboptimal concentrations of TGF-β (Figure 6A). As the negative effect of IL-2 on Th17 differentiation would predict, differences observed in IL-2 levels inversely correlated with levels of RORγt (Figure 6B) and the numbers of IL-17–producing cells (Figure 6C) at culture day 5. Analysis of Th1 and Th2 differentiation cultures showed that the differences in early IL-2 levels are specific to TGF-β–driven Th17 differentiation (supplemental Figure 7).
Increased TGF-β sensitivity is reflected in decreased IL-2 levels during Th17 differentiation but has no effect on iTreg differentiation. Naive CD4+ T cells isolated from TRAF6-ΔT and control mice were stimulated with plate-coated anti-CD3 and soluble anti-CD28 in Th17 (TGF-β1 [0, 0.1, 1.0 ng/mL] plus IL-6) or iTreg (TGF-β1 [0, 0.1, 1.0 ng/mL] plus IL-2) conditions. (A) Levels of IL-2 mRNA were analyzed by real-time PCR after 48-hour incubation. (B) Levels of RORγt mRNA were analyzed by real-time PCR after 5-day culture. (C) After 5 days of culture, cells were restimulated, and the amount of IL-17–producing cells was determined by flow cytometry. (D) After 5 days of culture, Foxp3 protein levels were determined by intracellular staining. Numbers and gates in the flow cytometry plots indicate percentages of Foxp3-expressing cells. (C-D) Right panels depict data from triplicate cultures. (E) Relative Foxp3 mRNA levels were determined by real-time PCR analysis after 5 days of culture. All experiments were repeated at least 2 times. *P < .05, **P < .005.
Increased TGF-β sensitivity is reflected in decreased IL-2 levels during Th17 differentiation but has no effect on iTreg differentiation. Naive CD4+ T cells isolated from TRAF6-ΔT and control mice were stimulated with plate-coated anti-CD3 and soluble anti-CD28 in Th17 (TGF-β1 [0, 0.1, 1.0 ng/mL] plus IL-6) or iTreg (TGF-β1 [0, 0.1, 1.0 ng/mL] plus IL-2) conditions. (A) Levels of IL-2 mRNA were analyzed by real-time PCR after 48-hour incubation. (B) Levels of RORγt mRNA were analyzed by real-time PCR after 5-day culture. (C) After 5 days of culture, cells were restimulated, and the amount of IL-17–producing cells was determined by flow cytometry. (D) After 5 days of culture, Foxp3 protein levels were determined by intracellular staining. Numbers and gates in the flow cytometry plots indicate percentages of Foxp3-expressing cells. (C-D) Right panels depict data from triplicate cultures. (E) Relative Foxp3 mRNA levels were determined by real-time PCR analysis after 5 days of culture. All experiments were repeated at least 2 times. *P < .05, **P < .005.
Inducible Treg cells are generated in vitro after stimulation in the presence of TGF-β and IL-2.21 To determine whether exogenous IL-2 can offset the effect of TGF-β in TRAF6-deficient cells, we stimulated cells in iTreg differentiation conditions. In contrast to Th17 differentiation, TRAF6-deficient cells generated normal numbers of Foxp3-expressing iTreg cells at all doses of TGF-β (Figure 6D-E). The data collectively suggest that TRAF6 acts as a negative regulator of TGF-β–induced IL-2 suppression by dampening Smad2/3 activation.
Discussion
TGF-β inhibits T-cell proliferation through down-regulation of IL-2 expression as well as by altering the levels of cell-cycle regulators.22-25 CD28 costimulation attenuates this inhibitory effect by a mechanism that depends on residual levels of IL-2 expression.26 In these conditions, TRAF6-deficient cells proved more sensitive to proliferation arrest than control cells. Interestingly, the addition of IL-2 (ie, iTreg differentiation conditions) restores the proliferation of TRAF6-deficient cells while generating normal numbers of Foxp3-expressing cells, suggesting that their higher sensitivity to TGF-β–induced proliferation arrest is the result of decreased IL-2 expression.
Down-regulation of IL-2 expression by TGF-β occurs by a Smad-mediated block of gene transcription.19,20,27 Previous studies have shown that TRAF6 binds constitutively to a consensus site in the TGF-β type I receptor, leading to Smad-independent activation of the TFG-β–activated kinase-1 (TAK1)–p38/c-Jun N-terminal kinase (JNK) pathway after receptor engagement.28,29 In those studies, TRAF6 deletion did not alter Smad2/3 phosphorylation or the activity of a Smad-specific luciferase reporter in the Hep3B human hepatoma cell line. TRAF6 expression is up-regulated promptly in CD4+ T cells after TCR stimulation.14 Interestingly, we detected increased Smad phosphorylation after TGF-β stimulation of TCR-activated TRAF6-deficient cells but not in naive (CD4+CD44loCD62LhiCD25−) TGF-β–treated cells (data not shown). Further studies are necessary to ascertain if TRAF6-mediated modulation of Smad2/3 activity depends on TRAF6 expression levels and/or if it represents a secondary effect specific for activated T cells. To this point, we have been unable to detect TGF-β–induced TAK1/p38 activation in T cells. However, Th17 differentiation did not increase when cells were treated with pharmacologic inhibitors of p38/JNK (data not shown), suggesting the increased differentiation observed from TRAF6-deficient cells is not a consequence of defective TAK1-p38/JNK signaling.
IL-6 has a positive effect on costimulation during CD4+ T-cell activation as well as in protection from activation-induced cell death. In both cases, IL-6 exerts its effect in an IL-2–independent manner.30-32 Control and TRAF6-deficient cells proliferate equally when cultured under Th17 conditions (supplemental Figure 3), indicating that IL-6 stimulation bypasses the stronger effect of TGF-β in the proliferation arrest of TRAF6-deficient cells. More importantly, cell proliferation is restored while maintaining lower IL-2 levels in TRAF6-deficient cells in a TGF-β–dependent manner.
On the basis of our observations, we conclude that during early Th17 differentiation, TGF-β has a significant role in the down-regulation of IL-2 expression. The data suggest that TRAF6 inhibits the Smad-mediated transcriptional down-regulation of IL-2 acting as a negative regulator of Th17 differentiation. Interestingly, in experiments in which we modulated IL-2 levels in the culture (Figures 3–4) TRAF6-deficient cells still generated more Th17 cells than controls. Although this difference might result from an additional TRAF6-mediated intrinsic mechanism affecting Th17 differentiation, we believe it might be explained by reduced autocrine/intracrine IL-2 signaling in TRAF6-deficient cells. Ongoing experiments should clarify this aspect of our findings.
Finally, it remains unclear why increased TGF-β–mediated Smad2/3 activation in TRAF6-deficient cells would induce stronger down-regulation of IL-2 transcription while failing to induce higher Foxp3 mRNA levels in iTreg differentiation conditions. The answer to this question may lie in the different mechanisms (repressive vs activating) and temporal requirements for Smad2/3 in IL-2 and Foxp3 transcriptional regulation. TGF-β blocks IL-2 transcription by inhibition of the activity of an Il2 enhancer-binding octamer and by recruitment of the corepressor Tob to a Smad-binding element in the Il2 promoter.18,20 Determining the molecular mechanism for TGF-β–mediated Foxp3 induction has been more difficult. Interestingly, the Foxp3 promoter region does not contain any Smad-binding element sequences, instead binding other transcription factors, including STAT5.33 A Foxp3 enhancer site that binds Smad3 and nuclear factor of activated T cell to induce promoter activity has recently been identified34 ; however, Smad3 binding to this enhancer seems to be required only at early stages of TGF-β–dependent Foxp3 induction. The different requirements might indicate that only transcriptional mechanisms requiring a more permanent Smad2/3 presence will be sensitive to the effects of TRAF6 in TGF-β signaling.
We found an effect of TRAF6 deficiency in Th17 differentiation while analyzing DSS-induced colitis in the TRAF6-ΔT mice. In this model it is thought that epithelial cell toxicity to DSS leads to increased permeability of the gut mucosal barrier with accumulation of neutrophils and macrophages.35,36 T cells can affect disease pathology by their contributions to the cytokine milieu and consequent alterations of the inflammatory process.37 In addition, IL-17, produced mainly by intestinal lamina propria T cells,38 has a protective effect in the mucosal barrier by inducing tight junction formation in epithelial cells and mucin secretion.39,40 The protective effect is evident in vivo, because either antibody-mediated IL-17 neutralization or genetic deletion of IL-17 aggravate DSS-induced acute colitis.15,16 Our findings indicate that the absence of TRAF6 expression in T cells has a beneficial effect on disease progression. It is important to note that, although increased Th17 cell numbers and IL-17 serum levels in DSS-treated TRAF6-ΔT mice could explain their resistance to disease, other contributory mechanisms cannot be excluded.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by National Institutes of Health grant AI43260 (Y.C., L.A.T.) and grants AI61570 and AI074878 (D.A.).
National Institutes of Health
Authorship
Contribution: P.J.C. designed and performed experiments, analyzed the data, and wrote the manuscript; M.C.W., E.L.P., and D.H. helped design experiments, assisted in data interpretation, and contributed to the preparation of the manuscript; G.M.H. performed experiments; and D.A., L.A.T., and Y.C. supervised planning of experiments and data interpretation and contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yongwon Choi, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 421 Curie Blvd BRB II/III Rm 308, Philadelphia, PA 19104; e-mail: ychoi3@mail.med.upenn.edu.




![Figure 6. Increased TGF-β sensitivity is reflected in decreased IL-2 levels during Th17 differentiation but has no effect on iTreg differentiation. Naive CD4+ T cells isolated from TRAF6-ΔT and control mice were stimulated with plate-coated anti-CD3 and soluble anti-CD28 in Th17 (TGF-β1 [0, 0.1, 1.0 ng/mL] plus IL-6) or iTreg (TGF-β1 [0, 0.1, 1.0 ng/mL] plus IL-2) conditions. (A) Levels of IL-2 mRNA were analyzed by real-time PCR after 48-hour incubation. (B) Levels of RORγt mRNA were analyzed by real-time PCR after 5-day culture. (C) After 5 days of culture, cells were restimulated, and the amount of IL-17–producing cells was determined by flow cytometry. (D) After 5 days of culture, Foxp3 protein levels were determined by intracellular staining. Numbers and gates in the flow cytometry plots indicate percentages of Foxp3-expressing cells. (C-D) Right panels depict data from triplicate cultures. (E) Relative Foxp3 mRNA levels were determined by real-time PCR analysis after 5 days of culture. All experiments were repeated at least 2 times. *P < .05, **P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/23/10.1182_blood-2009-09-242768/4/m_zh89991053600006.jpeg?Expires=1770934890&Signature=y1ayy1JpQyKdG68efhM3kd7jfa7gxiphn-TTEMG00qpygP2D4AZaCYw0OTA7SdjRhvdhbB6IrvUxyXLm6As6VFBc2Qzzl2p11eiJDtJiYnDV2DGxRwg2yo6wRhKsODvVDQI3rz6SQgN4L0gW7TlJfUV40caFNFIfzmqCVKLeuk66GA-uQivTOsy8lWqjnvZ6ZcbT~wzIPW0WBZPlCtKHLCxChmViYKakbI~rcosS6EIQgUuTdvdMu4FrNSpXHCuh6ZZtpSEGDEYCc5bY3bMQlDa0BrPPbc2GoyNMPCQpWAS~xPtX4mhk~fUUHaznHxSBLStWkhbv9gm7e6w-CxTJxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal